Interaction Between Enterococcus faecalis and Fusobacterium nucleatum Regulated Macrophage Transcriptional Profiling and Reprogrammed Cellular Immune and Metabolic Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Culture and Differentiation of THP-1 Cells
2.3. Establishment of Coaggregation Model
2.4. Co-Culture of dTHP-1 Cells and Bacterial Cells
2.5. Transmission Electron Microscopy (TEM) Imaging
2.6. Fluorescence Flow Cytometric Analysis (FACS) of Cell Surface Markers
2.7. ROS Determination
2.8. RNA Extraction
2.9. RNA Sequencing
2.10. Quantitative Reverse Transcription PCR (qRT-PCR)
2.11. Oil Red O Staining
2.12. Statistical Analysis
3. Results
3.1. Coaggregated E. faecalis and F. nucleatum Cause Nuclear Shrinkage and Increased Mitochondria in Mφ
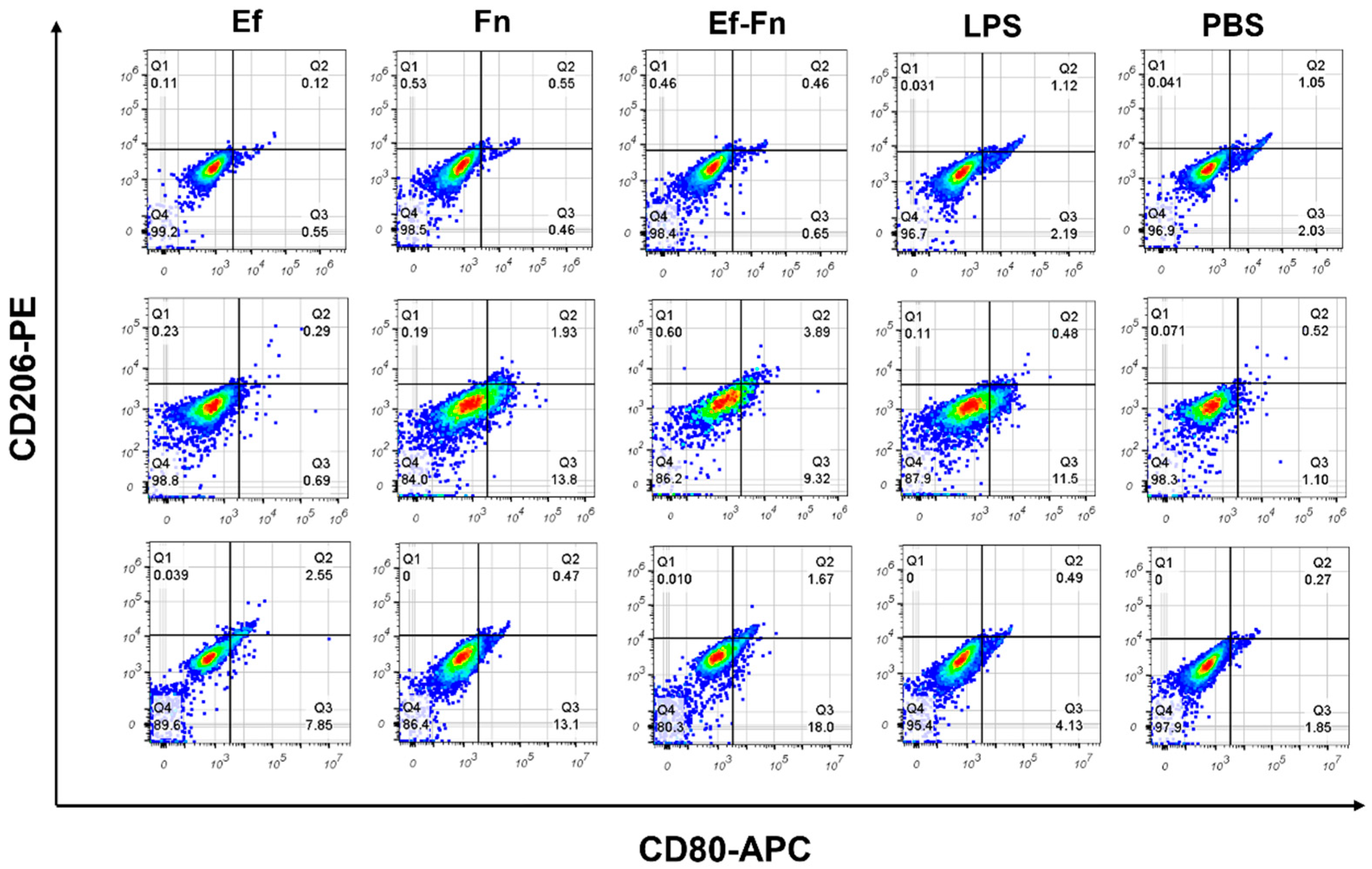
3.2. Coaggregated E. faecalis and F. nucleatum Induce M1 Polarization of Mφ
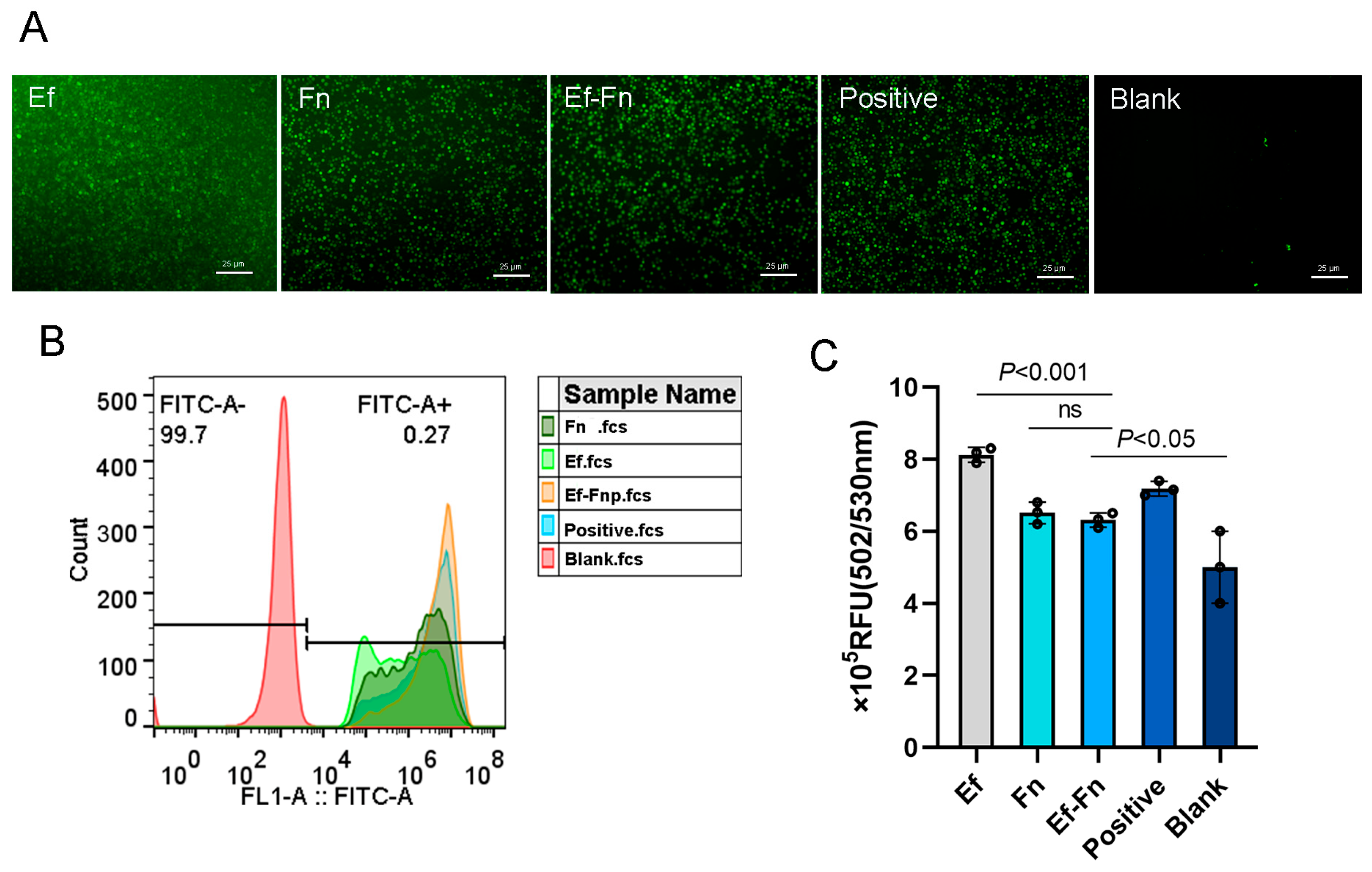
3.3. Coaggregated E. faecalis and F. nucleatum Promote Low-Level ROS Production in Mφ
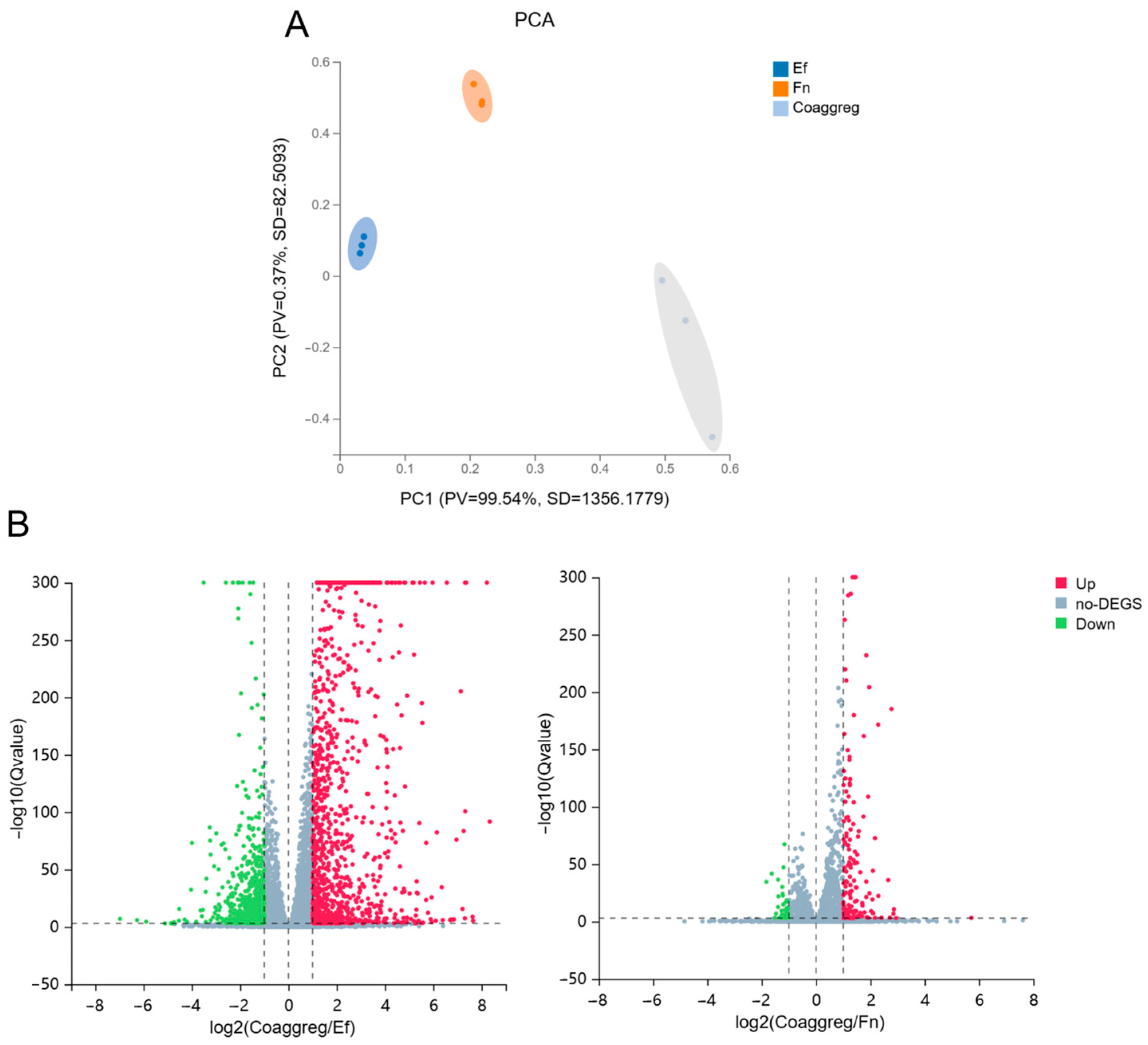
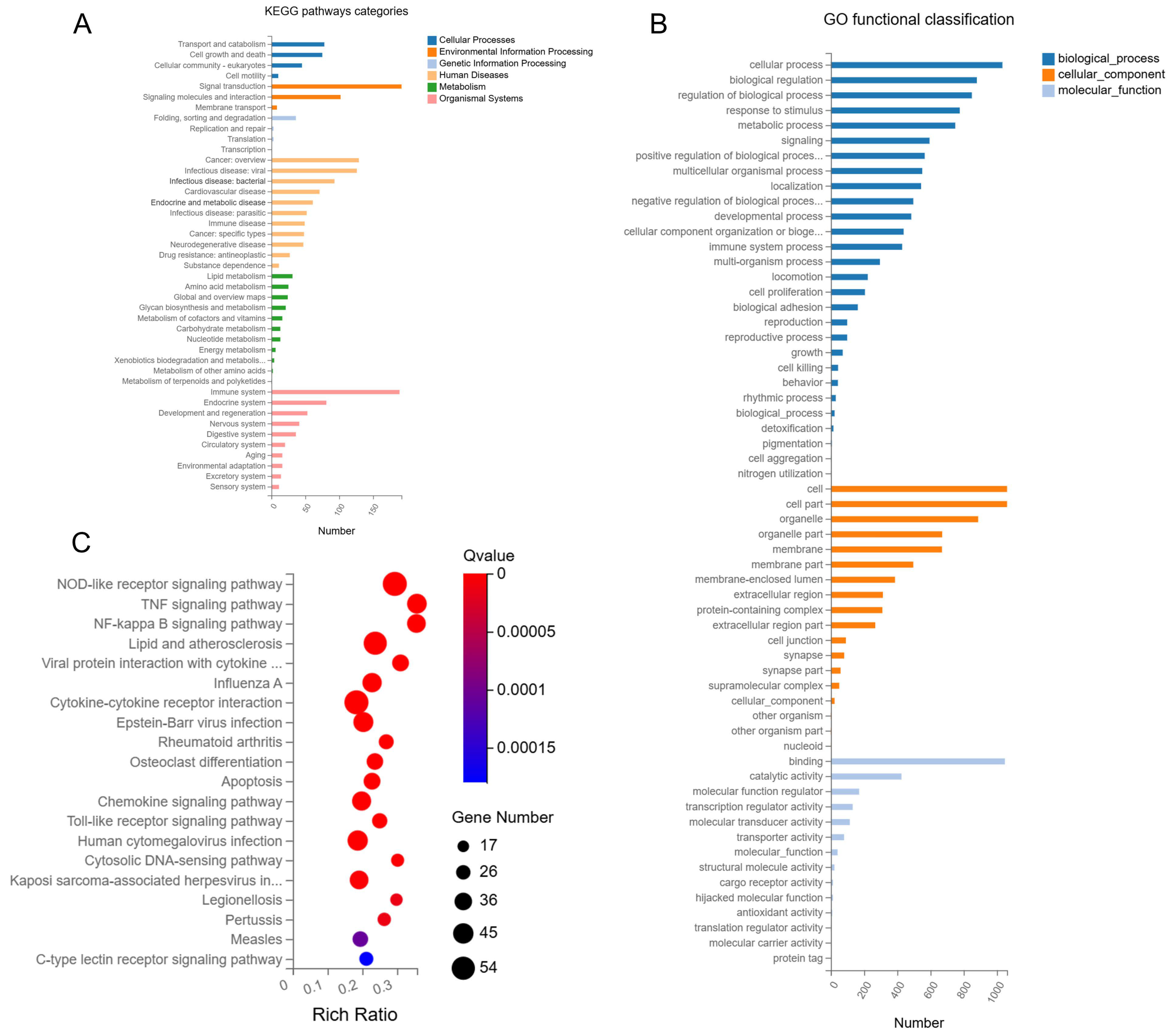
3.4. Influence of E. faecalis and F. nucleatum Coaggregation on the Transcriptome of Mφ
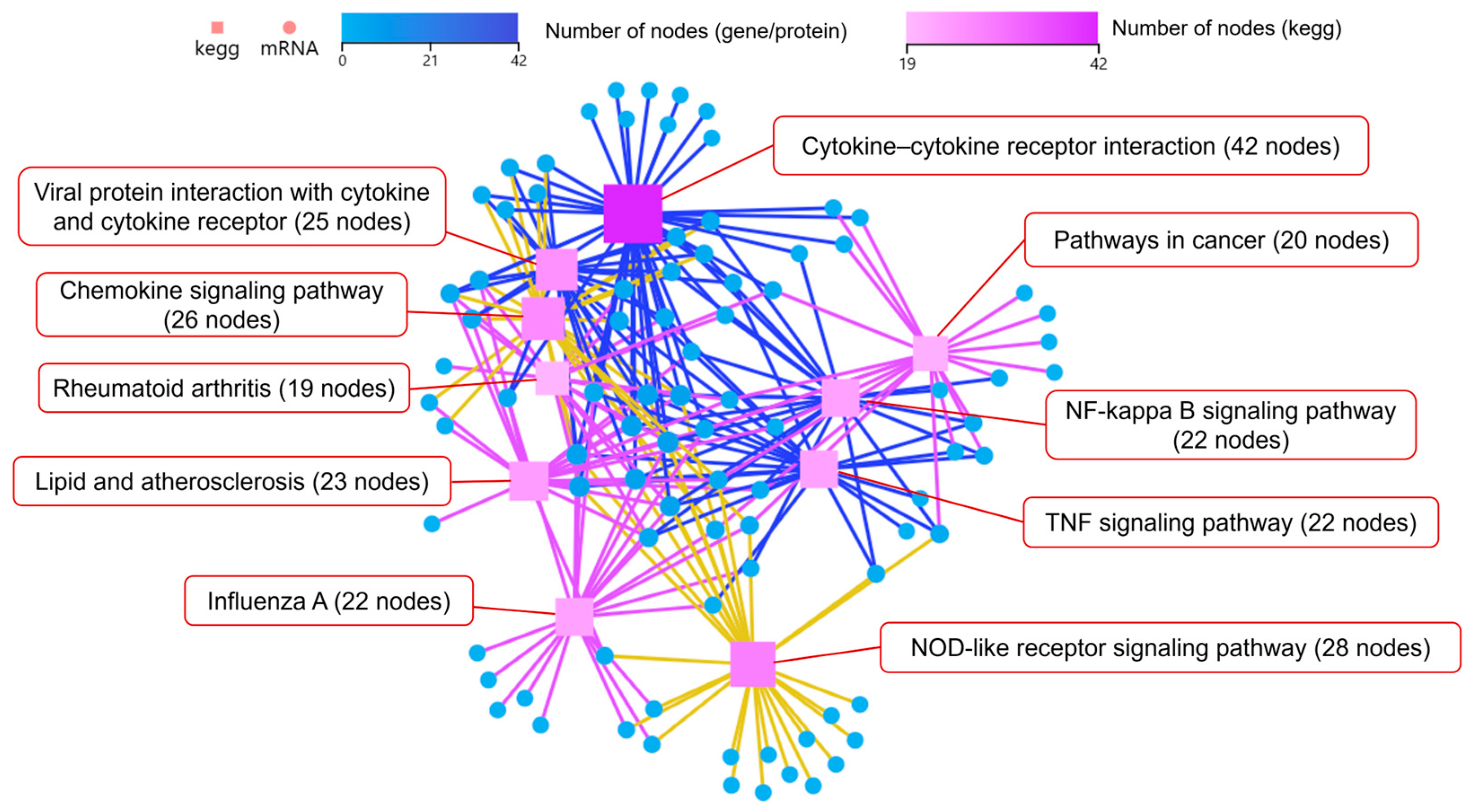
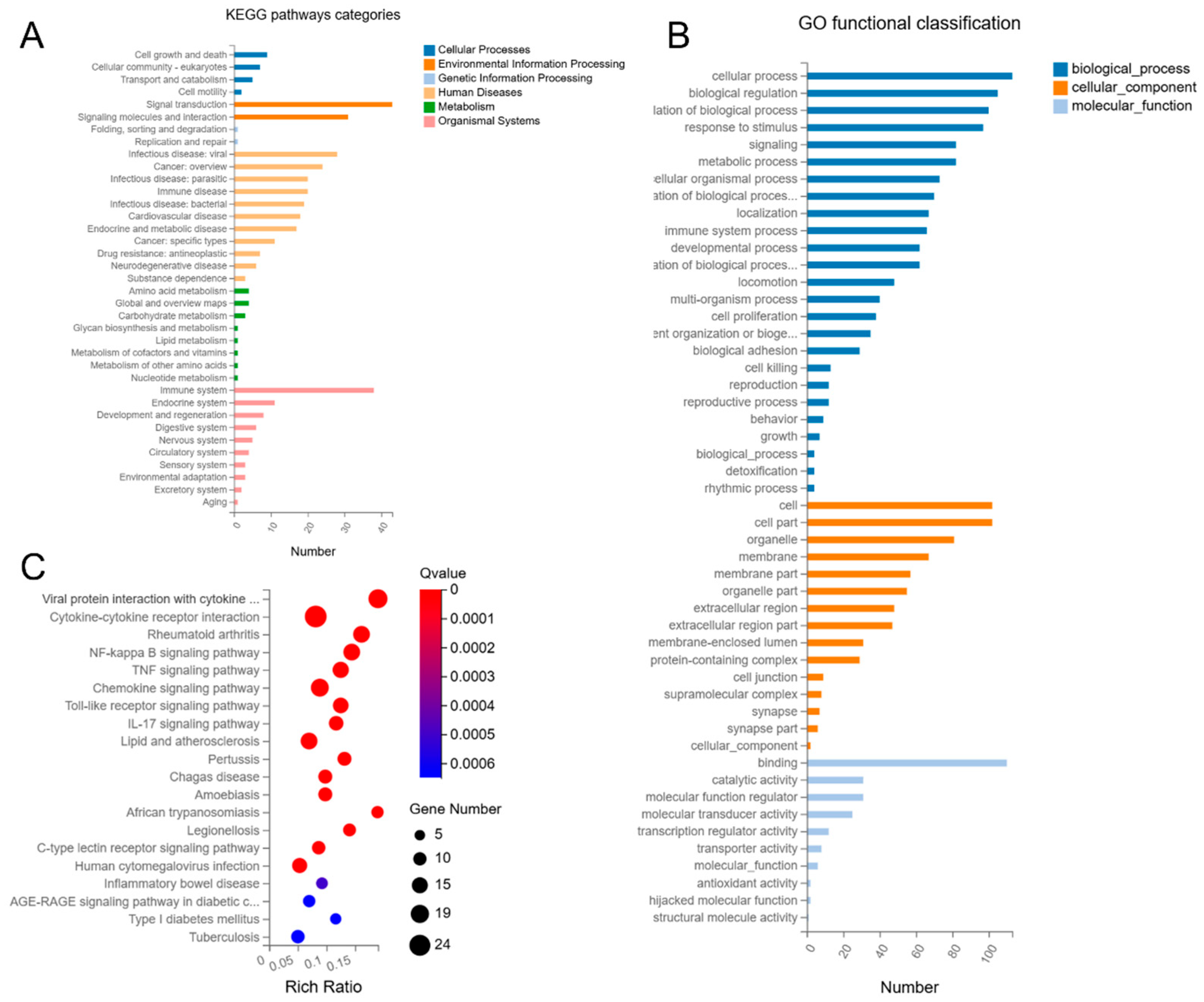
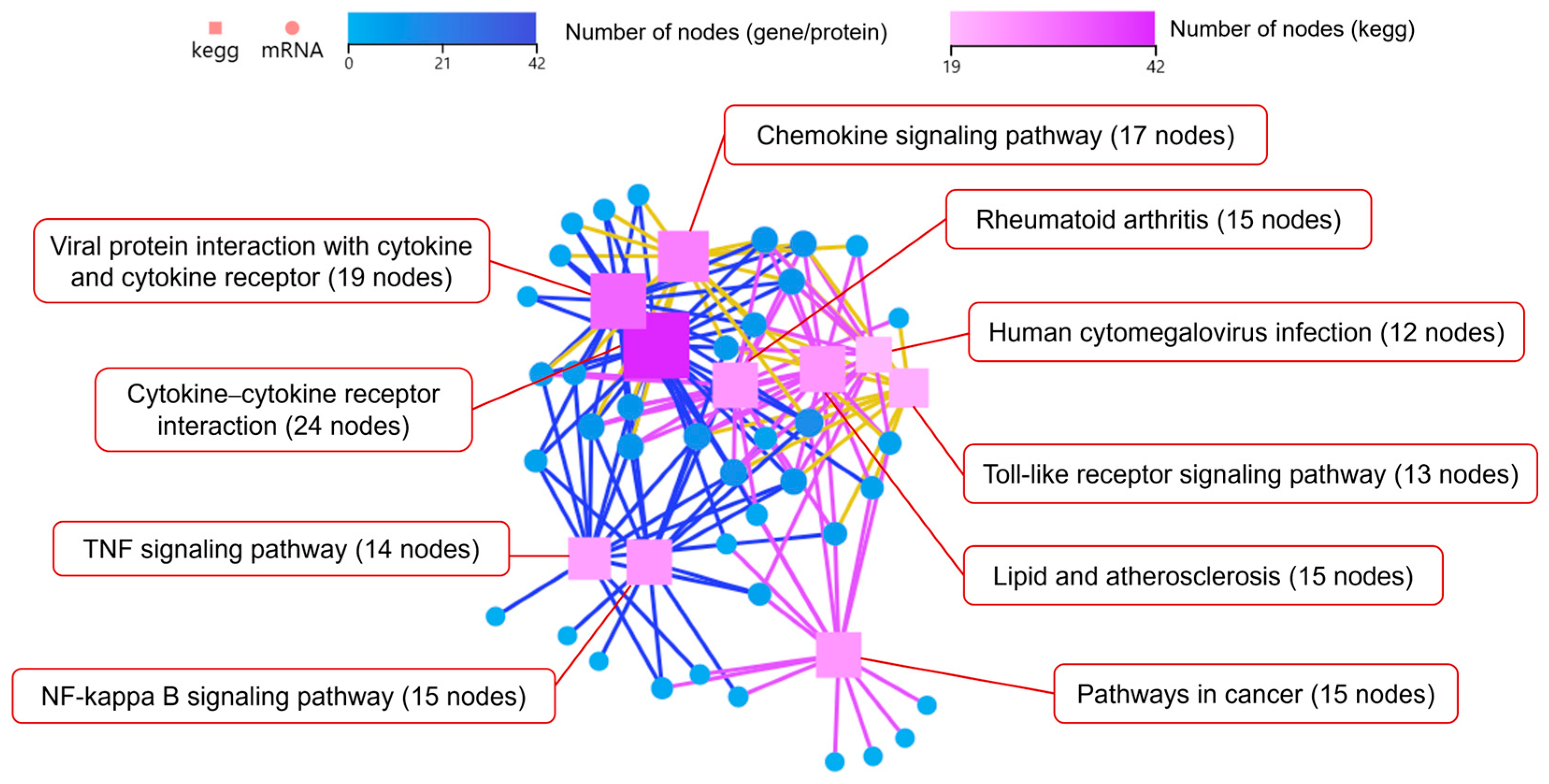
3.4.1. Coaggregated E. faecalis and F. nucleatum vs. E. faecalis
3.4.2. Coaggregated E. faecalis and F. nucleatum vs. F. nucleatum
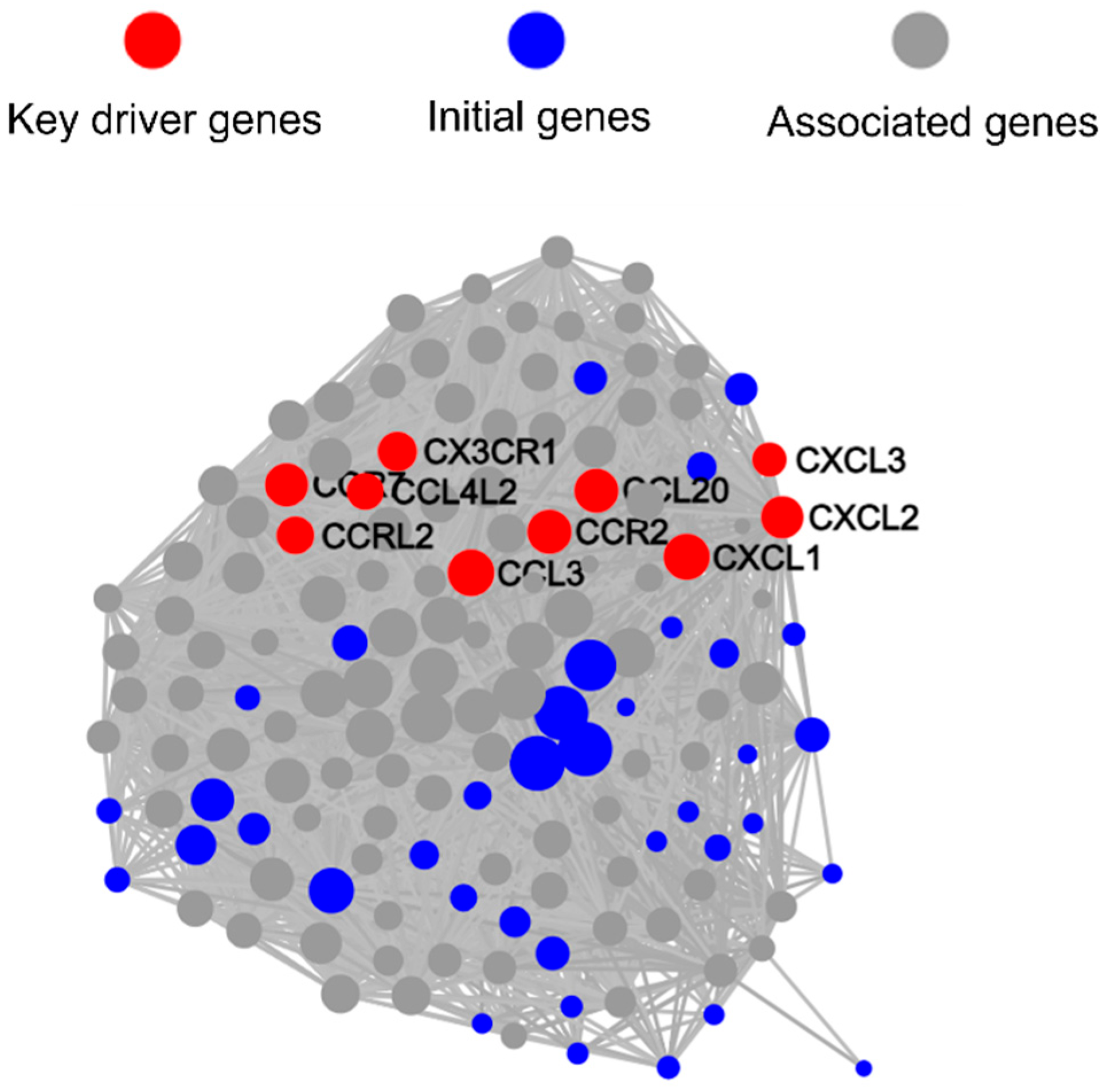
3.5. qRT-PCR Validation of Key DEGs Expression
3.6. Coaggregated E. faecalis and F. nucleatum Promote Lipid Accumulation in Mφ

