Membrane Vesicles of Enterococcus faecalis: In Vitro Composition Analysis and Macrophage Inflammatory Response Under Different pH Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strain and Growth Condition
2.2. Preparation of MVs-Enriched Fraction
2.3. Transmission Electron Microscopy (TEM)
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Proteomics
2.6. Metabolomics
2.7. Differentiation Culture of Human Macrophage In Vitro
2.8. Cell Counting Kit-8 (CCK-8) Assay
2.9. ELISA
2.10. Statistical Analysis
3. Results
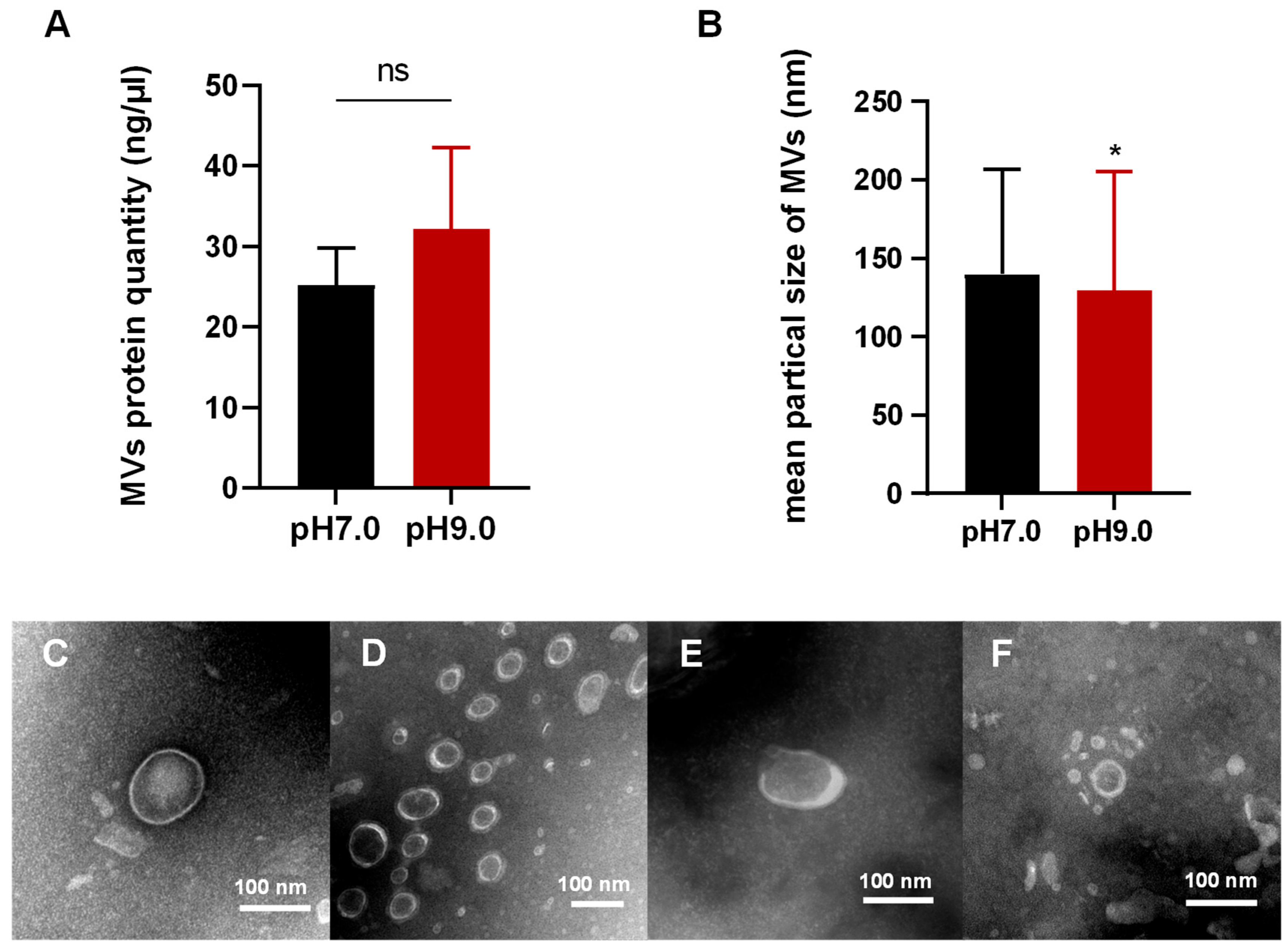
3.1. E. faecalis Releases MVs in Alkaline and Neutral Environments
3.2. Morphological Analysis of MVs in E. faecalis
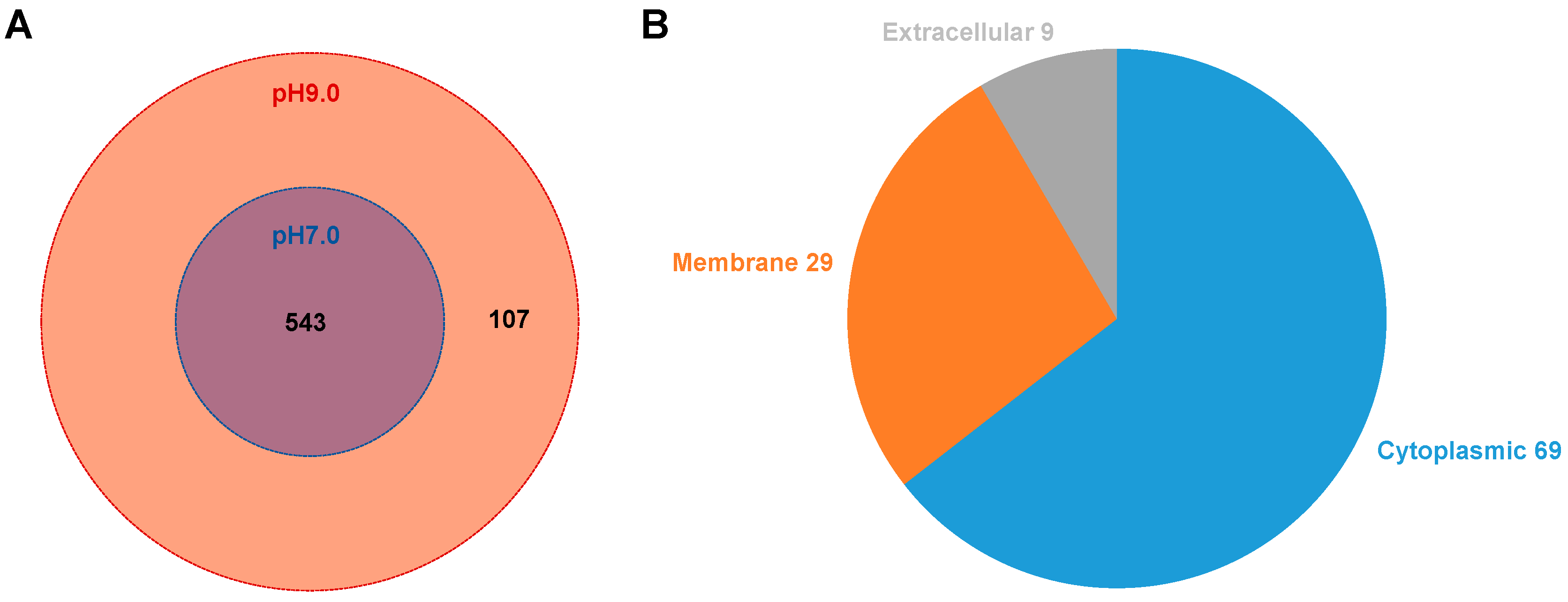
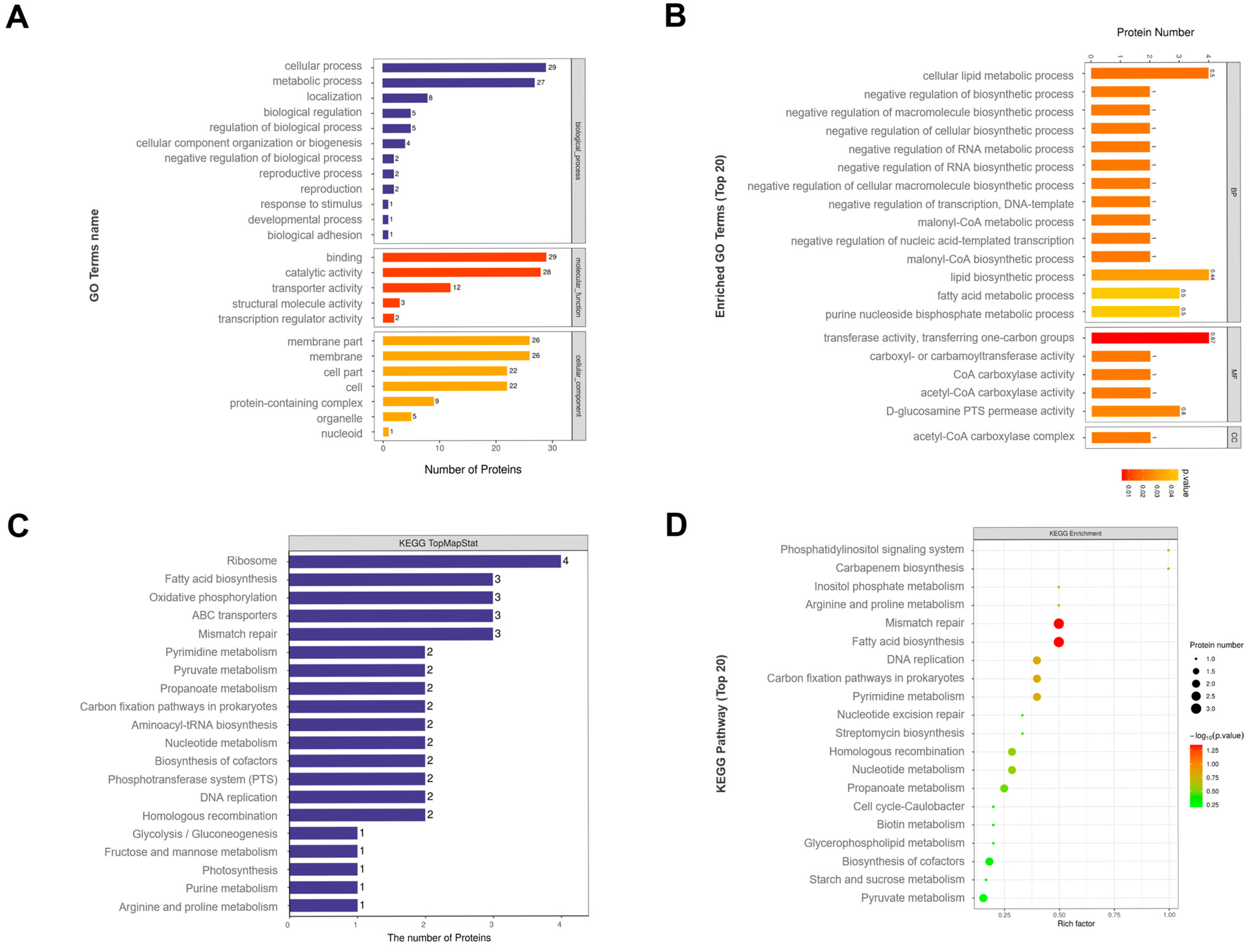
3.3. Proteomic Analysis of MVs
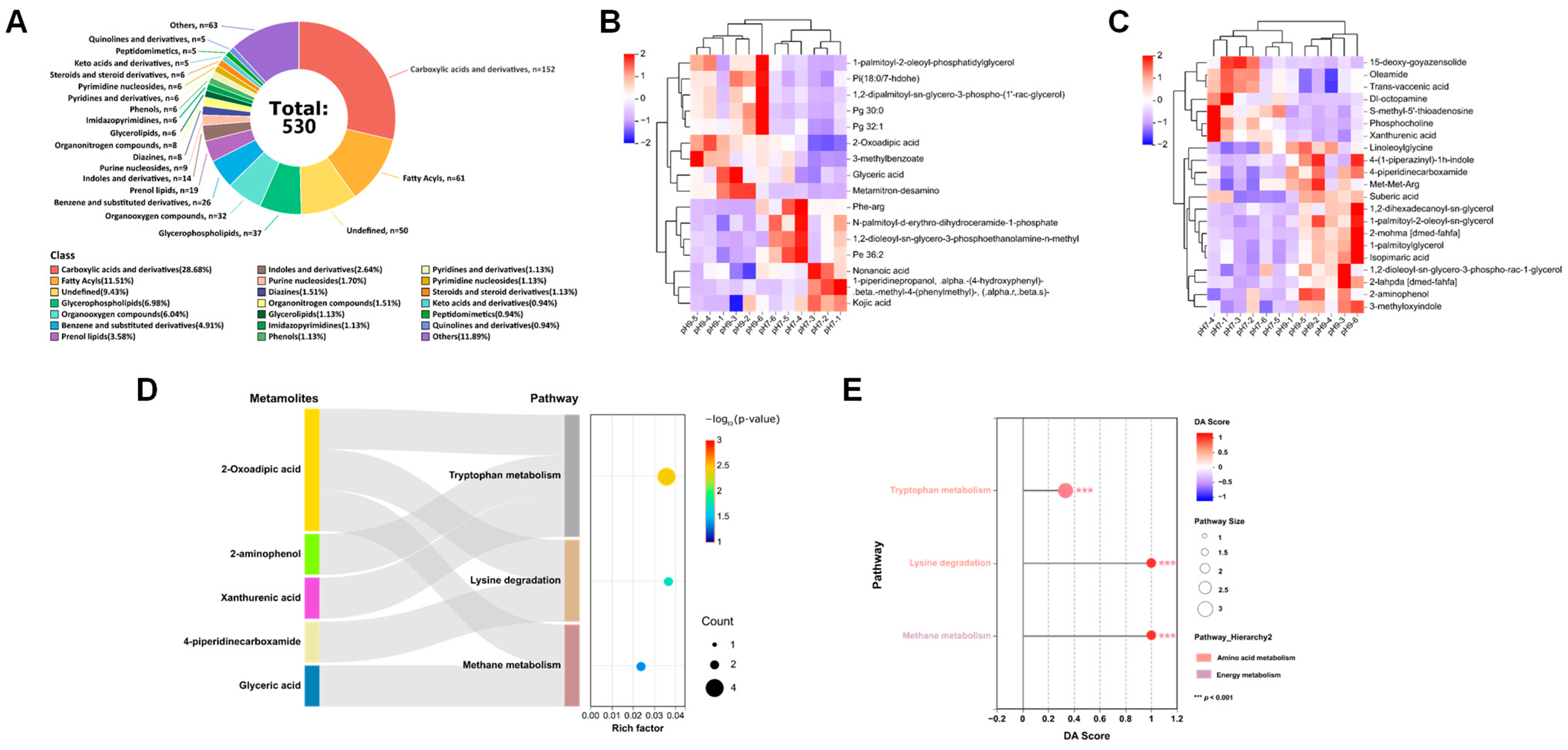
3.4. Metabolomic Analysis of MVs
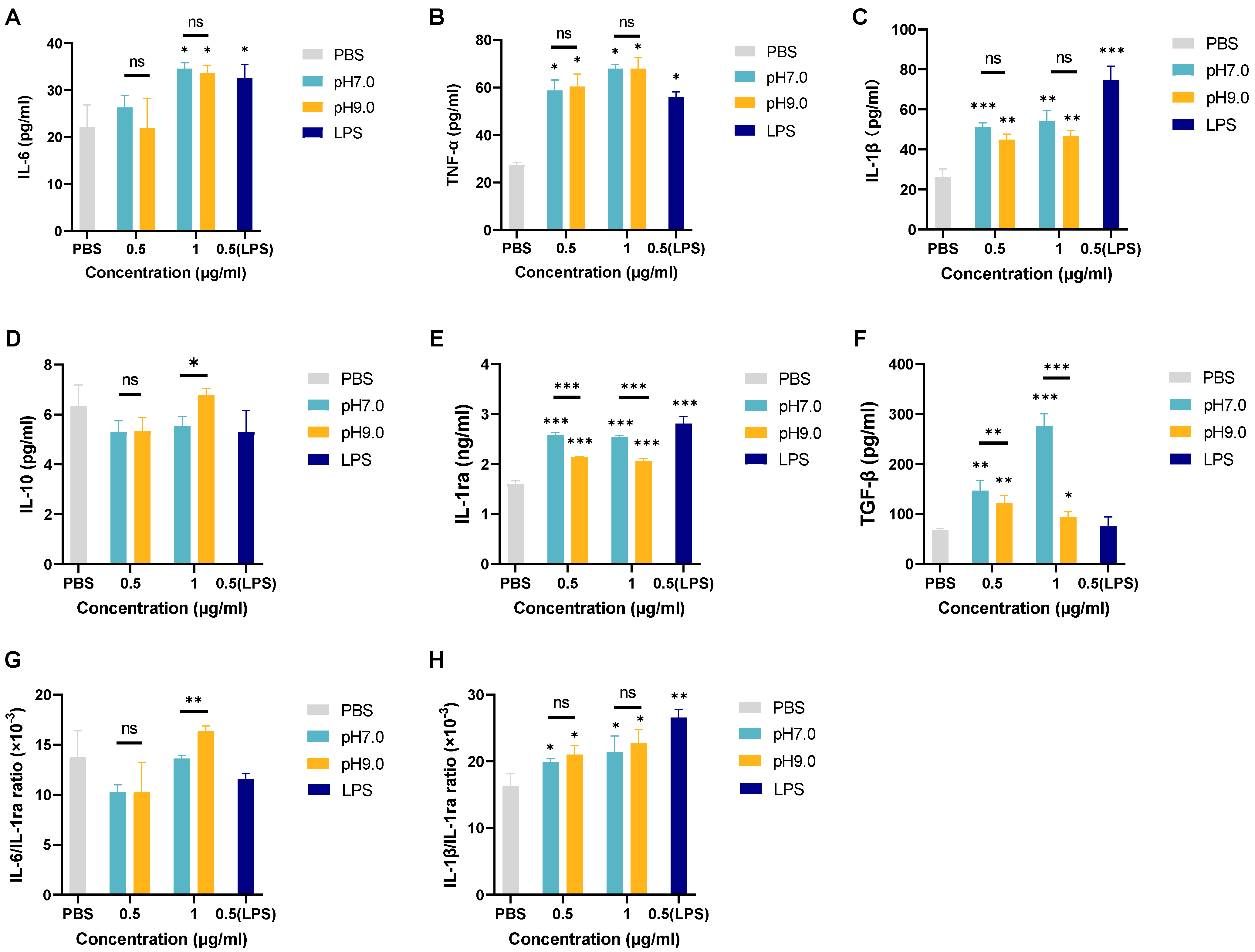
3.5. The Effect of E. faecalis MVs on dTHP-1 Inflammatory Response Regulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Ke, W.; Jiang, S.; Zhang, M.; Shan, K.; Li, C. Dietary Hemin Remodels Gut Microbiota and Mediates Tissue Inflammation and Injury in the Small Intestine. Mol. Nutr. Food Res. 2024, 68, e2300889. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wu, J.; Yang, W.; Lyu, R.; You, Q.; Li, J.; He, Q.; Zhuang, Y.; Wang, W.; Wang, Y.; et al. FTO-associated osteoclastogenesis promotes alveolar bone resorption in apical periodontitis male rat via the HK1/USP14/RANK pathway. Nat. Commun. 