Comparative Analysis of Bacterial Tick-Borne Pathogens in Questing Ticks from Sambia Peninsula, Kaliningrad Oblast, Russia: Spring and Autumn Prevalence and Public Health Risks
Abstract
1. Introduction
2. Materials and Methods
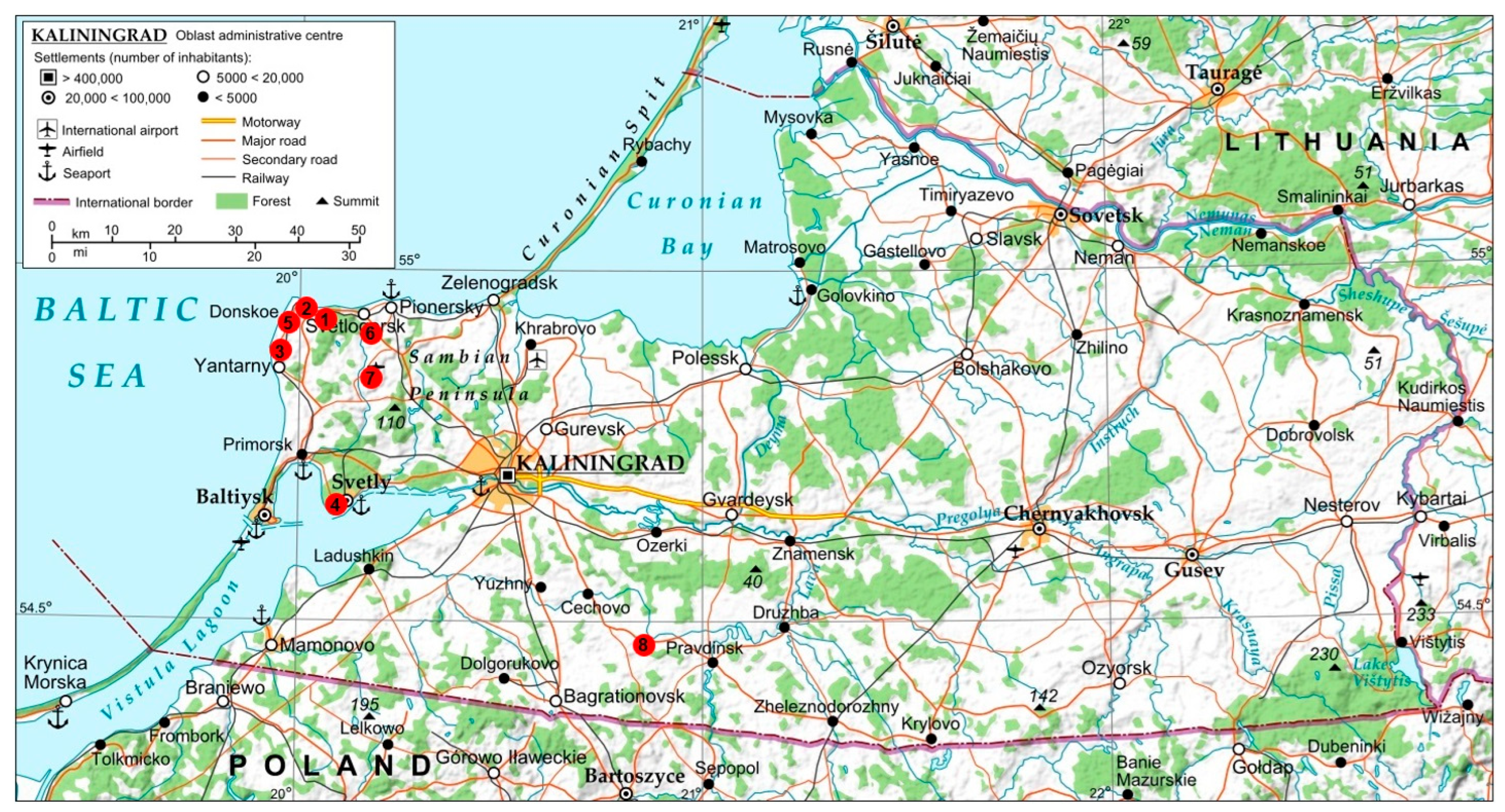
2.1. Study Area
2.2. Tick Collection
2.3. DNA Extraction and Quantitative PCR
2.4. Conventional PCR and Sanger Sequencing
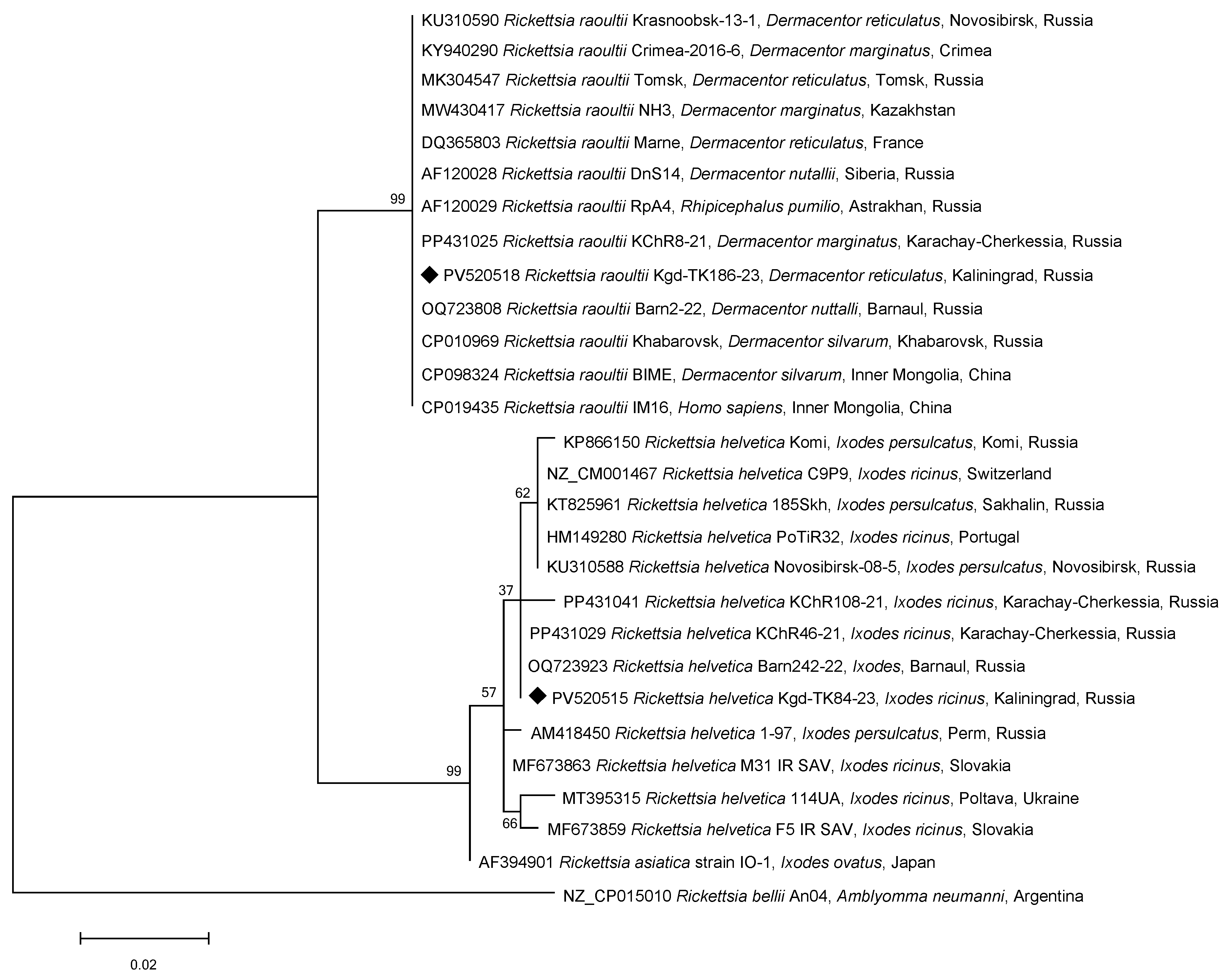
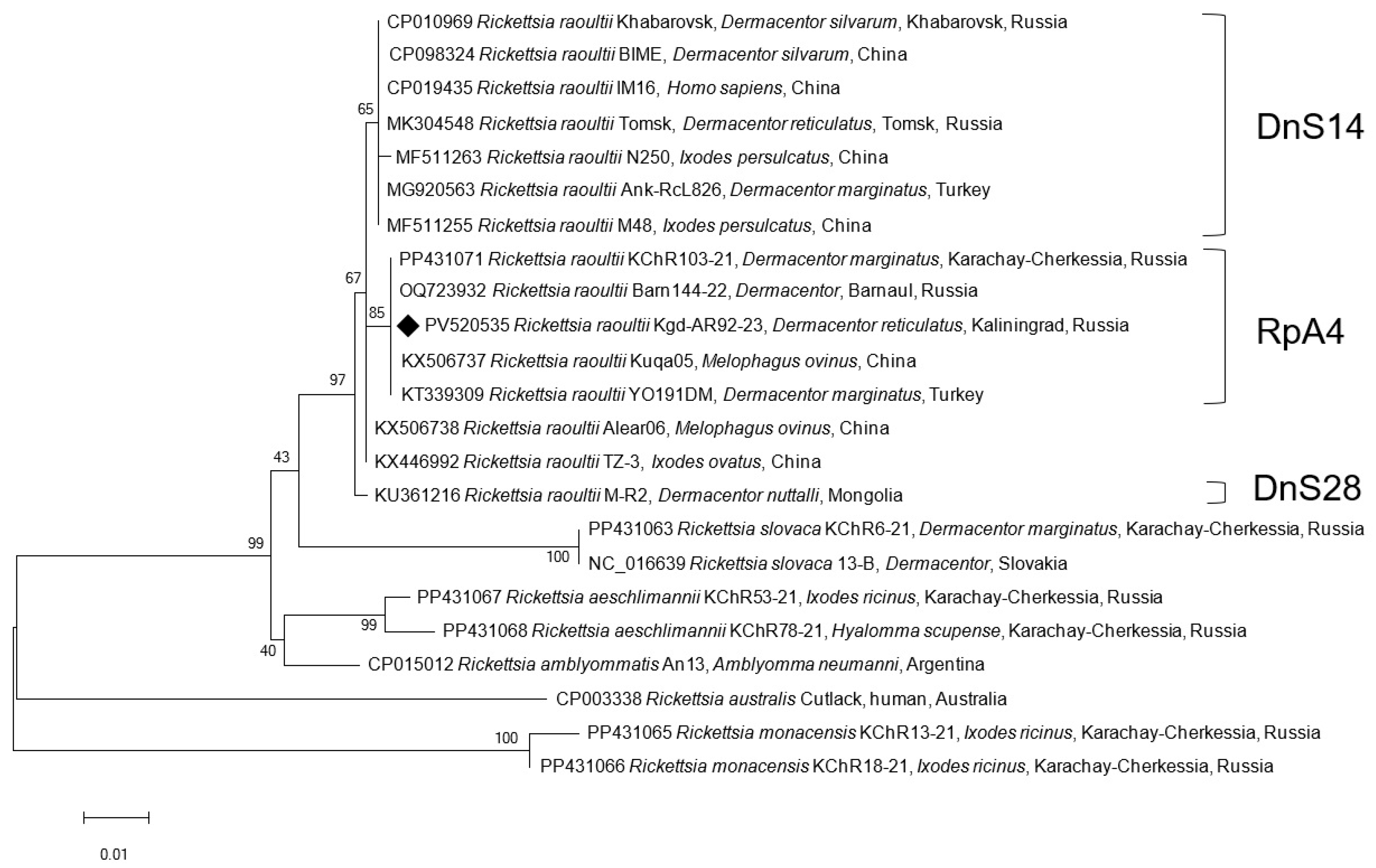
2.5. Phylogenetic and Statistical Analysis
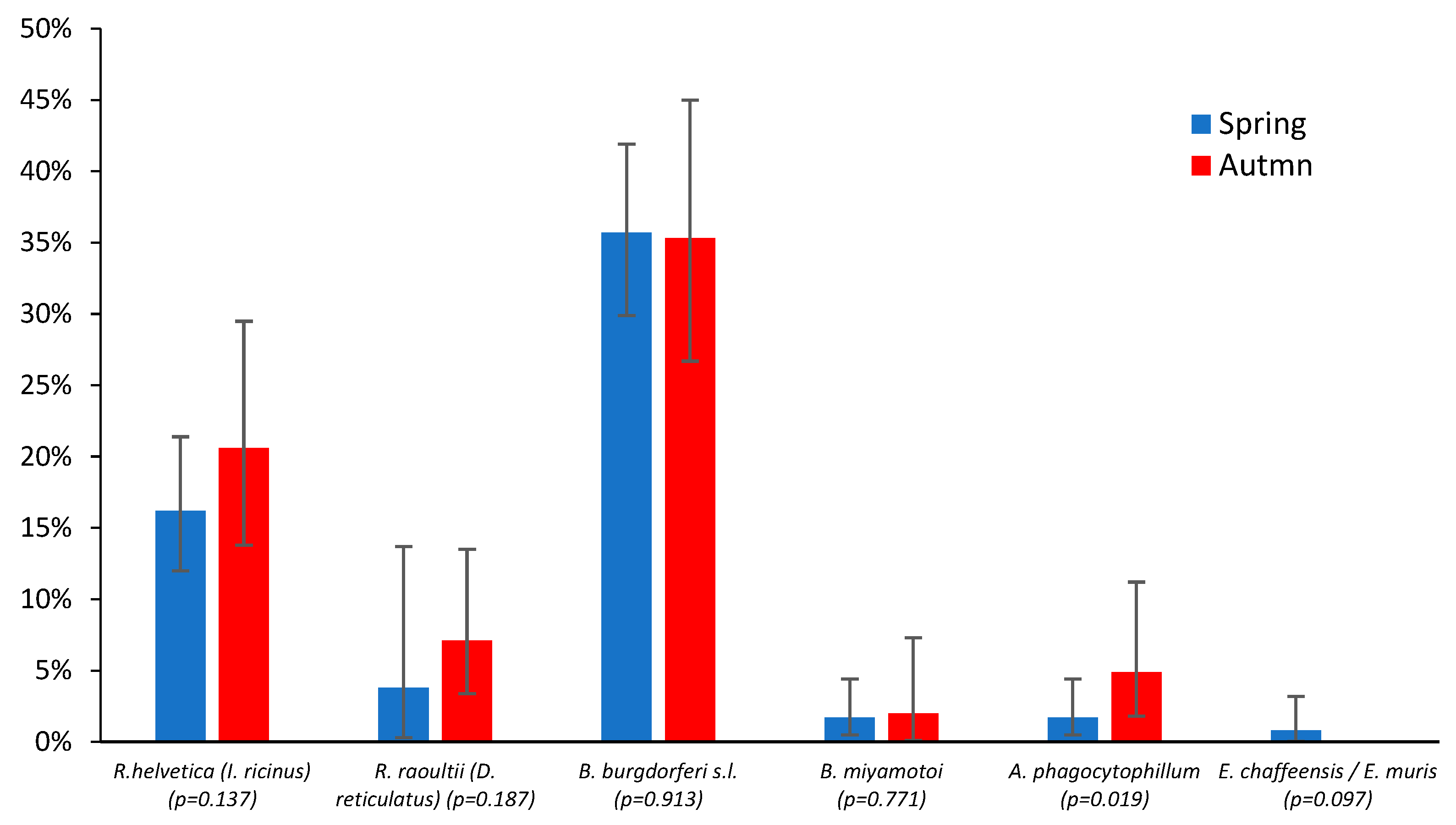
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBD | Tick-borne disease |
| TBEV | Tick-borne encephalitis virus |
| SFGR | Spotted fever group Rickettsia |
| TIBOLA | Tick-borne lymphadenopathy |
| DEBONEL | Dermacentor-borne necrosis erythema and lymphadenopathy |
| SENLAT | Scalp eschar and neck lymphadenopathy after tick bite |
| HGA | Human granulocytic anaplasmosis |