4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiburcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Babeer, A.; Liu, Y.; Ren, Z.; Xiang, Z.; Oh, M.J.; Pandey, N.K.; Simon-Soro, A.; Huang, R.; Karabucak, B.; Cormode, D.P.; et al. Ferumoxytol nanozymes effectively target chronic biofilm infections in apical periodontitis. J. Clin. Investig. 2024, 135, e183576. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lin, B.; Liu, F.; Zhao, W. Role of Enterococcus faecalis in refractory apical periodontitis: From pathogenicity to host cell response. J. Oral Microbiol. 2023, 15, 2184924. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ma, R.; Jiang, W.; Deng, Z.; Chen, L.; Liang, Y.; Shao, L.; Zhao, W. Enterococcus faecalis-induced macrophage necroptosis promotes refractory apical periodontitis. Microbiol. Spectr. 2022, 10, e0104522. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Huang, D.; Song, H. Macrophages in periapical lesions: Potential roles and future directions. Front. Immunol. 2022, 13, 949102. [Google Scholar] [CrossRef]
- Polak, D.; Yaya, A.; Levy, D.H.; Metzger, Z.; Abramovitz, I. Enterococcus faecalis sustained infection induces macrophage pro-resolution polarization. Int. Endod. J. 2021, 54, 1840–1849. [Google Scholar] [CrossRef]
- Ramirez, T.; Shrestha, A.; Kishen, A. Inflammatory potential of monospecies biofilm matrix components. Int. Endod. J. 2019, 52, 1020–1027. [Google Scholar] [CrossRef]
- Gomes, B.; Francisco, P.A.; Godoi, E.J.; Endo, M.S.; Barbosa-Ribeiro, M.; Delboni, M.G.; Pecorari, V. Identification of culturable and nonculturable microorganisms, lipopolysaccharides, and lipoteichoic acids from root canals of teeth with endodontic failure. J. Endod. 2021, 47, 1075–1086. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Fan, W.; Fan, B. Fusobacterium nucleatum triggers proinflammatory cell death via Z-DNA binding protein 1 in apical periodontitis. Cell Commun. Signal 2022, 20, 196. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Braeutigam, F.; Tonndorf-Martini, S.; Heyder, M.; Reise, M.; Sigusch, B. Antibacterial effect of endodontic disinfections on Enterococcus faecalis in dental root canals—An in-vitro model study. Materials 2021, 14, 2427. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Santa-Rosa, C.C.; Thebit, M.M.; Maciel, K.F.; Brito, L.; Vieira, L.Q.; Ribeiro-Sobrinho, A.P. Evaluation of chemokines and receptors in gnotobiotic root canal infection by F. nucleatum and E. faecalis. Braz. Oral Res. 2018, 32, e120. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.L.; Oliveira, R.R.; Tavares, W.L.; Saldanha, T.D.; Farias, L.M.; Vieira, L.Q.; Ribeiro, A.S. Murine experimental root canal infection: Cytokine expression in response to F. nucleatum and E. faecalis. Braz. Dent. J. 2016, 27, 578–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, Z.; Yang, R.; Liu, T.; Lu, X.; Huang, W.; Guo, L. Coaggregated, E. faecalis with F. nucleatum regulated environmental stress responses and inflammatory effects. Appl. Microbiol. Biotechnol. 2024, 108, 336. [Google Scholar] [CrossRef]
- Cisar, J.O.; Kolenbrander, P.E.; McIntire, F.C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect. Immun. 1979, 24, 742–752. [Google Scholar] [CrossRef]
- Lima, B.P.; Shi, W.; Lux, R. Identification and characterization of a novel Fusobacterium nucleatum adhesin involved in physical interaction and biofilm formation with Streptococcus gordonii. MicrobiologyOpen 2017, 6, e00444. [Google Scholar] [CrossRef]