2025, 16, 1519. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Tak, E.J.; Park, O.J.; Perinpanayagam, H.; Yoo, Y.J.; Lee, H.J.; Jeong, Y.S.; Lee, J.Y.; Kim, H.S.; Bae, J.W.; et al. Association between root canals and gingival sulci microbiota in secondary and persistent endodontic infections. Sci. Rep. 2025, 15, 11253. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Huang, D.; Li, R.; Peng, L.; Huan, T.; Ye, K.; Bian, Z.; Yin, W. Roles of Pyroptosis in the Progression of Pulpitis and Apical Periodontitis. J. Inflamm. Res. 2025, 18, 3361–3375. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Costalonga, M.; Dietz, M.; Lima, B.P.; Staley, C. The root canal microbiome diversity and function. A whole-metagenome shotgun analysis. Int. Endod. J. 2024, 57, 872–884. [Google Scholar] [CrossRef]
- Park, D.H.; Park, O.J.; Yoo, Y.J.; Perinpanayagam, H.; Cho, E.B.; Kim, K.; Park, J.; Noblett, W.C.; Kum, K.Y.; Han, S.H. Microbiota Association and Profiling of Gingival Sulci and Root Canals of Teeth with Primary or Secondary/Persistent Endodontic Infections. J. Endod. 2024, 50, 1124–1133. [Google Scholar] [CrossRef]
- Barbosa-Ribeiro, M.; Arruda-Vasconcelos, R.; Louzada, L.M.; Dos Santos, D.G.; Andreote, F.D.; Gomes, B. Microbiological analysis of endodontically treated teeth with apical periodontitis before and after endodontic retreatment. Clin. Oral. Investig. 2021, 25, 2017–2027. [Google Scholar] [CrossRef]