| HME | Human monocytotropic ehrlichiosis |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative polymerase chain reaction |
| CRIE | Central Research Institute of Epidemiology |
| CI | Confidence intervals |
| HTRF | Hard tick relapsing fever |
| BMD | Borrelia miyamotoi disease |
References
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Tsapko, N.V. List of species of ixodid ticks (Acari: Ixodidae) in Russia. Parazitologiya 2020, 54, 341–352. [Google Scholar] [CrossRef]
- Kartashov, M.Y.; Volchev, E.G.; Krivosheina, E.I.; Svirin, K.A.; Ternovoi, V.A.; Loktev, V.B. Genotyping of Borrelia, Rickettsia and Anaplasma in Ixodes ricinus and Dermacentor reticulatus ticks in the Kaliningrad region. J. Microbiol. Epidemiol. Immunobiol. 2024, 101, 227–236. [Google Scholar] [CrossRef]
- Kubiak, K.; Sielawa, H.; Dziekońska-Rynko, J.; Kubiak, D.; Rydzewska, M.; Dzika, E. Dermacentor reticulatus ticks (Acari: Ixodidae) distribution in north-eastern Poland: An endemic area of tick-borne diseases. Exp. Appl. Acarol. 2018, 75, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Ticks Tick. Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef]
- Asman, M.; Bartosik, K.; Jakubas-Zawalska, J.; Świętek, A.; Witecka, J. A New Endemic Locality of Dermacentor reticulatus in Central–Southern Poland and Its Potential Epidemiological Implications. Insects 2024, 15, 580. [Google Scholar] [CrossRef]
- Chmielewski, T.; Podsiadly, E.; Karbowiak, G.; Tylewska-Wierzbanowska, S. Rickettsia spp. in ticks, Poland. Emerg. Infect. Dis. 2009, 15, 486–488. [Google Scholar] [CrossRef]
- Radzijevskaja, J.; Paulauskas, A.; Aleksandraviciene, A.; Stanko, M.; Karbowiak, G.; Petko, B. New records of spotted fever group rickettsiae in Baltic region. Microbes Infect. 2015, 17, 874–878. [Google Scholar] [CrossRef]
- Reye, A.L.; Stegniy, V.; Mishaeva, N.P.; Velhin, S.; Hübschen, J.M.; Ignatyev, G.; Mulleret, C.P. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS ONE 2013, 8, e54476. [Google Scholar] [CrossRef]
- State Report “On the Status of Sanitary and Epidemiological Surveillance of the Population in the Kaliningrad Oblast in 2023”. Available online: https://39.rospotrebnadzor.ru/sites/default/files/gosudarstvennyy_doklad_2023.pdf (accessed on 6 June 2025).
- Piotrowski, M.; Rymaszewska, A. Expansion of Tick-Borne Rickettsioses in the World. Microorganisms 2020, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Estrada-Peña, A.; Rafael, M.; Almazán, C.; Bermúdez, S.; Abdelbaset, A.E.; Kasaija, P.D.; Kabi, F.; Akande, F.A.; Ajagbe, D.O.; et al. Perception of Ticks and Tick-Borne Diseases Worldwide. Pathogens 2023, 12, 1258. [Google Scholar] [CrossRef]
- Climate of the Kaliningrad Oblast. Available online: https://www.nbcrs.org/regions/kaliningradskaya-oblast/klimat (accessed on 6 June 2025).
