From Infection to Autoimmunity: S. pyogenes as a Model Pathogen
Abstract
1. Introduction
2. Streptococcus Pyogenes
2.1. Pathogenesis
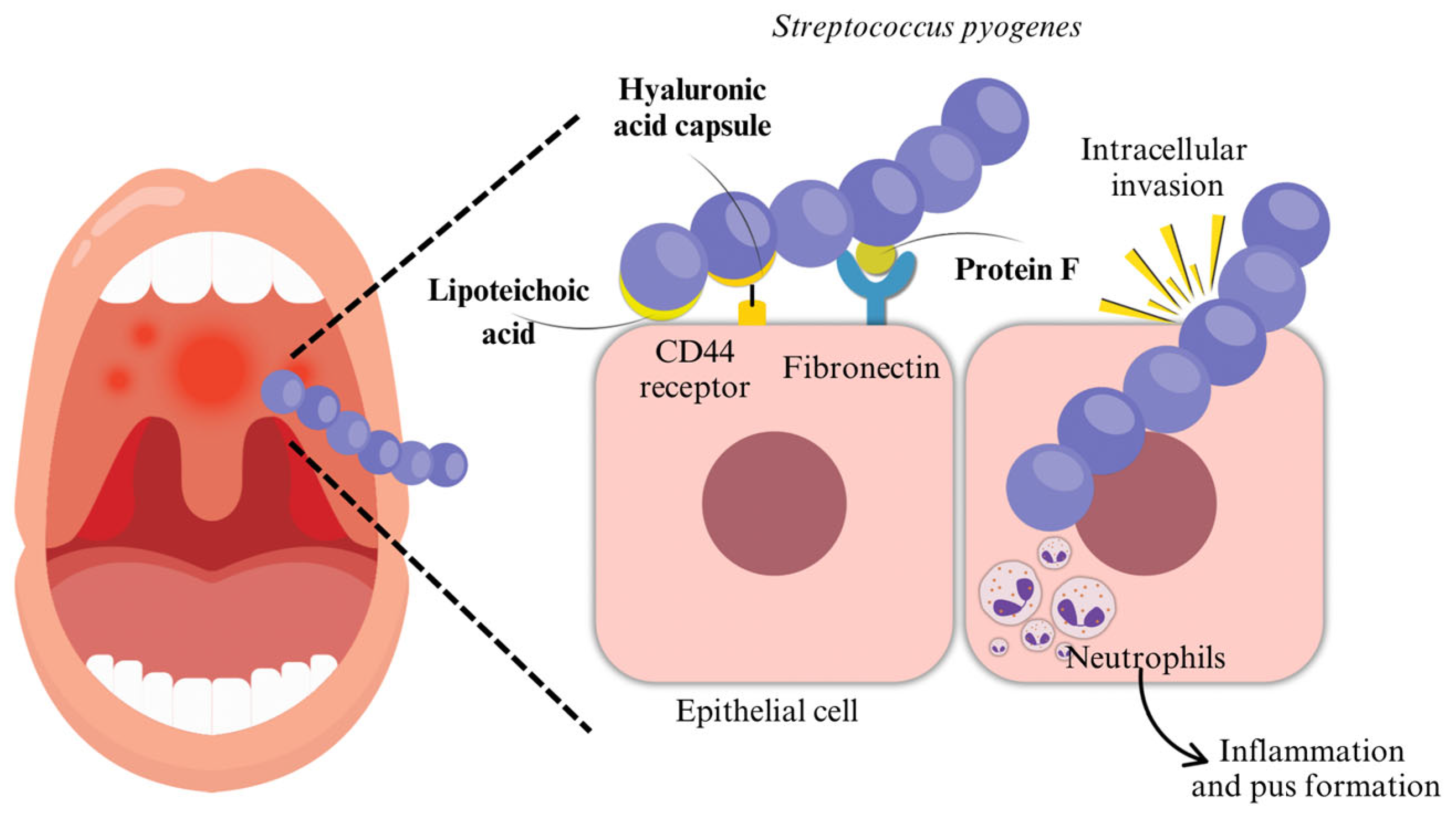
2.1.1. Adhesion
2.1.2. Invasion
2.1.3. Hyaluronic Acid Capsule
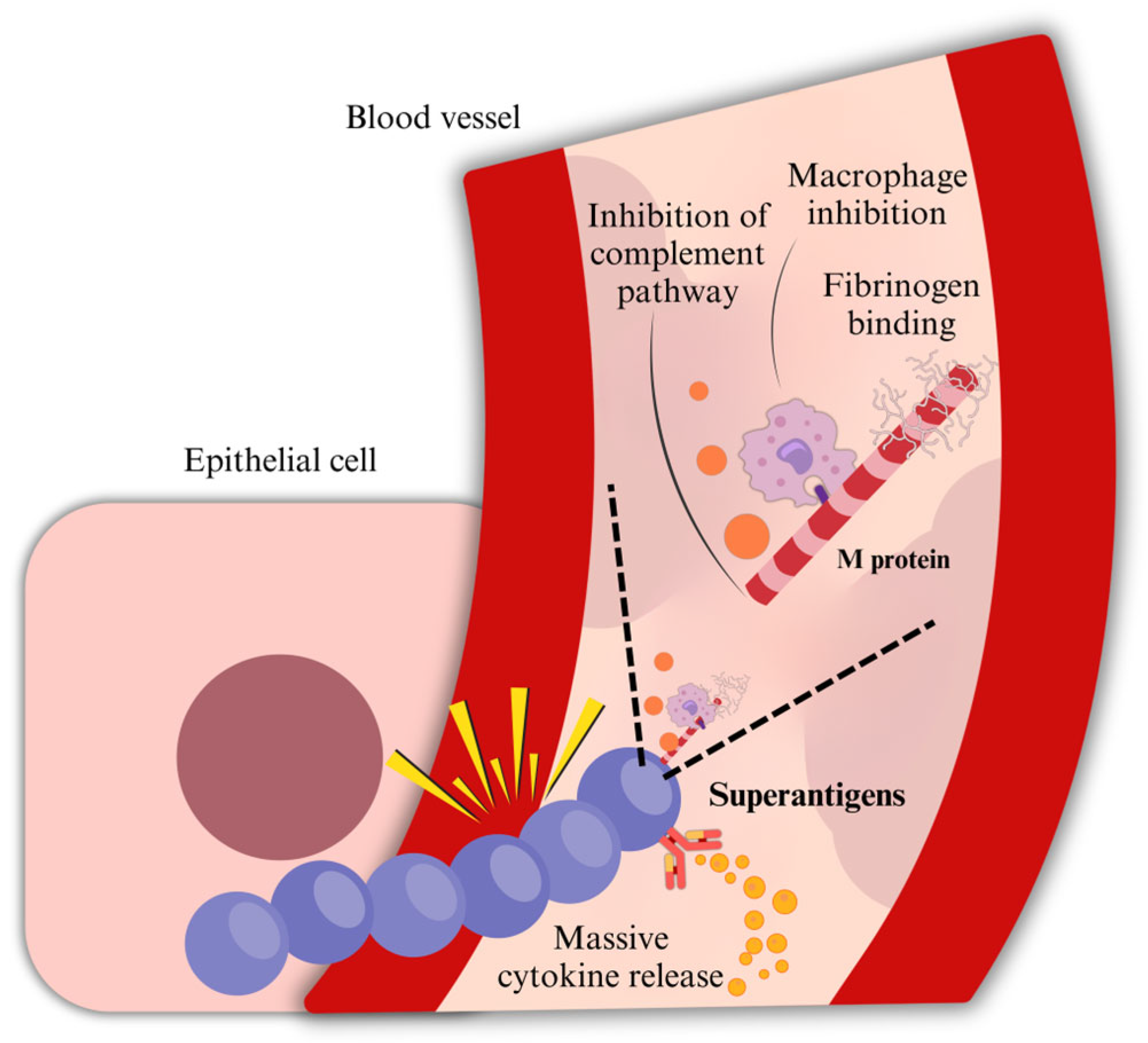
2.1.4. Opsonization
2.1.5. M Protein
2.1.6. Exotoxins and Superantigens
2.1.7. Exoenzymes
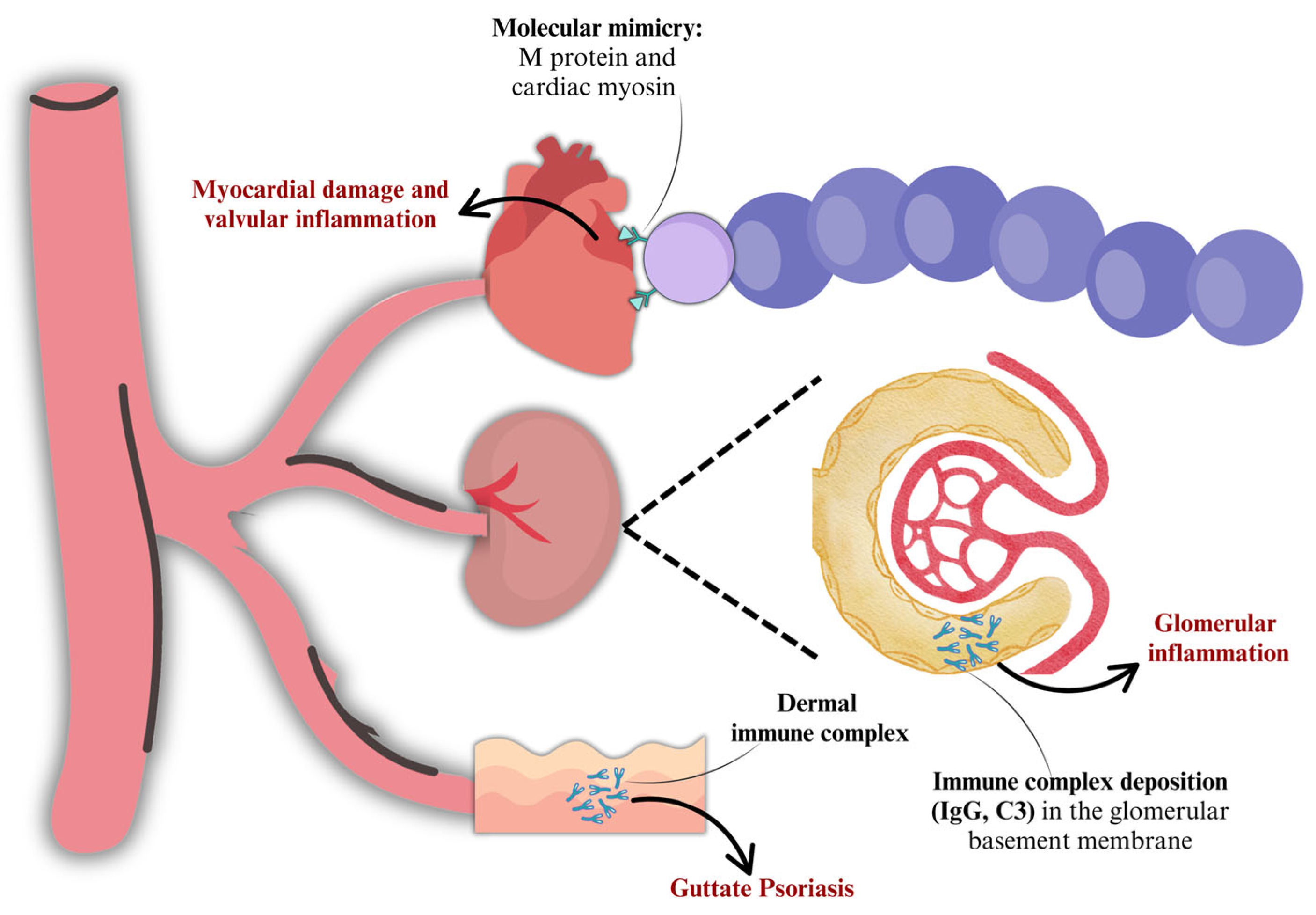
2.2. Molecular Mimicry
3. Group a Streptococcus-Associated Autoimmune Sequelae
3.1. Acute Rheumatic Fever (ARF) and Rheumatic Heart Disease (RHD)
3.1.1. Clinical Presentation
3.1.2. Diagnosis
3.1.3. Treatment and Primary Prevention
3.1.4. Secondary Prevention
3.2. Acute Post-Streptococcal Glomerulonephritis (APSGN)
3.2.1. Clinical Presentation
3.2.2. Diagnosis
3.2.3. Treatment
3.3. Guttate Psoriasis
4. Clinical and Therapeutic Implications
4.1. Strategies to Prevent Progression Toward Autoimmune Diseases
4.2. Potential Therapeutic Developments Targeting Pathogenic Mechanisms
Antimicrobial Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jespersen, M.G.; Lacey, J.A.; Tong, S.Y.C.; Davies, M.R. Global genomic epidemiology of Streptococcus pyogenes. Infect. Genet. Evol. 2020, 86, 104609. [Google Scholar] [CrossRef]
- Guerra, S.; LaRock, C. Group A Streptococcus interactions with the host across time and space. Curr. Opin. Microbiol. 2024, 77, 102420. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W. Pathogenesis of Group A Streptococcal Infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Caparon, M.G.; Stephens, D.S.; Olsén, A.; Scott, J.R. Role of M protein in adherence of group A streptococci. Infect. Immun. 1991, 59, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Baird, R.W.; Courtney, H.S.; Hasty, D.L.; Bronze, M.S. Passive Protection of Mice against Group A Streptococcal Pharyngeal Infection by Lipoteichoic Acid. J. Infect. Dis. 1994, 169, 319–323. [Google Scholar] [CrossRef]
- Courtney, H.S.; Von Hunolstein, C.; Dale, J.B.; Bronze, M.S.; Beachey, E.H.; Hasty, D.L. Lipoteichoic acid and M protein: Dual adhesins of group A streptococci. Microb. Pathog. 1992, 12, 199–208. [Google Scholar] [CrossRef]
- Hanski, E.; Caparon, M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 1992, 89, 6172–6176. [Google Scholar] [CrossRef] [PubMed]
- Courtney, H.S.; Hasty, D.L.; Dale, J.B.; Poirier, T.P. A 28-kilodalton fibronectin-binding protein of group a streptococci. Curr. Microbiol. 1992, 25, 245–250. [Google Scholar] [CrossRef]
- Pancholi, V.; Fischetti, V.A. A major surface protein on group A streptococci is a glyceraldehyde-3- phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 1992, 176, 415–426. [Google Scholar] [CrossRef]
- Valentin-Weigand, P.; Grulich-Henn, J.; Chhatwal, G.S.; Müller-Berghaus, G.; Blobel, H.; Preissner, K.T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin). Infect. Immun. 1988, 56, 2851–2855. [Google Scholar] [CrossRef]