- Mokhtari, F.; Modaresi, J.; Salmasi, A.H.; Khamisi, N.; Zandi, H.; Lesani, K. In vitro comparison of Enterococcus Faecalis survival in dentinal tubules following root canal therapy with AH plus, endoseal MTA, and cold ceramic sealers. BMC Oral. Health 2024, 24, 1488. [Google Scholar] [CrossRef]
- Torabinejad, M.; Fouad, A.F.; Shabahang, S. Endodontics e-Book: Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions-irrigants and irrigation methods. Int. Endod. J. 2022, 55 (Suppl. S3), 588–612. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Noblett, W.C.; Perez-Ron, A.; Ye, Z.; Vera, J. Present status and future directions of intracanal medicaments. Int. Endod. J. 2022, 55 (Suppl. S3), 613–636. [Google Scholar] [CrossRef]
- Wang, L.; Dong, M.; Zheng, J.; Song, Q.; Yin, W.; Li, J.; Niu, W. Relationship of biofilm formation and gelE gene expression in Enterococcus faecalis recovered from root canals in patients requiring endodontic retreatment. J. Endod. 2011, 37, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Hubble, T.S.; Hatton, J.F.; Nallapareddy, S.R.; Murray, B.E.; Gillespie, M.J. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol. Immunol. 2003, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Gunes, B.; Yeter, K.Y.; Altay, Y. Impact of different activation procedures on sodium hypochlorite penetration into dentinal tubules after endodontic retreatment via confocal laser scanning microscopy. BMC Oral Health 2024, 24, 1103. [Google Scholar] [CrossRef] [PubMed]