- Kaplan, A.; Kaplan, C.W.; He, X.; McHardy, I.; Shi, W.; Lux, R. Characterization of aid1, a novel gene involved in Fusobacterium nucleatum interspecies interactions. Microb. Ecol. 2014, 68, 379–387. [Google Scholar] [CrossRef]
- Liu, T.; Yang, R.; Zhou, J.; Lu, X.; Yuan, Z.; Wei, X.; Guo, L. Interactions between Streptococcus gordonii and Fusobacterium nucleatum altered bacterial transcriptional profiling and attenuated the immune responses of macrophages. Front. Cell Infect. Microbiol. 2022, 11, 783323. [Google Scholar] [CrossRef]
- Tan, H.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxidative Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Muchova, M.; Balacco, D.L.; Grant, M.M.; Chapple, I.L.C.; Kuehne, S.A.; Hirschfeld, J. Fusobacterium nucleatum subspecies differ in biofilm forming ability in vitro. Front. Oral Health 2022, 3, 853618. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, A.; Kuboniwa, M.; Shimma, S.; Alghamdi, S.A.; Mayumi, S.; Lamont, R.J.; Fukusaki, E.; Amano, A. Fusobacterium nucleatum metabolically integrates commensals and pathogens in oral biofilms. mSystems 2022, 7, e00170-22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More than just a periodontal pathogen—The research progress on Fusobacterium nucleatum. Front. Cell Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Dong, P.; Cen, L.; Bor, B.; Lux, R.; Shi, W.; Yu, Q.; He, X.; Wu, T. Antagonistic interaction between two key endodontic pathogens Enterococcus faecalis and Fusobacterium nucleatum. J. Oral Microbiol. 2023, 15, 2149448. [Google Scholar] [CrossRef]