- Visai, L.; Bozzini, S.; Raucci, G.; Toniolo, A.; Speziale, P. Isolation and Characterization of a Novel Collagen-binding Protein from Streptococcus pyogenes Strain 6414. J. Biol. Chem. 1995, 270, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.R.; Bronze, M.S. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc. Natl. Acad. Sci. USA 1994, 91, 12238–12242. [Google Scholar] [CrossRef] [PubMed]
- Molinari, G.; Chhatwal, G.S. Invasion and Survival of Streptococcus pyogenes in Eukaryotic Cells Correlates with the Source of the Clinical Isolates. J. Infect. Dis. 1998, 177, 1600–1607. [Google Scholar] [CrossRef]
- Österlund, A.; Popa, R.; Nikkilä, T.; Scheynius, A.; Engstrand, L. Intracellular Reservoir of Streptococcus pyogenes In Vivo: A Possible Explanation for Recurrent Pharyngotonsillitis. Laryngoscope 1997, 107, 640–647. [Google Scholar] [CrossRef]
- Logsdon, L.K.; Håkansson, A.P.; Cortés, G.; Wessels, M.R. Streptolysin O Inhibits Clathrin-Dependent Internalization of Group A Streptococcus. mBio 2011, 2, e00332-10. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Washburn, R.G.; Marques, M.B.; Wessels, M.R. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect. Immun. 1996, 64, 1495–1501. [Google Scholar] [CrossRef]
- Horstmann, R.D.; Sievertsen, H.J.; Leippe, M.; Fischetti, V.A. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect. Immun. 1992, 60, 5036–5041. [Google Scholar] [CrossRef]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef]
- Wilde, S.; Johnson, A.F.; LaRock, C.N. Playing With Fire: Proinflammatory Virulence Mechanisms of Group A Streptococcus. Front. Cell. Infect. Microbiol. 2021, 11, 704099. [Google Scholar] [CrossRef]
- Fischetti, V.A.; Pancholi, V.; Schneewind, O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol. Microbiol. 1990, 4, 1603–1605. [Google Scholar] [CrossRef]
- Barnett, T.C.; Patel, A.R.; Scott, J.R. A Novel Sortase, SrtC2, from Streptococcus Pyogenes Anchors a Surface Protein Containing a QVPTGV Motif to the Cell Wall. J Bacteriol 2004, 186, 5865–5875. [Google Scholar] [CrossRef] [PubMed]
- Bessen, D.E.; Fischetti, V.A. Differentiation between two biologically distinct classes of group A streptococci by limited substitutions of amino acids within the shared region of M protein-like molecules. J. Exp. Med. 1990, 172, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, J.P.; Maxted, W.R.; Grant, D.L. The Production of Opacity in Serum by Group A Streptococci and Its Relationship with the Presence of M Antigen. J. Gen. Microbiol. 1970, 61, 343–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Widdowson, J.P.; Maxted, W.R.; Grant, D.L.; Pinney, A.M. The Relationship between M-antigen and Opacity Factor in Group A Streptococci. J. Gen. Microbiol. 1971, 65, 69–80. [Google Scholar] [CrossRef][Green Version]
- Eison, T.M.; Ault, B.H.; Jones, D.P.; Chesney, R.W.; Wyatt, R.J. Post-streptococcal acute glomerulonephritis in children: Clinical features and pathogenesis. Pediatr. Nephrol. 2011, 26, 165–180. [Google Scholar] [CrossRef]