- Basak Erol, H.; Kaskatepe, B.; Gocmen, D.; Ziraman, F.G. The treatment of Enterococcus faecalis related root canal biofilms with phage therapy. Microb. Pathog. 2024, 197, 107081. [Google Scholar] [CrossRef]
- Sigusch, B.W.; Kranz, S.; Klein, S.; Völpel, A.; Harazim, S.; Sanchez, S.; Watts, D.C.; Jandt, K.D.; Schmidt, O.G.; Guellmar, A. Colonization of Enterococcus faecalis in a new SiO/SiO(2)-microtube in vitro model system as a function of tubule diameter. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 661–668. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, Z.; Hong, X.; Xu, Z.; Xu, Y.; He, Y.; Chen, S. Novel approaches on root canal disinfection methods against E. faecalis. J. Oral Microbiol. 2025, 17, 2475947. [Google Scholar] [CrossRef]
- Du, T.; Wang, Y.; Liu, X.; Zhao, W.; Yang, B.; Gan, K.; Zhu, J. Killing effect of antibacterial photodynamic therapy with long-term exposure against young and mature Enterococcus faecalis biofilms in dentin. BMC Oral Health 2025, 25, 287. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Braeutigam, F.; Tonndorf-Martini, S.; Heyder, M.; Reise, M.; Sigusch, B. Antibacterial Effect of Endodontic Disinfections on Enterococcus Faecalis in Dental Root Canals-An In-Vitro Model Study. Materials 2021, 14, 2427. [Google Scholar] [CrossRef]