- Flora of the Kaliningrad Oblast. Available online: https://www.nbcrs.org/regions/kaliningradskaya-oblast/flora (accessed on 6 June 2025).
- Filippova, N.A. Fauna of the USSR. Arachnida, Vol. 4, Part 4: Ixodid Ticks, Subfamily Ixodinae; Nauka: Leningrad, Russia, 1977; 396p. [Google Scholar]
- Rakov, A.V.; Chekanova, T.A.; Petremgvdlishvili, K.; Timonin, A.V.; Valdokhina, A.V.; Shirokostup, S.V.; Lukyanenko, N.V.; Akimkin, V.G. High Prevalence of Rickettsia raoultii Found in Dermacentor Ticks Collected in Barnaul, Altai Krai, Western Siberia. Pathogens 2023, 12, 914. [Google Scholar] [CrossRef]
- Sergeant, E.S.G. Epitools Epidemiological Calculators. Ausvet. 2018. Available online: https://epitools.ausvet.com.au (accessed on 6 June 2025).
- Rakov, A.V.; Chekanova, T.A.; Petremgvdlishvili, K.; Linnik, S.B.; Batchaev, K.K.; Akimkin, V.G. The Diversity of Spotted Fever Group Rickettsia Found in Ixodidae Hard Ticks Removed from Humans in Karachay-Cherkessia, North Caucasus, Russia. Microorganisms 2024, 12, 1293. [Google Scholar] [CrossRef]
- Rydkina, E.; Roux, V.; Rudakov, N.; Gafarova, M.; Tarasevich, I.; Raoult, D. New Rickettsiae in ticks collected in territories of the former Soviet Union. Emerg. Infect. Dis. 1999, 5, 811–814. [Google Scholar] [CrossRef]
- Buczek, W.; Buczek, A.; Asman, M.; Borzęcka-Sapko, A.; Minciel, E.; Grzeszczak, J.; Bartosik, K. Occurrence of Ticks and Tick-Borne Pathogens During Warm Winter—A Snapshot from Central Europe. Pathogens 2025, 14, 326. [Google Scholar] [CrossRef]
- Žygutienė, M.; Ranka, R.; Salmina, K. Genospecies of Borrelia Burgdorferi S.L. in Ixodes Ricinus Ticks in Lithuania. Acta Zool. Litu. 2003, 13, 385–389. [Google Scholar] [CrossRef]
- Turčinavičienė, J.; Ambrasiene, D.; Paulauskas, A.; Radzijevskaja, J.; Rosef, O.; Žygutienė, M. The prevalence and distribution of Borrelia burgdorferi sensu lato in host seeking Ixodes ricinus ticks in Lithuania. Biologija 2006, 52, 64–68. [Google Scholar]
- Paulauskas, A.; Ambrasiene, D.; Radzijevskaja, J.; Rosef, O.; Turcinaviciene, J. Diversity in prevalence and genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in Lithuania and Norway. Int. J. Med. Microbiol. 2008, 298 (Suppl. S1), 180–187. [Google Scholar] [CrossRef]
- Žygutienė, M. Tick-Borne Pathogens and Spread of Ixodes ricinus in Lithuania. EpiNorth 2009, 10, 63–71. [Google Scholar]
- Koczanowicz, S.; Nowak-Chmura, M.; Witecka, J.; Rączka, G.; Asman, M.M. The potential risk of human exposure to tick borne infection by Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti on the selected recreational areas of Poprad Landscape Park of southern Poland. Ann. Agric. Environ. Med. 2024, 31, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kiewra, D.; Stańczak, J.; Richter, M. Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in Lower Silesia, Poland—Preliminary study. Ticks Tick. Borne Dis. 2014, 5, 892–897. [Google Scholar] [CrossRef]
- Kubiak, K.; Dmitryjuk, M.; Dziekońska-Rynko, J.; Siejwa, P.; Dzika, E. The Risk of Exposure to Ticks and Tick-Borne Pathogens in a Spa Town in Northern Poland. Pathogens 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Dziekońska-Rynko, J.; Szymańska, H.; Kubiak, D.; Dmitryjuk, M.; Dzika, E. Questing Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in an urban area of north-eastern Poland. Exp. Appl. Acarol. 2019, 78, 113–126. [Google Scholar] [CrossRef]