- Spaulding, A.R.; Salgado-Pabón, W.; Kohler, P.L.; Horswill, A.R.; Leung, D.Y.M.; Schlievert, P.M. Staphylococcal and Streptococcal Superantigen Exotoxins. Clin. Microbiol. Rev. 2013, 26, 422–447. [Google Scholar] [CrossRef]
- Proft, T.; Fraser, J.D. Streptococcal Superantigens. In Chemical Immunology and Allergy; Marone, G., Ed.; Karger: Basel, Switzerland, 2007; pp. 1–23. ISBN 978-3-8055-8266-7. [Google Scholar]
- Kim, Y.B.; Watson, D.W. A purified Group A Streptococcal pyrogenic exotocin. J. Exp. Med. 1970, 131, 611–628. [Google Scholar] [CrossRef]
- Galvin, J.E.; Hemric, M.E.; Ward, K.; Cunningham, M.W. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. J. Clin. Investig. 2000, 106, 217–224. [Google Scholar] [CrossRef]
- Faé, K.C.; Da Silva, D.D.; Oshiro, S.E.; Tanaka, A.C.; Pomerantzeff, P.M.A.; Douay, C.; Charron, D.; Toubert, A.; Cunningham, M.W.; Kalil, J.; et al. Mimicry in Recognition of Cardiac Myosin Peptides by Heart-Intralesional T Cell Clones from Rheumatic Heart Disease. J. Immunol. 2006, 176, 5662–5670. [Google Scholar] [CrossRef]
- Ellis, N.M.J.; Li, Y.; Hildebrand, W.; Fischetti, V.A.; Cunningham, M.W. T Cell Mimicry and Epitope Specificity of Cross-Reactive T Cell Clones from Rheumatic Heart Disease. J. Immunol. 2005, 175, 5448–5456. [Google Scholar] [CrossRef]
- Cunningham, M.W.; Antone, S.M.; Gulizia, J.M.; McManus, B.A.; Gauntt, C.J. α-Helical Coiled-Coil Molecules: A Role in Autoimmunity against the Heart. Clin. Immunol. Immunopathol. 1993, 68, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W. Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Zabriskie, J.B. Mimetic Relationships Between Group A Streptococci And Mammalian Tissues. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 1967; Volume 7, pp. 147–188. ISBN 978-0-12-022407-4. [Google Scholar]
- Zabriskie, J.B.; Freimer, E.H. An immunological relationship between the group. A streptococcus and mammalian muscle. J. Exp. Med. 1966, 124, 661–678. [Google Scholar] [CrossRef]
- Zabriskie, J.B.; Hsu, K.C.; Seegal, B.C. Heart-reactive antibody associated with rheumatic fever: Characterization and diagnostic significance. Clin. Exp. Immunol. 1970, 7, 147–159. [Google Scholar]
- Kaplan, M.H.; Meyeserian, M. An immunological cross-reaction between group-A streptococcal cells and human heart tissue. Lancet Lond. Engl. 1962, 1, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Suchy, M.L. Immunologic relation of streptococcal and tissue antigens. II. Cross reactions of antisera to mammalian heart tissue with a cell wall constituent of certain strains of group A streptococci. J. Exp. Med. 1964, 119, 643–650. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Svec, K.H. Immunologic relation of streptococcal and tissue antigens. III. Presence in human sera of stretpcoccal antibody cross reactive with heart tissue. Association with streptococcal infection, rheumatic fever, and glomerulonephritis. J. Exp. Med. 1964, 119, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Van De Rijn, I.; Sabriskie, J.; McCarty, M. Group a streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J. Exp. Med. 1977, 146, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Halpern, B.; Robert, L. Immunological Relationship between Streptococcus A Polysaccharide and the Structural Glycoproteins of Heart Valve. Nature 1967, 213, 44–47. [Google Scholar] [CrossRef]