- Da Silva, R.A.G.; Tay, W.H.; Ho, F.K.; Tanoto, F.R.; Chong, K.K.L.; Choo, P.Y.; Ludwig, A.; Kline, K.A. Enterococcus faecalis alters endo-lysosomal trafficking to replicate and persist within mammalian cells. PLoS Pathog. 2022, 18, e1010434. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Brodská, B.; Holoubek, A. Generation of reactive oxygen species during apoptosis induced by DNA-damaging agents and/or histone deacetylase inhibitors. Oxidative Med. Cell Longev. 2011, 2011, 253529. [Google Scholar] [CrossRef]
- Almeida, P.E.D.; Pereira de Sousa, N.M.; Rampinelli, P.G.; Silva, R.V.D.S.; Correa, J.R.; D’Avila, H. Lipid droplets as multifunctional organelles related to the mechanism of evasion during mycobacterial infection. Front. Cell Infect. Microbiol. 2023, 13, 1102643. [Google Scholar] [CrossRef]
- Acevedo Ospina, H.; Guay-Vincent, M.; Descoteaux, A. Macrophage mitochondrial biogenesis and metabolic reprogramming induced by Leishmania donovani require lipophosphoglycan and type I interferon signaling. mBio 2022, 13, e0257822. [Google Scholar] [CrossRef]
- Roque, N.R.; Lage, S.L.; Navarro, R.; Fazolini, N.; Maya-Monteiro, C.M.; Rietdorf, J.; Melo, R.C.N.; D’Avila, H.; Bozza, P.T. Rab7 controls lipid droplet-phagosome association during mycobacterial infection. Biochim. Biophys. Acta–Mol. Cell Biol. Lipids. 2020, 1865, 158703. [Google Scholar] [CrossRef]
- Rabhi, S.; Rabhi, I.; Trentin, B.; Piquemal, D.; Regnault, B.; Goyard, S.; Lang, T.; Descoteaux, A.; Enninga, J.; Guizani-Tabbane, L. Lipid droplet formation, their localization and dynamics during Leishmania major macrophage infection. PLoS ONE 2016, 11, e0148640. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.Y.; Deng, Z.L.; Du, Q.Y.; Dai, M.Q.; Luo, Y.Y.; Liang, Y.E.; Dai, X.Z.; Guo, S.M.; Zhao, W.H. Enterococcus faecalis extracellular vesicles promote apical periodontitis. J. Dent. Res. 2024, 103, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Moots, R.J.; Edwards, S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, D.; Nishimoto, S.; Aini, K.; Tanaka, A.; Nishiguchi, T.; Kim Kaneyama, J.R.; Lei, X.F.; Masuda, K.; Naruto, T.; Tanaka, K.; et al. Toll-like receptor 9 plays a pivotal role in angiotensin II–induced atherosclerosis. J. Am. Heart Assoc. 2019, 8, e010860. [Google Scholar] [CrossRef]
- Zahlten, J.; Steinicke, R.; Bertrams, W.; Hocke, A.C.; Scharf, S.; Schmeck, B.; Witzenrath, M.; Hammerschmidt, S.; Suttorp, N.; Hippenstiel, S. TLR9- and Src-dependent expression of Krueppel-like factor 4 controls interleukin-10 expression in pneumonia. Eur. Respir. J. 2013, 41, 384–391. [Google Scholar] [CrossRef]
- Zhang, Q.; Green, M.D.; Lang, X.; Lazarus, J.; Parsels, J.D.; Wei, S.; Parsels, L.A.; Shi, J.; Ramnath, N.; Wahl, D.R.; et al. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Res. 2019, 79, 3940–3951. [Google Scholar] [CrossRef]
- Li, X.; Yang, M.; Yu, Z.; Tang, S.; Wang, L.; Cao, X.; Chen, T. The tyrosine kinase Src promotes phosphorylation of the kinase TBK1 to facilitate type I interferon production after viral infection. Sci. Signal 2017, 10, aae0435. [Google Scholar] [CrossRef]
- Liu, S.; Guan, L.; Peng, C.; Cheng, Y.; Cheng, H.; Wang, F.; Ma, M.; Zheng, R.; Ji, Z.; Cui, P.; et al. Mycobacterium tuberculosis suppresses host DNA repair to boost its intracellular survival. Cell Host Microbe. 2023, 31, 1820–1836. [Google Scholar] [CrossRef]
