A Study on the Changing Law of Bacterial Communities in the Milk of Bactrian Camels with Subclinical Mastitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Specimen Collection
2.2. DNA Extraction, Polymerase Chain Reaction Amplification, and Sequencing for the v3–v4 Region of the 16S rRNA Gene
2.3. Control of Data Quality
2.4. Amplitude Sequence Variant Denoising and Species Annotation
2.5. Statistical Analyses
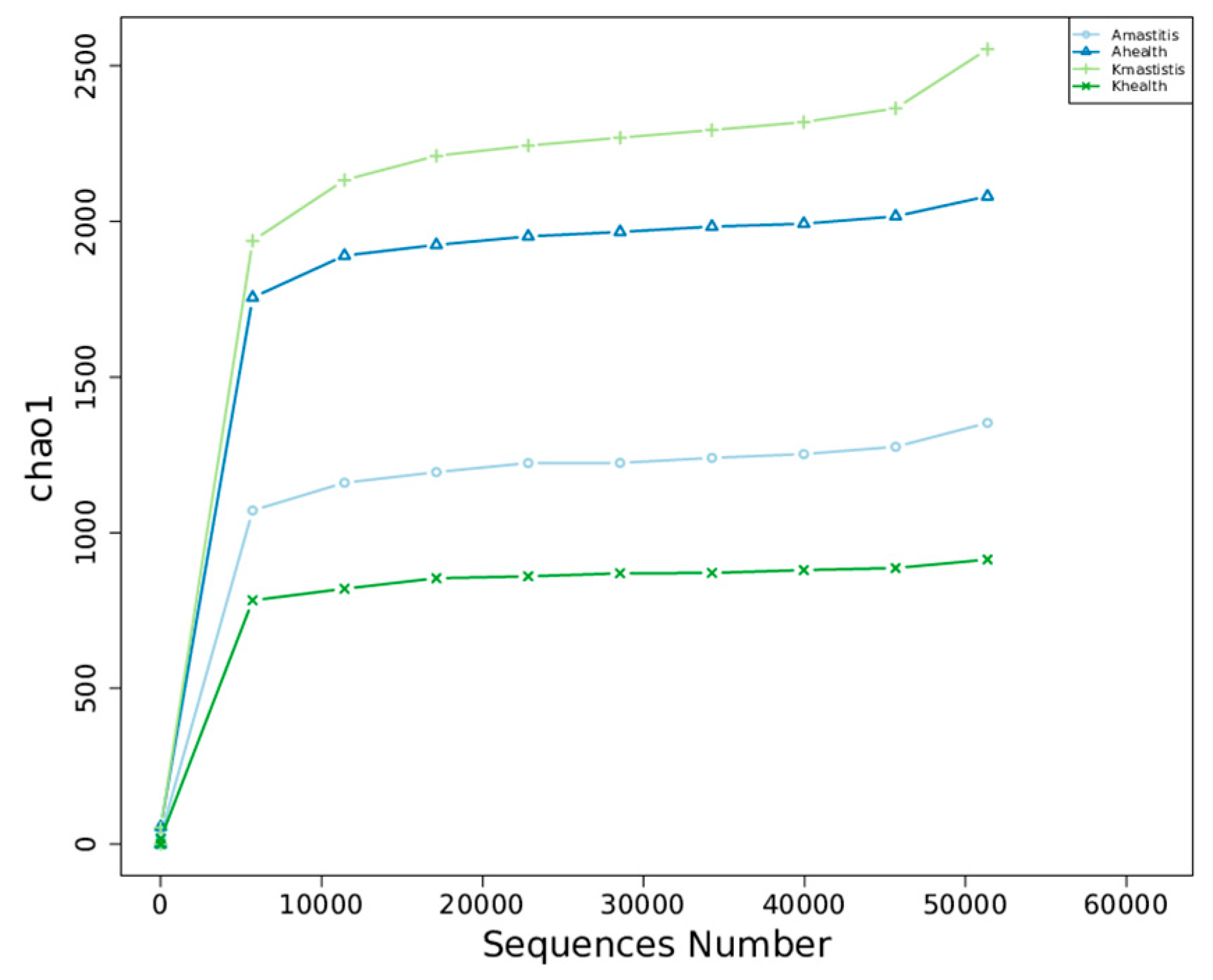
2.5.1. Analysis of Alpha Diversity
2.5.2. Analysis of Beta Diversity
2.5.3. Analysis of Community Differences and Functional Prediction Between Samples
3. Results
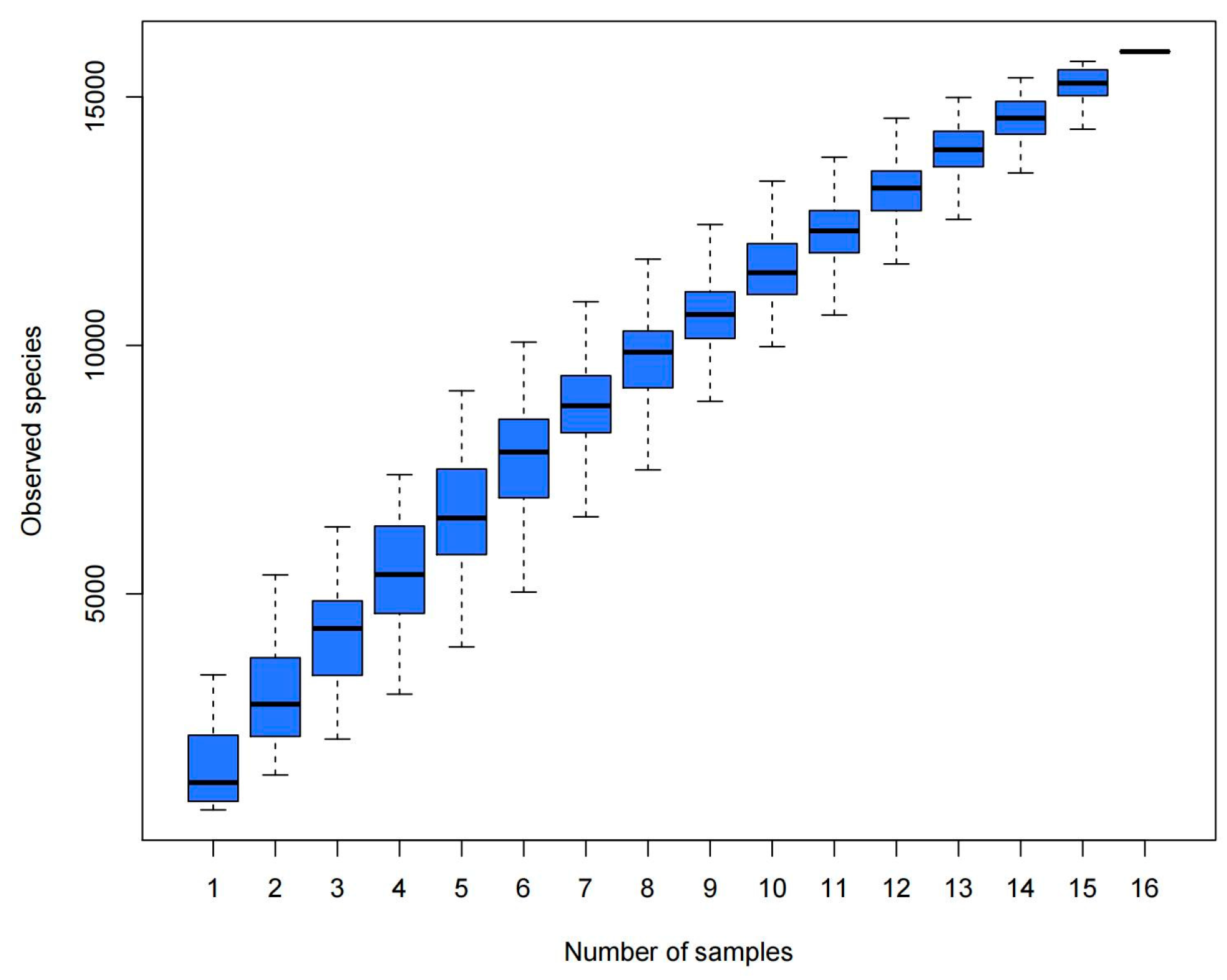
3.1. Results of Data Quality Control
3.2. Annotation Results of ASVs
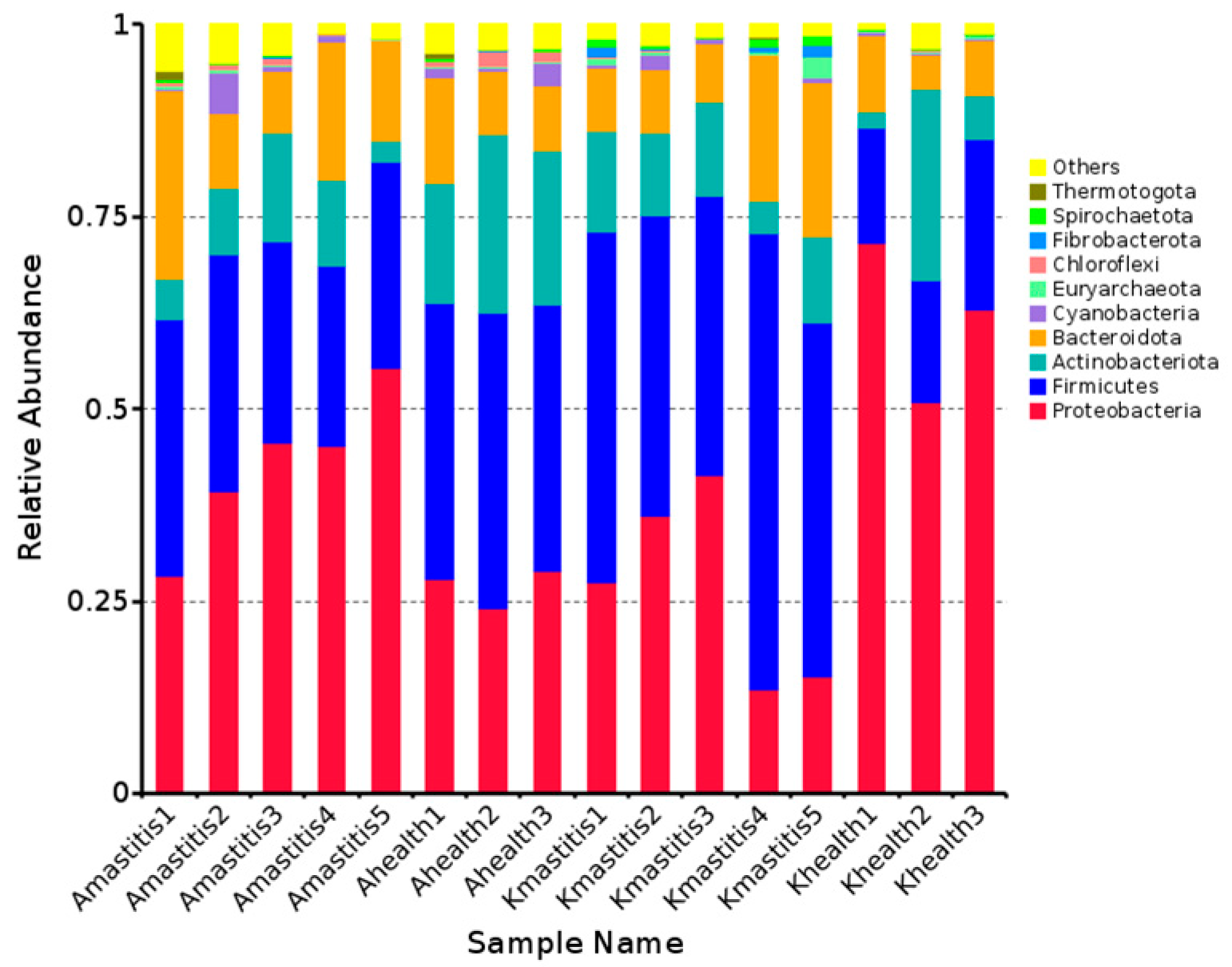
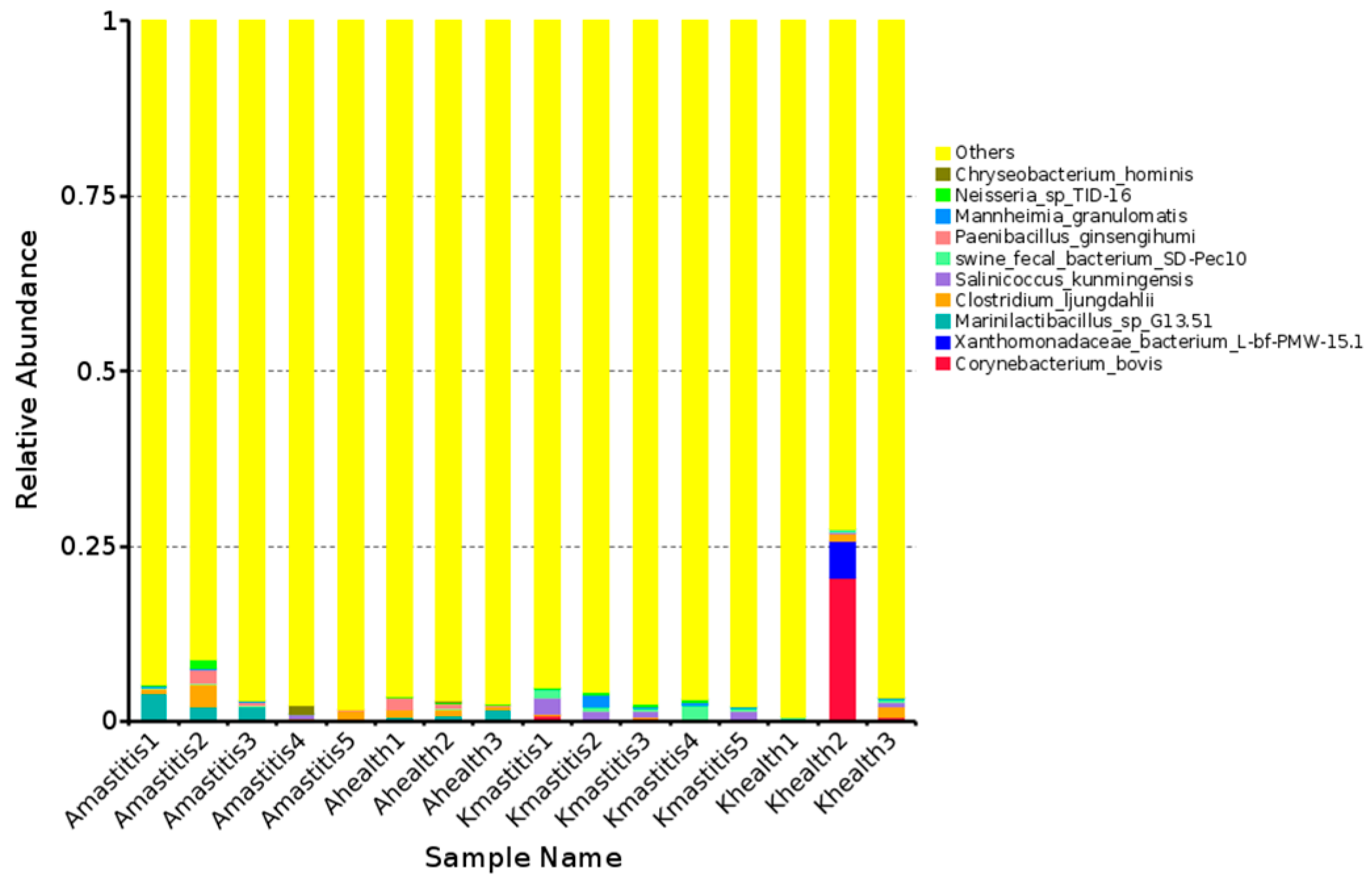
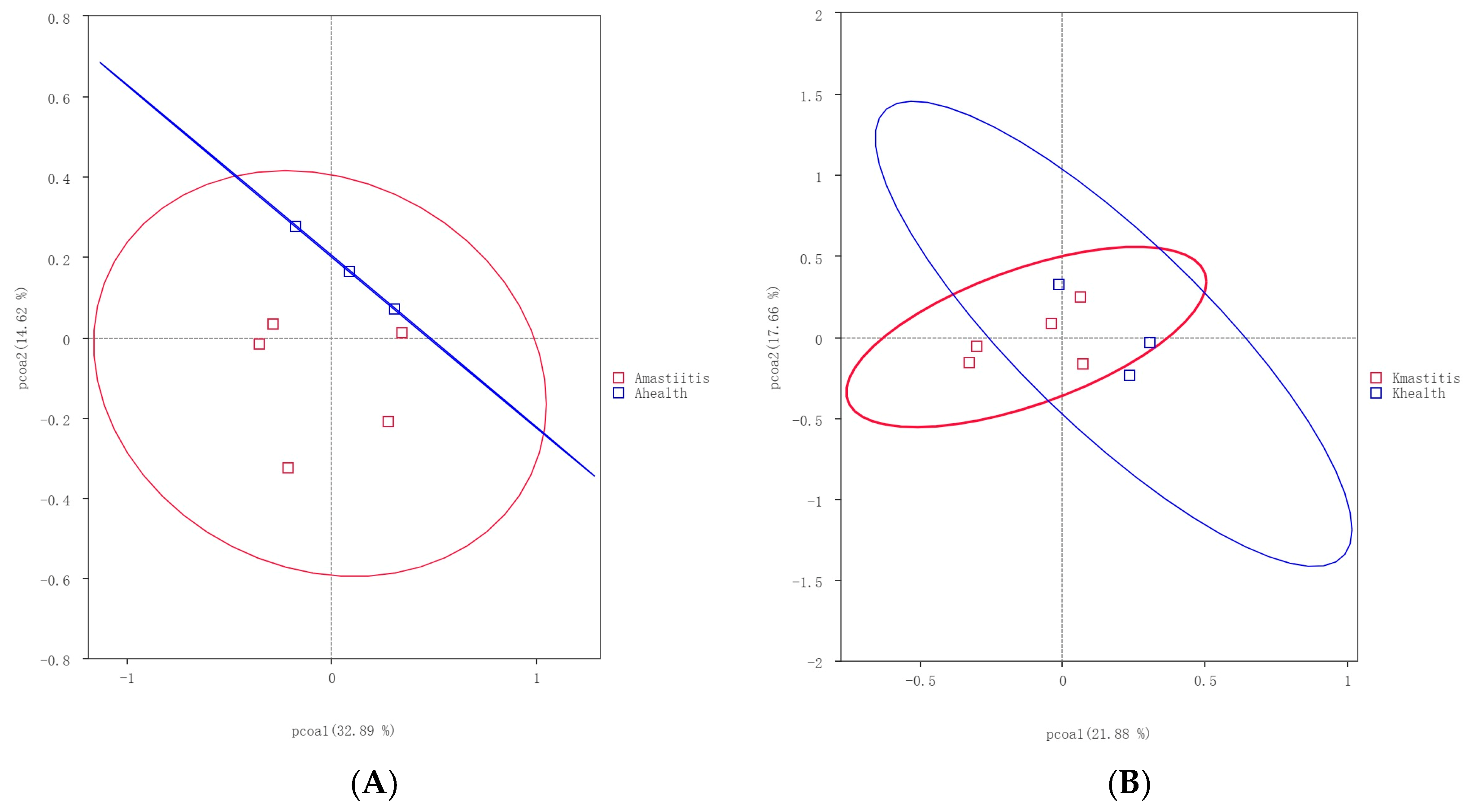
3.3. Analysis of Bacterial Abundance and Diversity in Camel Milk Samples in Healthy and Subclinical Mastitis Conditions
3.4. Analysis of Community Differences Among Samples and Functional Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMT | California Mastitis Test |
| PCR | Polymerase Chain Reaction |
| ASV | Amplitude Sequence Variant |
| PICRUSt2 | Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 |
| CTAB | Cetyltrimethylammonium Bromide |
| UPGMA | Unweighted Pair Group Method with Arithmetic Mean |
References
- Wang, Z.; Zhang, W.; Wang, B.; Zhang, F.; Shao, Y. Influence of Bactrian Camel Milk on the Gut Microbiota. J. Dairy Sci. 2018, 101, 5758–5769. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-D.; Xie, F.; Zhang, W.-D.; Zeng, W.-W.; Lu, J.; Cheng, Y.-J.; Wang, W.-H. Age-Dependent Distribution of IgA and IgG Antibody-Secreting Cells in the Pharyngeal Tonsil of the Bactrian Camel. Vet. J. 2024, 305, 106131. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liang, X.; Dou, Z.; Zhao, Z.; Ma, W.; Hao, Z.; Yan, H.; Wang, Y.; Wu, Z.; Chen, G.; et al. Transcriptome Analysis to Identify Candidate Genes Related to Mammary Gland Development of Bactrian Camel (Camelus bactrianus). Front. Vet. Sci. 2023, 10, 1196950. [Google Scholar] [CrossRef] [PubMed]
- Seifu, E. Camel Milk Products: Innovations, Limitations and Opportunities. Food Prod. Process. Nutr. 2023, 5, 15. [Google Scholar] [CrossRef]
- Miao, J.; Xiao, S.; Wang, J. Comparative Study of Camel Milk from Different Areas of Xinjiang Province in China. Food Sci. Anim. Resour. 2023, 43, 674–684. [Google Scholar] [CrossRef]