- Dudding, B.A.; Ayoub, E.M. Persistence of Streptococcal Group A antibody in patients with Rheumatic Valvular Disease. J. Exp. Med. 1968, 128, 1081–1098. [Google Scholar] [CrossRef]
- Lyampert, I.M.; Beletskaya, L.V.; Borodiyuk, N.A.; Gnezditskaya, E.V.; Rassokhina, I.I.; Danilova, T.A. A cross-reactive antigen of thymus and skin epithelial cells common with the polysaccharide of group A streptococci. Immunology 1976, 31, 47–55. [Google Scholar] [PubMed]
- Lyampert, I.M.; Borodiyuk, N.A.; Ugryumova, G.A. The reaction of heart and other organ extracts with the sera of animals immunized with group A streptococci. Immunology 1968, 15, 845–854. [Google Scholar] [PubMed]
- Lyampert, I.M.; Vvedenskaya, O.I.; Danilova, T.A. Study on streptococcus group A antigens common with heart tissue elements. Immunology 1966, 11, 313–320. [Google Scholar]
- McCarty, M. Missing Links in the Streptococcal Chain Leading to Rheumatic Fever: The T. Duckett Jones Memorial Lecture. Circulation 1964, 29, 488–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunningham, M.W.; Krisher, K.; Graves, D.C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect. Immun. 1984, 46, 34–41. [Google Scholar] [CrossRef]
- Phillips, G.N.; Flicker, P.F.; Cohen, C.; Manjula, B.N.; Fischetti, V.A. Streptococcal M protein: Alpha-helical coiled-coil structure and arrangement on the cell surface. Proc. Natl. Acad. Sci. USA 1981, 78, 4689–4693. [Google Scholar] [CrossRef]
- Manjula, B.N.; Fischetti, V.A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem. Biophys. Res. Commun. 1986, 140, 684–690. [Google Scholar] [CrossRef]
- Barnett, L.A.; Cunningham, M.W. A New Heart-Cross-Reactive Antigen in Streptococcus pyogenes Is Not M Protein. J. Infect. Dis. 1990, 162, 875–882. [Google Scholar] [CrossRef]
- Kil, K.S.; Cunningham, M.W.; Barnett, L.A. Cloning and sequence analysis of a gene encoding a 67-kilodalton myosin-cross-reactive antigen of Streptococcus pyogenes reveals its similarity with class II major histocompatibility antigens. Infect. Immun. 1994, 62, 2440–2449. [Google Scholar] [CrossRef]
- Roberts, S.; Kosanke, S.; Terrence Dunn, S.; Jankelow, D.; Duran, C.M.G.; Cunningham, M.W. Pathogenic Mechanisms in Rheumatic Carditis: Focus on Valvular Endothelium. J. Infect. Dis. 2001, 183, 507–511. [Google Scholar] [CrossRef]