- Kiewra, D.; Dyczko, D.; Žákovská, A.; Nejezchlebova, H. Prevalence of Borrelia and Rickettsia in Ixodes ricinus from Chosen Urban and Protected Areas in Poland and the Czech Republic. Insects 2024, 15, 785. [Google Scholar] [CrossRef] [PubMed]
- Kirczuk, L.; Piotrowski, M.; Rymaszewska, A. Detection of Tick-Borne Pathogens of the Genera Rickettsia, Anaplasma and Francisella in Ixodes ricinus Ticks in Pomerania (Poland). Pathogens 2021, 10, 901. [Google Scholar] [CrossRef]
- Rymaszewska, A.; Piotrowski, M. Use of DNA sequences for Rickettsia identification in Ixodes ricinus ticks: The first detection of Rickettsia monacensis in Poland. Microbes Infect. 2013, 15, 140–146. [Google Scholar] [CrossRef]
- Biernat, B.; Stańczak, J.; Michalik, J.; Sikora, B.; Wierzbicka, A. Prevalence of infection with Rickettsia helvetica in Ixodes ricinus ticks feeding on non-rickettsiemic rodent hosts in sylvatic habitats of west-central Poland. Ticks Tick. Borne Dis. 2016, 7, 135–141. [Google Scholar] [CrossRef]
- Stańczak, J.; Biernat, B.; Matyjasek, A.; Racewicz, M.; Zalewska, M.; Lewandowska, D. Kampinos National Park: A risk area for spotted fever group rickettsioses, central Poland? Exp. Appl. Acarol. 2016, 70, 395–410. [Google Scholar] [CrossRef]
- Kubiak, K.; Szymańska, H.; Dziekońska-Rynko, J.; Tylkowska, A.; Dmitryjuk, M.; Dzika, E. Tick-borne pathogens in questing adults Dermacentor reticulatus from the Eastern European population (north-eastern Poland). Sci. Rep. 2024, 14, 698. [Google Scholar] [CrossRef]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Kiewra, D.; Czułowska, A.; Robak, A.; Bajer, A. Update on prevalence of Babesia canis and Rickettsia spp. in adult and juvenile Dermacentor reticulatus ticks in the area of Poland (2016–2018). Sci. Rep. 2022, 12, 5755. [Google Scholar] [CrossRef] [PubMed]
- Stańczak, J. Detection of spotted fever group (SFG) rickettsiae in Dermacentor reticulatus (Acari: Ixodidae) in Poland. Int. J. Med. Microbiol. 2006, 296 (Suppl. S40), 144–148. [Google Scholar] [CrossRef]
- Mierzejewska, E.J.; Pawełczyk, A.; Radkowski, M.; Welc-Falęciak, R.; Bajer, A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit. Vectors 2015, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fatla, A.; Cisak, E.; Zając, V.; Sroka, J.; Sawczyn, A.; Dutkiewicz, J. Study on tick-borne rickettsiae in eastern Poland. I. Prevalence in Dermacentor reticulatus (Acari: Amblyommidae). Ann. Agric. Environ. Med. 2013, 20, 276–279. [Google Scholar] [PubMed]
- Król, N.; Obiegala, A.; Pfeffer, M.; Lonc, E.; Kiewra, D. Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration, South-West Poland. Parasit. Vectors 2016, 9, 351. [Google Scholar] [CrossRef]
- Katargina, O.; Geller, J.; Alekseev, A.; Dubinina, H.; Efremova, G.; Mishaeva, N.; Vasilenko, V.; Kuznetsova, T.; Järvekülg, L.; Vene, S.; et al. Identification of Anaplasma phagocytophilum in tick populations in Estonia, the European part of Russia and Belarus. Clin. Microbiol. Infect. 2012, 18, 40–46. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Kowalec, M.; Karbowiak, G.; Bajer, A.; Behnke, J.M.; Siński, E. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasit. Vectors 2014, 7, 121. [Google Scholar] [CrossRef]
- Alekseev, A.N.; Dubinina, H.V.; Van De Pol, I.; Schouls, L.M. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 2001, 39, 2237–2242. [Google Scholar] [CrossRef]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- Dumler, J.S.; Choi, K.S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Gygax, L.; Schudel, S.; Kositz, C.; Kuenzli, E.; Neumayr, A. Human monocytotropic ehrlichiosis—A systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 2024, 18, e0012377. [Google Scholar] [CrossRef] [PubMed]