- Ebrahimi, K.H.; Gilbert-Jaramillo, J.; James, W.S.; McCullagh, J.S.O. Interferon-stimulated gene products as regulators of central carbon metabolism. FEBS J. 2021, 288, 3715–3726. [Google Scholar] [CrossRef]
- Kiarely Souza, E.; Pereira Dutra, F.S.; Rajão, M.A.; Ferraro Moreira, F.; Goltara Gomes, T.C.; Cunha Fernandes, T.; Santos, J.D.C.; Prestes, E.B.; Andrade, W.A.; Zamboni, D.S.; et al. Lipid droplet accumulation occurs early following Salmonella infection and contributes to intracellular bacterial survival and replication. Mol. Microbiol. 2022, 117, 293–306. [Google Scholar] [CrossRef]
- Liang, J.J.; Fraser, I.D.C.; Bryant, C.E. Lipid regulation of NLRP3 inflammasome activity through organelle stress. Trends Immunol. 2021, 42, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Laabei, M.; Duggan, S. CidA and LrgA: A “hole” lot more than programmed cell death. mBio 2022, 13, e00761-22. [Google Scholar] [CrossRef] [PubMed]
- Endres, J.L.; Chaudhari, S.S.; Zhang, X.; Prahlad, J.; Wang, S.Q.; Foley, L.A.; Luca, S.; Bose, J.L.; Thomas, V.C.; Bayles, K.W. The Staphylococcus aureus CidA and LrgA proteins are functional holins involved in the transport of by-products of carbohydrate metabolism. mBio 2021, 13, e0282721. [Google Scholar] [CrossRef] [PubMed]
- Tanouchi, Y.; Lee, A.J.; Meredith, H.; You, L. Programmed cell death in bacteria and implications for antibiotic therapy. Trends Microbiol. 2013, 21, 265–270. [Google Scholar] [CrossRef]
- Smith, R.P.; Barraza, I.; Quinn, R.J.; Fortoul, M.C. The mechanisms and cell signaling pathways of programmed cell death in the bacterial world. Int. Rev. Cell Mol. Biol. 2020, 352, 1–53. [Google Scholar] [CrossRef]