- Aschoff, L. The Rheumatic Nodules in the Heart. Ann. Rheum. Dis. 1939, 1, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.J.; Haffejee, Z.; Jankelow, D.; Wadee, A.; Cooper, K. Rheumatic Aschoff nodules revisited. II: Cytokine expression corroborates recently proposed sequential stages. Histopathology 1997, 31, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.J.; Haffejee, Z.; Cooper, K. Rheumatic Aschoff nodules revisited: An immunohistological reappraisal of the cellular component. Histopathology 1995, 27, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Kefalides, N.A.; Ohno, N.; Wilson, C.B.; Fillit, H.; Zabriski, J.; Rosenbloom, J. Identification of antigenic epitopes in type IV collagen by use of synthetic peptides. Kidney Int. 1993, 43, 94–100. [Google Scholar] [CrossRef]
- Markowitz, A.S. Streptococcal Related Glomerulonephritis: I. Isolation, Immunochemistry and Comparative Chemistry of Soluble Fractions from Type 12 Nephritogenic Streptococci and Human Glomeruli. J. Immunol. 1964, 148, 3110–3116. [Google Scholar] [CrossRef]
- Kraus, W.; Beachey, E.H. Renal autoimmune epitope of group A streptococci specified by M protein tetrapeptide Ile-Arg-Leu-Arg. Proc. Natl. Acad. Sci. USA 1988, 85, 4516–4520. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Primer 2016, 2, 15084. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Guilherme, L. Acute rheumatic fever. Lancet 2018, 392, 161–174. [Google Scholar] [CrossRef]
- Guilherme, L.; Cury, P.; Demarchi, L.M.F.; Coelho, V.; Abel, L.; Lopez, A.P.; Oshiro, S.E.; Aliotti, S.; Cunha-Neto, E.; Pomerantzeff, P.M.A.; et al. Rheumatic Heart Disease: Proinflammatory Cytokines Play a Role in the Progression and Maintenance of Valvular Lesions. Am. J. Pathol. 2004, 165, 1583–1591. [Google Scholar] [CrossRef]
- Tubridy-Clark, M.; Carapetis, J.R. Subclinical carditis in rheumatic fever: A systematic review. Int. J. Cardiol. 2007, 119, 54–58. [Google Scholar] [CrossRef]
- Vijayan, V.; Sukumaran, S. Erythema Marginatum. J. Pediatr. 2023, 258, 113330. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.E.; Leonard, H.L.; Casey, B.J.; Mannheim, G.B.; Lenane, M.C.; Rettew, D.C.; Schapiro, M.B. Sydenham’s Chorea: Physical and Psychological Symptoms of St Vitus Dance. Pediatrics 1993, 91, 706–713. [Google Scholar] [PubMed]
- Cox, C.J.; Sharma, M.; Leckman, J.F.; Zuccolo, J.; Zuccolo, A.; Kovoor, A.; Swedo, S.E.; Cunningham, M.W. Brain Human Monoclonal Autoantibody from Sydenham Chorea Targets Dopaminergic Neurons in Transgenic Mice and Signals Dopamine D2 Receptor: Implications in Human Disease. J. Immunol. 2013, 191, 5524–5541. [Google Scholar] [CrossRef]
- Kirvan, C.A.; Swedo, S.E.; Heuser, J.S.; Cunningham, M.W. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med. 2003, 9, 914–920. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Hornig, M.; Serge, R.; De Miranda, J.; Baghban, A.; Villar, G.; Lipkin, W.I. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol. Psychiatry 2010, 15, 712–726. [Google Scholar] [CrossRef]
- Gewitz, M.H.; Baltimore, R.S.; Tani, L.Y.; Sable, C.A.; Shulman, S.T.; Carapetis, J.; Remenyi, B.; Taubert, K.A.; Bolger, A.F.; Beerman, L.; et al. Revision of the Jones Criteria for the Diagnosis of Acute Rheumatic Fever in the Era of Doppler Echocardiography: A Scientific Statement From the American Heart Association. Circulation 2015, 131, 1806–1818. [Google Scholar] [CrossRef]