- Ashraf, D.; Shaaban, M.I.; Hassan, R.; El-Aziz, A.M.A. Polidocanol inhibits Enterococcus faecalis virulence factors by targeting fsr quorum sensing system. BMC Microbiol. 2024, 24, 411. [Google Scholar] [CrossRef]
- Liu, R.; Liu, P.; Luo, Y.; Fan, W.; Fan, B. Metformin reduced the alkaline resistance of Enterococcus faecalis against calcium hydroxide via Man-PTS EII: In vitro and in vivo studies. Clin. Oral Investig. 2024, 28, 520. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Bu, Y.; Ren, X.; Dong, Z. Review on bacterial outer membrane vesicles: Structure, vesicle formation, separation and biotechnological applications. Microb. Cell Factories 2025, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nature reviews. Microbiology 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Joshi, B.; Janice, J.; Askarian, F.; Škalko-Basnet, N.; Hagestad, O.C.; Mekhlif, A.; Wai, S.N.; Hegstad, K.; Johannessen, M. Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. J. Proteom. 2018, 187, 28–38. [Google Scholar] [CrossRef]
- Lehmkuhl, J.; Schneider, J.S.; Werth, K.L.V.; Scherff, N.; Mellmann, A.; Kampmeier, S. Role of membrane vesicles in the transmission of vancomycin resistance in Enterococcus faecium. Sci. Rep. 2024, 14, 1895. [Google Scholar] [CrossRef]