- Goodman, S.D. Extracellular DNA-protein interactions. Curr. Opin. Struct. Biol. 2024, 89, 102943. [Google Scholar] [CrossRef]
- Vandana; Priyadarshanee, M.; Das, S. Bacterial extracellular polymeric substances: Biosynthesis and interaction with environmental pollutants. Chemosphere 2023, 332, 138876. [Google Scholar] [CrossRef]
- Bayles, K.W. Bacterial programmed cell death: Making sense of a paradox. Nat. Rev. Microbiol. 2014, 12, 63–69. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Huang, W.; Chan, P.; Guo, L. Interaction Between Enterococcus faecalis and Fusobacterium nucleatum Regulated Macrophage Transcriptional Profiling and Reprogrammed Cellular Immune and Metabolic Response. Microorganisms 2025, 13, 1351. https://doi.org/10.3390/microorganisms13061351
Liang J, Huang W, Chan P, Guo L. Interaction Between Enterococcus faecalis and Fusobacterium nucleatum Regulated Macrophage Transcriptional Profiling and Reprogrammed Cellular Immune and Metabolic Response. Microorganisms. 2025; 13(6):1351. https://doi.org/10.3390/microorganisms13061351
Chicago/Turabian StyleLiang, Jingheng, Wenling Huang, Poukei Chan, and Lihong Guo. 2025. "Interaction Between Enterococcus faecalis and Fusobacterium nucleatum Regulated Macrophage Transcriptional Profiling and Reprogrammed Cellular Immune and Metabolic Response" Microorganisms 13, no. 6: 1351. https://doi.org/10.3390/microorganisms13061351
APA StyleLiang, J., Huang, W., Chan, P., & Guo, L. (2025). Interaction Between Enterococcus faecalis and Fusobacterium nucleatum Regulated Macrophage Transcriptional Profiling and Reprogrammed Cellular Immune and Metabolic Response. Microorganisms, 13(6), 1351. https://doi.org/10.3390/microorganisms13061351