- Gerber, M.A.; Baltimore, R.S.; Eaton, C.B.; Gewitz, M.; Rowley, A.H.; Shulman, S.T.; Taubert, K.A. Prevention of Rheumatic Fever and Diagnosis and Treatment of Acute Streptococcal Pharyngitis: A Scientific Statement From the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation 2009, 119, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 70, 776–803. [Google Scholar] [CrossRef]
- World Health Organization. The Current Evidence for the Burden of Group A Streptococcal Diseases; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Satoskar, A.A.; Parikh, S.V.; Nadasdy, T. Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nat. Rev. Nephrol. 2020, 16, 32–50. [Google Scholar] [CrossRef]
- Roy, S.; Murphy, W.M.; Arant, B.S. Poststreptococcal crescenteric glomerulonephritis in children: Comparison of quintuple therapy versus supportive care. J. Pediatr. 1981, 98, 403–410. [Google Scholar] [CrossRef]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F. Childhood guttate psoriasis: An updated review. Drugs Context 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Han, L.; Deng, H.; Fang, X.; Zhang, Z.; Huang, G.; Zheng, Z.Z.; Huang, Q.; Xu, J. The distinct role and regulatory mechanism of IL-17 and IFN-γ in the initiation and development of plaque vs guttate psoriasis. J. Dermatol. Sci. 2018, 92, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M. Childhood psoriasis. Curr. Opin. Pediatr. 2002, 14, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.; Chauhan, P.; Sethi, S. Dermoscopic characterization of guttate psoriasis, pityriasis rosea, and pityriasis lichenoides chronica in dark skin phototypes: An observational study. Dermatol. Ther. 2021, 34. [Google Scholar] [CrossRef] [PubMed]
- Makhecha, M.; Singh, T.; Khatib, Y. Dermoscopy Differentiates Guttate Psoriasis from a Mimicker—Pityriasis Rosea. Dermatol. Pract. Concept. 2021, 11, e2021138. [Google Scholar] [CrossRef]
- De Jager, M.E.A.; De Jong, E.M.G.J.; Van De Kerkhof, P.C.M.; Seyger, M.M.B. Efficacy and safety of treatments for childhood psoriasis: A systematic literature review. J. Am. Acad. Dermatol. 2010, 62, 1013–1030. [Google Scholar] [CrossRef]
- Fotiadou, C.; Lazaridou, E.; Ioannides, D. Management of psoriasis in adolescence. Adolesc. Health Med. Ther. 2014, 5, 25–34. [Google Scholar] [CrossRef]
- Eisert, L.; Augustin, M.; Bach, S.; Dittmann, M.; Eiler, R.; Fölster-Holst, R.; Gerdes, S.; Hamm, H.; Höger, P.; Horneff, G.; et al. S2k guidelines for the treatment of psoriasis in children and adolescents–Short version part 1. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 856–870. [Google Scholar] [CrossRef]
- Pavlovsky, M.; Baum, S.; Shpiro, D.; Pavlovsky, L.; Pavlotsky, F. Narrow band UVB: Is it effective and safe for paediatric psoriasis and atopic dermatitis? J. Eur. Acad. Dermatol. Venereol. 2011, 25, 727–729. [Google Scholar] [CrossRef]