- He, J.; Guo, K.; Chen, Q.; Wang, Y.; Jirimutu, N. Camel Milk Modulates the Gut Microbiota and Has Anti-Inflammatory Effects in a Mouse Model of Colitis. J. Dairy Sci. 2022, 105, 3782–3793. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, G.B.; Shah, A.H.; Marghazani, I.B.; Barham, G.S.; Shah, Q.A.; Khand, F.M.; Buzdar, J.A.; Soomro, F.; Fazlani, S.A. Nutritional Significance and Promising Therapeutic/Medicinal Application of Camel Milk as a Functional Food in Human and Animals: A Comprehensive Review. Anim. Biotechnol. 2023, 34, 1988–2005. [Google Scholar] [CrossRef]
- Behrouz, S.; Saadat, S.; Memarzia, A.; Sarir, H.; Folkerts, G.; Boskabady, M.H. The Antioxidant, Anti-Inflammatory and Immunomodulatory Effects of Camel Milk. Front. Immunol. 2022, 13, 855342. [Google Scholar] [CrossRef]
- Al Nohair, S.F. Medical Benefits of Camel’s Milk: A Comprehensive Review. JPMA J. Pak. Med. Assoc. 2021, 71, 933–937. [Google Scholar] [CrossRef]
- Yao, H.; Pan, Z.; Ma, W.; Zhao, Z.; Su, Z.; Yang, J. Whole-Genome Resequencing Analysis of the Camelus Bactrianus (Bactrian camel) Genome Identifies Mutations and Genes Affecting Milk Production Traits. Int. J. Mol. Sci. 2024, 25, 7836. [Google Scholar] [CrossRef]
- Bakry, I.A.; Yang, L.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Cacciotti, I.; Ziedan, N.I.; Jin, J.; Jin, Q.; Wei, W.; et al. A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat. Foods 2021, 10, 2158. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Salman, H.M.; Ali, M.; Khaskheli, G.B.; Barham, G.S.; Marghazani, I.B.; Ahmed, S. A Review on Camel Milk Composition, Techno-Functional Properties and Processing Constraints. Food Sci. Anim. Resour. 2024, 44, 739–757. [Google Scholar] [CrossRef]
- Alhaj, O.A.; Altooq, N.J.; Alenezi, A.F.; Janahi, A.I.; Janahi, M.I.; Humood, A.M.; AlRasheed, M.M.; Bragazzi, N.L.; Jahrami, H.A.; Faye, B. Camel Milk Composition by Breed, Season, Publication Year, and Country: A Global Systematic Review, Meta-Analysis, and Meta-Regression. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2520–2559. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Narnaware, S.D.; Prakash, V. Incidence, Risk Factors and Economic Impact of Clinical Mastitis in Dromedary Camel (Camelus dromedarius). Trop. Anim. Health Prod. 2021, 54, 31. [Google Scholar] [CrossRef]
- Oselu, S.; Ebere, R.; Arimi, J.M. Camels, Camel Milk, and Camel Milk Product Situation in Kenya in Relation to the World. Int. J. Food Sci. 2022, 2022, 1237423. [Google Scholar] [CrossRef] [PubMed]
- Seligsohn, D.; Nyman, A.-K.; Younan, M.; Sake, W.; Persson, Y.; Bornstein, S.; Maichomo, M.; de Verdier, K.; Morrell, J.M.; Chenais, E. Subclinical Mastitis in Pastoralist Dairy Camel Herds in Isiolo, Kenya: Prevalence, Risk Factors, and Antimicrobial Susceptibility. J. Dairy Sci. 2020, 103, 4717–4731. [Google Scholar] [CrossRef]