- Zhao, M.; He, S.; Wen, R.; Li, C.; Chen, X.; Lin, X.; Wang, H.; Tang, Y. Membrane vesicles derived from Enterococcus faecalis promote the co-transfer of important antibiotic resistance genes located on both plasmids and chromosomes. J. Antimicrob. Chemother. 2024, 79, 320–326. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.Y.; Son, J.H.; Kim, S.I.; Lee, H.; Kim, S.; Shin, M.; Lee, J.C. Production of Membrane Vesicles by Enterococcus faecium Cultured with or Without Subinhibitory Concentrations of Antibiotics and Their Pathological Effects on Epithelial Cells. Front. Cell. Infect. Microbiol. 2019, 9, 295. [Google Scholar] [CrossRef]
- Wagner, T.M.; Romero-Saavedra, F.; Laverde, D.; Johannessen, M.; Hübner, J.; Hegstad, K. Enterococcal Membrane Vesicles as Vaccine Candidates. Int. J. Mol. Sci. 2023, 24, 16051. [Google Scholar] [CrossRef]
- Ma, R.Y.; Deng, Z.L.; Du, Q.Y.; Dai, M.Q.; Luo, Y.Y.; Liang, Y.E.; Dai, X.Z.; Guo, S.M.; Zhao, W.H. Enterococcus faecalis Extracellular Vesicles Promote Apical Periodontitis. J. Dent. Res. 2024, 103, 672–682. [Google Scholar] [CrossRef]
- Luo, M.; Sun, J.; Li, S.; Wei, L.; Sun, R.; Feng, X.; Zhang, H.; Chen, T.; Xi, Q.; Zhang, Y.; et al. Protective effect of Enterococcus faecium against ethanol-induced gastric injury via extracellular vesicles. Microbiol. Spectr. 2024, 12, e0389423. [Google Scholar] [CrossRef]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Deo, P.; Stuart, I.; Naderer, T. Bacterial outer membrane vesicles and host cell death signaling. Trends Microbiol. 