- Zamberk, P.; Velázquez, D.; Campos, M.; Hernanz, J.; Lázaro, P. Paediatric psoriasis–narrowband UVB treatment. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 415–419. [Google Scholar] [CrossRef]
- Bronckers, I.M.G.J.; Seyger, M.M.B.; West, D.P.; Lara-Corrales, I.; Tollefson, M.; Tom, W.L.; Hogeling, M.; Belazarian, L.; Zachariae, C.; Mahé, E.; et al. Safety of Systemic Agents for the Treatment of Pediatric Psoriasis. JAMA Dermatol. 2017, 153, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Denny, F.W. A 45-Year Perspective on the Streptococcus and Rheumatic Fever: The Edward H. Kass Lecture in Infectious Disease History. Clin. Infect. Dis. 1994, 19, 1100–1122. [Google Scholar] [CrossRef]
- Dale, J.B.; Walker, M.J. Update on Group A Streptococcal Vaccine Development. Curr. Opin. Infect. Dis. 2020, 33, 244–250. [Google Scholar] [CrossRef]
- Steer, A.C.; Carapetis, J.R.; Dale, J.B.; Fraser, J.D.; Good, M.F.; Guilherme, L.; Moreland, N.J.; Mulholland, E.K.; Schodel, F.; Smeesters, P.R. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 2016, 34, 2953–2958. [Google Scholar] [CrossRef]
- Nagarajan, G.; Govindan, R.; Poomarimuthu, M.; Andiappan, R.; Elango, S.; Maruthamuthu, S.; Kadiam, S. The microbiome and rheumatic heart disease: Current knowledge and future perspectives. Acta Cardiol. 2023, 78, 525–533. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; De Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Yu, D.; Guo, D.; Zheng, Y.; Yang, Y. A review of penicillin binding protein and group A Streptococcus with reduced-β-lactam susceptibility. Front. Cell. Infect. Microbiol. 2023, 13, 1117160. [Google Scholar] [CrossRef]
- Metcalf, B.J.; Chochua, S.; Gertz, R.E.; Hawkins, P.A.; Ricaldi, J.; Li, Z.; Walker, H.; Tran, T.; Rivers, J.; Mathis, S.; et al. Short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiae recovered in the USA. Clin. Microbiol. Infect. 2017, 23, e7–e574. [Google Scholar] [CrossRef]
- Hayes, K.; O’Halloran, F.; Cotter, L. A review of antibiotic resistance in Group B Streptococcus: The story so far. Crit. Rev. Microbiol. 2020, 46, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Chochua, S.; Metcalf, B.; Li, Z.; Mathis, S.; Tran, T.; Rivers, J.; Fleming-Dutra, K.E.; Li, Y.; McGee, L.; Beall, B. Invasive Group A Streptococcal Penicillin Binding Protein 2× Variants Associated with Reduced Susceptibility to β-Lactam Antibiotics in the United States, 2015–2021. Antimicrob. Agents Chemother. 2022, 66, e00802–e00822. [Google Scholar] [CrossRef]
- Abraham, T.; Sistla, S. Trends in Antimicrobial Resistance Patterns of Group A Streptococci, Molecular Basis and Implications. Indian J. Med. Microbiol. 2018, 36, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Ozberk, V.; Pandey, M.; Good, M.F. Contribution of Cryptic Epitopes in Designing a Group A Streptococcal Vaccine. Hum. Vaccines Immunother. 2018, 14, 2034–2052. [Google Scholar] [CrossRef]