- Mohamud, A.I.; Mohamed, Y.A.; Jama, O.S.A.; Mishra, P.; Mohamed, M.I. Prevalence and Major Pathogens Associated with Clinical and Subclinical Mastitis in Dairy Camel (Camelus dromedarius) in Benadir Region of Somalia. Vet. Sci. Res. Rev. 2020, 6, 132–137. [Google Scholar] [CrossRef]
- Mwangi, W.E.; Gitau, G.K.; Ikiror, D.; Kimeli, P.; Gakuru, M.I.; Machuchu, D.; Kingori, W. The Prevalence, Antimicrobial Sensitivity, and Factors Associated with Camel Mastitis in Isiolo County, Kenya. Vet. World 2022, 15, 2962–2970. [Google Scholar] [CrossRef]
- Aqib, A.I.; Muzammil, I.; Naseer, M.A.; Shoaib, M.; Bakht, P.; Zaheer, T.; Khan, Y.R.; Khan, R.L.; Usman, M.; Shafeeq, M.; et al. Pathological Insights into Camel Mastitis. Acta Trop. 2022, 231, 106415. [Google Scholar] [CrossRef]
- Mayila; Yerken; Adrijiang; Gulizia; Kurabek; Shaylanbek. Survey on the detection of cryptic mastitis in dairy camels in Fuhai County. Xinjiang Livest. Ind. 2015, S1, 22–23. [Google Scholar] [CrossRef]
- Li, B.; Ma, W.; Pei, W.; Zheng, Y.; Duan, Q.; Lu, Y.; Li, J.; Su, Z. Analysis of microbial diversity and identification of main pathogenic bacteria in camel mastitis milk-samples from some areas of Altay, Xinjiang. Chin. J. Vet. Sci. 2023, 43, 1935–1940. [Google Scholar] [CrossRef]
- Darwish, A.A. Clinicopathological Study on Camel Mastitis at Matrouh Governorate. J. Adv. Vet. Anim. Res. 2023, 10, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Maichomo, M.; Gachogo, R.; Masila, E.; Ogali, I.; Otieno, L.; Langat, N.; Onywera, R.; Malonza, V.; Inguyesi, C.; Onyambu, F.; et al. Draft Genome Sequences of Enterococcus Faecium, Enterococcus Gallinarum, and Lactococcus Lactis Strains Isolated from a Mastitis-Infected Camel in Isiolo County, Kenya. Microbiol. Resour. Announc. 2023, 12, e0108322. [Google Scholar] [CrossRef]
- Ali, M.M.; Helmy, S.M.; Fahmy, H.A.; Elaadli, H.; Eldesoukey, I.E. Molecular Characterization of Antimicrobial Resistance Genes of Staphylococcus Aureus Isolated from Mastitic Camel Milk in Egypt. Vet. Res. Forum Int. Q. J. 2024, 15, 267–274. [Google Scholar] [CrossRef]
- Jama, M.M.; Hussein, H.A.; Darod, Z.A.; Ahad, A.A. Determination of Prevalence of Subclinical Mastitis, Characterization of Intra-Mammary Infection-Causing Bacteria, and Antibiotic Susceptibility in Dairy Camels in Jigjiga City, Somali Region, Ethiopia. Front. Vet. Sci. 2024, 11, 1398118. [Google Scholar] [CrossRef] [PubMed]
- Balemi, A.; Gumi, B.; Amenu, K.; Girma, S.; Gebru, M.; Tekle, M.; Ríus, A.A.; D’Souza, D.H.; Agga, G.E.; Kerro Dego, O. Prevalence of Mastitis and Antibiotic Resistance of Bacterial Isolates from CMT Positive Milk Samples Obtained from Dairy Cows, Camels, and Goats in Two Pastoral Districts in Southern Ethiopia. Animals 2021, 11, 1530. [Google Scholar] [CrossRef]