- Czułowska, A.; Kiewra, D.; Dyczko, D. Infections of Ixodes ricinus ticks with bacteria of Rickettsia and Borrelia genus in selected Forest Inspectorates (Lower Silesia, SW Poland). Ann. Parasitol. 2019, 65 (Suppl. S1), s177. [Google Scholar]
- Raulf, M.K.; Jordan, D.; Fingerle, V.; Strube, C. Association of Borrelia and Rickettsia spp. and bacterial loads in Ixodes ricinus ticks. Ticks Tick-Borne Dis. 2018, 9, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Movila, A.; Reye, A.L.; Dubinina, H.V.; Tolstenkov, O.O.; Toderas, I.; Hübschen, J.M.; Muller, C.P.; Alekseev, A.N. Detection of Babesia Sp. EU1 and members of spotted fever group rickettsiae in ticks collected from migratory birds at Curonian Spit, North-Western Russia. Vector Borne Zoonotic Dis. 2011, 11, 89–91. [Google Scholar] [CrossRef]
- Movila, A.; Alekseev, A.N.; Dubinina, H.V.; Toderas, I. Detection of tick-borne pathogens in ticks from migratory birds in the Baltic region of Russia. Med. Vet. Entomol. 2013, 27, 113–117. [Google Scholar] [CrossRef]
| Tick Species | Number of Ticks | Number of Ticks Infected by (%, 95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Rickettsia spp. SFG | B. burgdorferi s.l. | B. miyamotoi | C. burnetii | A. phagocytophillum | E. chaffeensis/E. muris | ||
| I. ricinus | 343 | 60 (17.5%, 13.8–21.9%) | 122 (35.6%, 30.7–40.8%) | 6 (1.7%, 0.7–3.9%) | 0 | 9 (2.6%, 1.3–5.0%) | 2 (0.6%, 0.02–2.2%) |
| D. reticulatus | 165 | 10 (6.1%, 3.2–10.9%) | 0 | 0 | 0 | 0 | 0 |
| Total | 508 | 70 (13.8%, 11.0–17.1%) | 122 (24.0%, 20.5–27.9%) | 6 (1.2%, 0.5–2.6%) | 0 | 9 (1.8%, 0.9–3.4%) | 2 (0.4%, 0.01–1.5%) |
| Tick Species | Number of Ticks | Number of Ticks Infected by SFGR (%, 95% CI) | |||
|---|---|---|---|---|---|
| R. raoultii | R. helvetica | Not Sequenced | Total Rickettsia spp. | ||
| I. ricinus | 343 | 0 | 13 (3.8%, 2.2–6.4%) | 47 (13.7%, 10.4–17.8%) | 60 (17.5%, 13.8–21.9%) |
| D. reticulatus | 165 | 10 (6.1%, 3.2–10.9%) | 0 | 0 | 10 (6.1%, 3.2–10.9%) |
| Total | 508 | 10 (2.0%, 1.0–3.6%) | 13 (2.6%, 1.5–4.4%) | 47 (9.2%, 7.0–12.1%) | 70 (13.8%, 11.0–17.1%) |
| Tick Species | Collection Zone | Number of Ticks | Number of Ticks Infected by (%, 95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Rickettsia spp. SFG | B. burgdorferi s.l. | B. miyamotoi | A. phagocytophillum | E. chaffeensis/E. muris | |||
| I. ricinus | 1 * | 20 | 1 (5.0%, 0–25.4%) | 4 (20.0%, 7.5–42.2%) | 0 | 1 (5.0%, 0–25.4%) | 0 |
| 2 * | 23 | 6 (26.1%, 12.3–46.8%) | 9 (39.1%, 22.1–59.3%) | 2 (8.7%, 1.2–28.0%) | 0 | 0 | |
| 3 * | 110 | 18 (16.4%, 10.5–24.5%) | 44 (40.0%, 31.3–49.3%) | 2 (1.8%, 0.1–6.8%) | 0 | 0 | |
| 4 * | 7 | 2 (28.6%, 7.6–64.8%) | 3 (42.9%, 15.7–75.0%) | 0 | 0 | 0 | |
| 5 * | 78 | 11 (14.1%, 7.9–23.7%) | 25 (32.0%, 22.7–43.1%) | 0 | 3 (3.8%, 0.9–11.2%) | 2 (2.6%, 0.2–9.4%) | |
| 6 * | 3 | 1 (33.3%, 5.6–79.8%) | 1 (33.3%, 5.6–79.8%) | 0 | 0 | 0 | |
| Subtotal | 241 | 39 (16.2%, 12.0–21.4%) | 86 (35.7%, 29.9–41.9%) | 4 (1.7%, 0.5–4.4%) | 4 (1.7%, 0.5–4.4%) | 2 (0.8%, 0.03–3.2%) | |
| D. reticulatus | 1 * | 4 | 0 | 0 | 0 | 0 | 0 |
| 2 * | 20 | 0 | 0 | 0 | 0 | 0 | |
| 3 * | 20 | 2 (10.0%, 15.7–31.3%) | 0 | 0 | 0 | 0 | |
| 4 * | - | - | - | - | - | - | |
| 5 * | 1 | 0 | 0 | 0 | 0 | 0 | |