- Zeppa, J.J.; Kasper, K.J.; Mohorovic, I.; Mazzuca, D.M.; Haeryfar, S.M.M.; McCormick, J.K. Nasopharyngeal Infection by Streptococcus Pyogenes Requires Superantigen-Responsive Vβ-Specific T Cells. Proc. Natl. Acad. Sci. USA 2017, 114, 10226–10231. [Google Scholar] [CrossRef]
| B. Major Criteria | C. Minor Criteria |
|---|---|
| Clinical and/or subclinical carditis 1 | Monoarthralgia |
| Mono-/polyarthritis | Fever (≥38 °C) |
| Polyarthralgia | ESR ≥ 30 mm/h and/or CRP ≥ 3.0 mg/dL |
| Sydenham Corea | Prolonged PR interval |
| Erythema marginatum | |
| Subcutaneous nodules |
| B. Major Criteria | C. Minor Criteria |
|---|---|
| Clinical and/or subclinical carditis | Polyarthralgia |
| Polyarthritis | Fever (≥38.5 °C) |
| Sydenham Corea | ESR ≥ 60 mm/h and/or CRP ≥ 3.0 mg/dL |
| Erythema marginatum | Prolonged PR interval |
| Subcutaneous nodules |
| Preceding GAS Infection | Criteria |
|---|---|
| Diagnosis of initial ARF | 2 major criteria 1 major criteria + 2 minor criteria |
| Diagnosis of recurrent ARF | 2 major criteria 1 major criteria + 2 minor criteria 3 minor criteria |
| Agent | Dose | Duration |
|---|---|---|
| Benzathine penicillin G | ≤27 kg: 600,000 U i.m. >27 kg: 1,200,000 U i.m. | Single dose |
| Phenoxymethyl penicillin (Penicillin V) | ≤27 kg: 250 mg (2–3 times/day orally) >27 kg: 500 mg (2–3 times/day orally) | 10 days |
| Amoxicillin | 50 mg/kg daily orally (max. 1 g) | 10 days |
| Azithromycin | 12 mg/kg/day (max. 500 mg) | 5 days |
| Clarithromycin | 15 mg/kg/day in 2 doses (max. 250 mg BID) | 10 days |
| Clindamycin | 20 mg/kg/day in 3 doses (max. 1.8 g/d) | 10 days |
| Clinical Presentation | Duration |
|---|---|
| ARF with carditis and residual heart disease (persistent valvular disease 3) | 10 years or until 40 years of age (longer option), sometimes lifelong. |
| ARF with carditis but no residual heart disease (no valvular disease) | 10 years or until 21 years of age (longer option) |
| ARF without carditis | 5 years or until 21 years of age (longer option) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girlando, V.; De Angelis, L.; D’Egidio, G.; Di Ludovico, A.; Breda, L. From Infection to Autoimmunity: S. pyogenes as a Model Pathogen. Microorganisms 2025, 13, 1398. https://doi.org/10.3390/microorganisms13061398
Girlando V, De Angelis L, D’Egidio G, Di Ludovico A, Breda L. From Infection to Autoimmunity: S. pyogenes as a Model Pathogen. Microorganisms. 2025; 13(6):1398. https://doi.org/10.3390/microorganisms13061398
Chicago/Turabian StyleGirlando, Virginia, Luisa De Angelis, Gianluca D’Egidio, Armando Di Ludovico, and Luciana Breda. 2025. "From Infection to Autoimmunity: S. pyogenes as a Model Pathogen" Microorganisms 13, no. 6: 1398. https://doi.org/10.3390/microorganisms13061398
APA StyleGirlando, V., De Angelis, L., D’Egidio, G., Di Ludovico, A., & Breda, L. (2025). From Infection to Autoimmunity: S. pyogenes as a Model Pathogen. Microorganisms, 13(6), 1398. https://doi.org/10.3390/microorganisms13061398