- Geresu, M.A.; Abera Leliso, S.; Liben, G.W. Camel Mastitis: Prevalence, Risk Factors, and Isolation of Major Bacterial Pathogens in Gomole District of Borena Zone, Southern Ethiopia. Vet. Med. Int. 2021, 2021, 9993571. [Google Scholar] [CrossRef]
- El Tigani-Asil, E.T.A.; Abdelwahab, G.E.; Veedu, J.T.V.P.; Khalafalla, A.I.; Mohamed, Z.S.A.; Ishag, H.Z.A.; Shah, A.A.M.; Alhosani, M.A.A.; Al Muhairi, S.S.M. Gangrenous Mastitis in Dromedary Camels in UAE Caused by Streptococcus Agalactiae. BMC Vet. Res. 2020, 16, 174. [Google Scholar] [CrossRef]
- Taponen, S.; Salmikivi, L.; Simojoki, H.; Koskinen, M.T.; Pyörälä, S. Real-Time Polymerase Chain Reaction-Based Identification of Bacteria in Milk Samples from Bovine Clinical Mastitis with No Growth in Conventional Culturing. J. Dairy Sci. 2009, 92, 2610–2617. [Google Scholar] [CrossRef]
- Catozzi, C.; Sanchez Bonastre, A.; Francino, O.; Lecchi, C.; De Carlo, E.; Vecchio, D.; Martucciello, A.; Fraulo, P.; Bronzo, V.; Cuscó, A.; et al. The Microbiota of Water Buffalo Milk during Mastitis. PLoS ONE 2017, 12, e0184710. [Google Scholar] [CrossRef]
- Rahmeh, R.; Akbar, A.; Alomirah, H.; Kishk, M.; Al-Ateeqi, A.; Shajan, A.; Alonaizi, T.; Esposito, A. Assessment of Mastitis in Camel Using High-Throughput Sequencing. PLoS ONE 2022, 17, e0278456. [Google Scholar] [CrossRef] [PubMed]
- Imanian, B.; Donaghy, J.; Jackson, T.; Gummalla, S.; Ganesan, B.; Baker, R.C.; Henderson, M.; Butler, E.K.; Hong, Y.; Ring, B.; et al. The Power, Potential, Benefits, and Challenges of Implementing High-Throughput Sequencing in Food Safety Systems. npj Sci. Food 2022, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Dreier, M.; Meola, M.; Berthoud, H.; Shani, N.; Wechsler, D.; Junier, P. High-Throughput qPCR and 16S rRNA Gene Amplicon Sequencing as Complementary Methods for the Investigation of the Cheese Microbiota. BMC Microbiol. 2022, 22, 48. [Google Scholar] [CrossRef]
- Kadri, Z.; Spitaels, F.; Cnockaert, M.; Amar, M.; Joossens, M.; Vandamme, P. The Bacterial Diversity of Raw Moroccon Camel Milk. Int. J. Food Microbiol. 2021, 341, 109050. [Google Scholar] [CrossRef] [PubMed]
- Rahmeh, R.; Akbar, A.; Alomirah, H.; Kishk, M.; Al-Ateeqi, A.; Al-Milhm, S.; Shajan, A.; Akbar, B.; Al-Merri, S.; Alotaibi, M.; et al. Data on microbial diversity of camel milk microbiota determined by 16S rRNA gene sequencing. Data Brief 2022, 45, 108744. [Google Scholar] [CrossRef]
- Sun, M.; Shao, W.; Liu, Z.; Ma, X.; Chen, H.; Zheng, N.; Zhao, Y. Microbial Diversity in Camel Milk from Xinjiang, China as Revealed by Metataxonomic Analysis. Front. Microbiol. 2024, 15, 1367116. [Google Scholar] [CrossRef]