| 6 * | 7 | 0 | 0 | 0 | 0 | 0 | |
| Subtotal | 52 | 2 (3.8%, 0.3–13.7%) | 0 | 0 | 0 | 0 | |
| Total | 293 | 41 (14.0%, 10.5–18.5%) | 86 (29.3%, 24.4–34.8%) | 4 (1.4%, 0.4–3.7%) | 4 (1.4%, 0.4–3.7%) | 2 (0.7%, 0.02–2.6%) | |
| Tick Species | Collection Zone | Number of Ticks | Number of Ticks Infected by (%, 95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Rickettsia spp. SFG | B. burgdorferi s.l. | B. miyamotoi | A. phagocytophillum | E. chaffeensis/E. muris | |||
| I. ricinus | 1 * | 3 | 0 | 0 | 0 | 0 | 0 |
| 2 * | 1 | 0 | 0 | 0 | 0 | 0 | |
| 3 * | 22 | 3 (13.6%, 3.9–34.2%) | 6 (27.3%, 12.9–48.4%) | 1 (4.5%, 0–23.5%) | 0 | 0 | |
| 4 * | 4 | 0 | 3 (75.0%, 28.9–96.6%) | 0 | 0 | 0 | |
| 5 * | 70 | 18 (25.7%, 16.8–37.1%) | 26 (37.1%, 26.7–48.9%) | 1 (1.4%, 0–8.4%) | 5 (7.1%, 2.7–16.0%) | 0 | |
| 6 * | 2 | 0 | 1 (50.0%, 9.4–90.5%) | 0 | 0 | 0 | |
| Subtotal | 102 | 21 (20.6%, 13.8–29.5%) | 36 (35.3%, 26.7–45.0%) | 2 (2.0%, 0.1–7.3%) | 5 (4.9%, 1.8–11.2%) | 0 | |
| D. reticulatus | 1 * | - | - | - | - | - | - |
| 2 * | 44 | 7 (15.9%, 7.6–29.7%) | 0 | 0 | 0 | 0 | |
| 3 * | 38 | 0 | 0 | 0 | 0 | 0 | |
| 4 * | - | - | - | - | - | - | |
| 5 * | 31 | 1 (3.2%, 0–17.6%) | 0 | 0 | 0 | 0 | |
| 6 * | - | - | - | - | - | - | |
| Subtotal | 113 | 8 (7.1%, 3.4–13.5%) | 0 | 0 | 0 | 0 | |
| Total | 215 | 29 (13.5%, 9.5–18.7%) | 36 (16.7%, 12.3–22.3%) | 2 (1.0%, 0–3.5%) | 5 (2.3%, 0.8–5.5%) | 0 | |
| Pathogen Co-Infections | No. of Positive | Positive Rate (95% CI) |
|---|---|---|
| R. helvetica + B. miyamotoi + B. burgdorferi s.l. | 1 | 0.3% (0.28–0.86%) |
| R. helvetica + B. burgdorferi s.l. | 2 | 0.6% (0.22–1.4%) |
| R. helvetica + B. miyamotoi | 1 | 0.3% (0.28–0.86%) |
| R. helvetica + E. chaffeensis/E. muris | 1 | 0.3% (0.28–0.86%) |
| R. spp. + B. burgdorferi s.l. | 14 | 4.1% (2.0–6.2%) |
| R. spp. + A. phagocytophilum | 1 | 0.3% (0.28–0.86%) |
| B. burgdorferi s.l. + A. phagocytophilum | 2 | 0.6% (0.2–1.4%) |
| B. burgdorferi s.l. + B. miyamotoi | 3 | 0.9% (0.1–1.9%) |
| Total | 25 | 7.3% (4.6–10.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakov, A.V.; Volchev, E.G.; Petremgvdlishvili, K.; Chekanova, T.A. Comparative Analysis of Bacterial Tick-Borne Pathogens in Questing Ticks from Sambia Peninsula, Kaliningrad Oblast, Russia: Spring and Autumn Prevalence and Public Health Risks. Microorganisms 2025, 13, 1403. https://doi.org/10.3390/microorganisms13061403
Rakov AV, Volchev EG, Petremgvdlishvili K, Chekanova TA. Comparative Analysis of Bacterial Tick-Borne Pathogens in Questing Ticks from Sambia Peninsula, Kaliningrad Oblast, Russia: Spring and Autumn Prevalence and Public Health Risks. Microorganisms. 2025; 13(6):1403. https://doi.org/10.3390/microorganisms13061403
Chicago/Turabian StyleRakov, Alexey V., Evgenii G. Volchev, Ketevan Petremgvdlishvili, and Tatiana A. Chekanova. 2025. "Comparative Analysis of Bacterial Tick-Borne Pathogens in Questing Ticks from Sambia Peninsula, Kaliningrad Oblast, Russia: Spring and Autumn Prevalence and Public Health Risks" Microorganisms 13, no. 6: 1403. https://doi.org/10.3390/microorganisms13061403
APA StyleRakov, A. V., Volchev, E. G., Petremgvdlishvili, K., & Chekanova, T. A. (2025). Comparative Analysis of Bacterial Tick-Borne Pathogens in Questing Ticks from Sambia Peninsula, Kaliningrad Oblast, Russia: Spring and Autumn Prevalence and Public Health Risks. Microorganisms, 13(6), 1403. https://doi.org/10.3390/microorganisms13061403