- Qin, Y.; Huang, W.; Yang, J.; Zhao, Y.; Zhao, M.; Xu, H.; Zhang, M. The Antibiotic Resistome and Its Association with Bacterial Communities in Raw Camel Milk from Altay Xinjiang. Foods 2023, 12, 3928. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, H.; Kwok, L.-Y.; Guo, F.; Ji, R.; Ya, M.; Chen, Y. Analyses of Physicochemical Properties, Bacterial Microbiota, and Lactic Acid Bacteria of Fresh Camel Milk Collected in Inner Mongolia. J. Dairy Sci. 2020, 103, 106–116. [Google Scholar] [CrossRef]
- Rahmeh, R.; Akbar, A.; Alomirah, H.; Kishk, M.; Al-Ateeqi, A.; Al-Milhm, S.; Shajan, A.; Akbar, B.; Al-Merri, S.; Alotaibi, M.; et al. Camel Milk Microbiota: A Culture-Independent Assessment. Food Res. Int. 2022, 159, 111629. [Google Scholar] [CrossRef]
- Yao, H.; Wang, H.; Ma, Q.; Munila, R.M.; Zheng, T.; Zhang, Y.; Ma, Y.; Chen, B.; Wan, Z.; Wu, Z.; et al. Investigation and Analysis of Plant Feeding by Bactrian Camels in Junggar, Xinjiang. Heilongjiang Anim. Husb. Vet. Med. 2021, 24, 89–95. [Google Scholar] [CrossRef]
- Alhafiz, G.A.; Alghatam, F.H.; Almohammed, H.; Hussen, J. Milk Immune Cell Composition in Dromedary Camels with Subclinical Mastitis. Front. Vet. Sci. 2022, 9, 885523. [Google Scholar] [CrossRef] [PubMed]
- Alebie, A.; Molla, A.; Adugna, W.; Tesfaye, A.; Ejo, M. Prevalence, Isolation, Identification, and Risk Factors of Major Bacterial Cause of Camel Subclinical Mastitis. Biomed Res. Int. 2021, 2021, 5522331. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, A.C.; Davis, J.W.; Spollen, W.; Bivens, N.; Givan, S.; Hagan, C.E.; McIntosh, M.; Franklin, C.L. Effects of Vendor and Genetic Background on the Composition of the Fecal Microbiota of Inbred Mice. PLoS ONE 2015, 10, e0116704. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Passey, T.; Wei, F.; Saville, R.; Harrison, R.J. Amplicon-Based Metagenomics Identified Candidate Organisms in Soils That Caused Yield Decline in Strawberry. Hortic. Res. 2015, 2, 15022. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-Filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Gao, X.; Wang, J. The Intratumor Microbiota Signatures Associate with Subtype, Tumor Stage, and Survival Status of Esophageal Carcinoma. Front. Oncol. 2021, 11, 754788. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Guo, F.; Wu, W.; Zhang, T. Characterization of Tetracycline Resistant Bacterial Community in Saline Activated Sludge Using Batch Stress Incubation with High-Throughput Sequencing Analysis. Water Res. 2013, 47, 4207–4216. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative Beta Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Amrouche, T.; Mounier, J.; Pawtowski, A.; Thomas, F.; Picot, A. Microbiota Associated with Dromedary Camel Milk from Algerian Sahara. Curr. Microbiol. 2020, 77, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hadef, L.; Hamad, B.; Aggad, H. Risk Factors Associated with Subclinical Mastitis and Its Effect on Physico-Mineral Features of Camel Milk. Trop. Anim. Health Prod. 2022, 54, 224. [Google Scholar] [CrossRef]
- Hadef, L.; Hamad, B.; Aggad, H. Effect of Subclinical Mastitis on Milk Yield and Milk Composition Parameters in Dairy Camels. Acta Biol. Szeged. 2020, 63, 83–90. [Google Scholar] [CrossRef]
- Wang, A.; Diana, A.; Rahmannia, S.; Gibson, R.S.; Houghton, L.A.; Slupsky, C.M. Impact of Milk Secretor Status on the Fecal Metabolome and Microbiota of Breastfed Infants. Gut Microbes 2023, 15, 2257273. [Google Scholar] [CrossRef]
- Papademas, P.; Kamilari, E.; Aspri, M.; Anagnostopoulos, D.A.; Mousikos, P.; Kamilaris, A.; Tsaltas, D. Investigation of Donkey Milk Bacterial Diversity by 16S rDNA High-Throughput Sequencing on a Cyprus Donkey Farm. J. Dairy Sci. 2021, 104, 167–178. [Google Scholar] [CrossRef]
- Jiang, C.; Hou, X.; Gao, X.; Liu, P.; Guo, X.; Hu, G.; Li, Q.; Huang, C.; Li, G.; Fang, W.; et al. The 16S rDNA High-Throughput Sequencing Correlation Analysis of Milk and Gut Microbial Communities in Mastitis Holstein Cows. BMC Microbiol. 2023, 23, 180. [Google Scholar] [CrossRef]
- Vayssier-Taussat, M.; Albina, E.; Citti, C.; Cosson, J.-F.; Jacques, M.-A.; Lebrun, M.-H.; Le Loir, Y.; Ogliastro, M.; Petit, M.-A.; Roumagnac, P.; et al. Shifting the Paradigm from Pathogens to Pathobiome: New Concepts in the Light of Meta-Omics. Front. Cell. Infect. Microbiol. 2014, 4, 29. [Google Scholar] [CrossRef]
- Salman, M.M.; Nawaz, M.; Yaqub, T.; Mushtaq, M.H. Investigation of Milk Microbiota of Healthy and Mastitic Sahiwal Cattle. BMC Microbiol. 2023, 23, 304. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Mao, W.; Wang, C.; Yin, S. Functional Potential Differences between Firmicutes and Proteobacteria in Response to Manure Amendment in a Reclaimed Soil. Can. J. Microbiol. 2020, 66, 689–697. [Google Scholar] [CrossRef]
- Du, X.; Li, F.; Kong, F.; Cui, Z.; Li, D.; Wang, Y.; Zhu, Q.; Shu, G.; Tian, Y.; Zhang, Y.; et al. Altitude-Adaption of Gut Microbiota in Tibetan Chicken. Poult. Sci. 2022, 101, 101998. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Lootah, M.; Tahlak, M.; Venema, K. Profiles of Human Milk Oligosaccharides and Their Relations to the Milk Microbiota of Breastfeeding Mothers in Dubai. Nutrients 2020, 12, 1727. [Google Scholar] [CrossRef]
- Andriyanov, P.A.; Kashina, D.D.; Menshikova, A.N. Genomic Analysis of Multidrug-Resistant Delftia Tsuruhatensis Isolated from Raw Bovine Milk. Front. Microbiol. 2023, 14, 1321122. [Google Scholar] [CrossRef]
- Luoyizha, W.; Wu, X.; Zhang, M.; Guo, X.; Li, H.; Liao, X. Compared Analysis of Microbial Diversity in Donkey Milk from Xinjiang and Shandong of China through High-Throughput Sequencing. Food Res. Int. 2020, 137, 109684. [Google Scholar] [CrossRef]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the All-Bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef]
- Mangutov, E.O.; Kharseeva, G.G.; Alutina, E.L. Corynebacterium Spp.—Problematic Pathogens of the Human Respiratory Tract (Review of Literature). Klin. Lab. Diagn. 2021, 66, 502–508. [Google Scholar] [CrossRef]
- Oliveira, A.; Oliveira, L.C.; Aburjaile, F.; Benevides, L.; Tiwari, S.; Jamal, S.B.; Silva, A.; Figueiredo, H.C.P.; Ghosh, P.; Portela, R.W.; et al. Insight of Genus Corynebacterium: Ascertaining the Role of Pathogenic and Non-Pathogenic Species. Front. Microbiol. 2017, 8, 1937. [Google Scholar] [CrossRef] [PubMed]
- Nicola, I.; Cerutti, F.; Grego, E.; Bertone, I.; Gianella, P.; D’Angelo, A.; Peletto, S.; Bellino, C. Characterization of the Upper and Lower Respiratory Tract Microbiota in Piedmontese Calves. Microbiome 2017, 5, 152. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, Types, Etiological Agents, Prevalence, Diagnosis, Treatment, Prevention, Effects on Human Health and Future Aspects of Bovine Mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.; El-Hajjaji, S.; Burteau, S.; Fall, P.A.; Pirard, B.; Taminiau, B.; Daube, G.; Sindic, M. Study of the Microbial Diversity of a Panel of Belgian Artisanal Cheeses Associated with Challenge Studies for Listeria Monocytogenes. Food Microbiol. 2021, 100, 103861. [Google Scholar] [CrossRef]
- Banerjee, A.; Leang, C.; Ueki, T.; Nevin, K.P.; Lovley, D.R. Lactose-Inducible System for Metabolic Engineering of Clostridium ljungdahlii. Appl. Environ. Microbiol. 2014, 80, 2410–2416. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Alsulaimany, F.A.S.; Alattas, S.G.; Al-Garni, S.M. Molecular Diagnosis for Camel Raw Milk Microbiota. Cell. Mol. Biol. 2024, 70, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Kommadath, A.; Tingley, J.P.; Abbott, D.W. Novel Insights into the Pig Gut Microbiome Using Metagenome-Assembled Genomes. Microbiol. Spectr. 2022, 10, e0238022. [Google Scholar] [CrossRef]
- Gluecks, I.V.; Bethe, A.; Younan, M.; Ewers, C. Molecular Study on Pasteurella Multocida and Mannheimia Granulomatis from Kenyan Camels (Camelus dromedarius). BMC Vet. Res. 2017, 13, 265. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of Adaptive Immunity by the Innate Immune System. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Wang, X.; Su, F.; Yu, X.; Geng, N.; Li, L.; Wang, R.; Zhang, M.; Liu, J.; Liu, Y.; Han, B. RNA-Seq Whole Transcriptome Analysis of Bovine Mammary Epithelial Cells in Response to Intracellular Staphylococcus Aureus. Front. Vet. Sci. 2020, 7, 642. [Google Scholar] [CrossRef]
- Kulbacka, J.; Choromańska, A.; Rossowska, J.; Weżgowiec, J.; Saczko, J.; Rols, M.-P. Cell Membrane Transport Mechanisms: Ion Channels and Electrical Properties of Cell Membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino Acid Metabolism in Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Csapó, J.; Csapó-Kiss, Z.; Stefler, J.; Martin, T.G.; Némethy, S. Influence of Mastitis on D-Amino Acid Content of Milk. J. Dairy Sci. 1995, 78, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Bochniarz, M.; Kocki, T.; Dąbrowski, R.; Szczubiał, M.; Wawron, W.; Turski, W.A. Tryptophan, Kynurenine, Kynurenic Acid Concentrations and Indoleamine 2,3-Dioxygenase Activity in Serum and Milk of Dairy Cows with Subclinical Mastitis Caused by Coagulase-Negative Staphylococci. Reprod. Domest. Anim. Zuchthyg. 2018, 53, 1491–1497. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Dunn, S.M.; Ametaj, B.N. Innate Immunity and Carbohydrate Metabolism Alterations Precede Occurrence of Subclinical Mastitis in Transition Dairy Cows. J. Anim. Sci. Technol. 2015, 57, 46. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Dunn, S.M.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. GC-MS Metabolomics Identifies Metabolite Alterations That Precede Subclinical Mastitis in the Blood of Transition Dairy Cows. J. Proteome Res. 2017, 16, 433–446. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Zhang, L.; Yao, H.; Zhang, Y.; Wang, W.; Liu, Y.; Zhao, X.; Su, Z. A Study on the Changing Law of Bacterial Communities in the Milk of Bactrian Camels with Subclinical Mastitis. Microorganisms 2025, 13, 1394. https://doi.org/10.3390/microorganisms13061394
Ma W, Zhang L, Yao H, Zhang Y, Wang W, Liu Y, Zhao X, Su Z. A Study on the Changing Law of Bacterial Communities in the Milk of Bactrian Camels with Subclinical Mastitis. Microorganisms. 2025; 13(6):1394. https://doi.org/10.3390/microorganisms13061394
Chicago/Turabian StyleMa, Wanpeng, Lin Zhang, Huaibing Yao, Yi Zhang, Wei Wang, Yifan Liu, Xueting Zhao, and Zhanqiang Su. 2025. "A Study on the Changing Law of Bacterial Communities in the Milk of Bactrian Camels with Subclinical Mastitis" Microorganisms 13, no. 6: 1394. https://doi.org/10.3390/microorganisms13061394
APA StyleMa, W., Zhang, L., Yao, H., Zhang, Y., Wang, W., Liu, Y., Zhao, X., & Su, Z. (2025). A Study on the Changing Law of Bacterial Communities in the Milk of Bactrian Camels with Subclinical Mastitis. Microorganisms, 13(6), 1394. https://doi.org/10.3390/microorganisms13061394