2021, 29, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Meng, L.; Chen, Y.; Dong, Z.; Peng, Q. Bacterial outer membrane vesicles as potential biological nanomaterials for antibacterial therapy. Acta Biomater. 2022, 140, 102–115. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; et al. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 1482–1490. [Google Scholar] [CrossRef]
- Evans, M.; Davies, J.K.; Sundqvist, G.; Figdor, D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int. Endod. J. 2002, 35, 221–228. [Google Scholar] [CrossRef]
- Ran, S.; Liu, B.; Jiang, W.; Sun, Z.; Liang, J. Transcriptome analysis of Enterococcus faecalis in response to alkaline stress. Front. Microbiol. 2015, 6, 795. [Google Scholar] [CrossRef]
- Tang, M.; Lei, Y.; Chen, K.; Ding, M.; Ou, Q.; Tang, J.; Zhang, Y.; Tang, T.; Wang, C. Reducing the degree of crosslinking of peptidoglycan in Listeria monocytogenes promoted the secretion of membrane vesicles. Biotechnol. Bioeng. 2024, 121, 3629–3641. [Google Scholar] [CrossRef]
- Li, J.; Zhu, K.; Li, C.; Huang, W.; Tian, X.; Yan, H.; Zhao, Y.; Zhou, J.; Gao, X.; Rao, X.; et al. Alkaline shock protein 23 (Asp23)-controlled cell wall imbalance promotes membrane vesicle biogenesis in Staphylococcus aureus. J. Extracell. Vesicles 2024, 13, e12501. [Google Scholar] [CrossRef]
- Werner Lass, S.; Smith, B.E.; Camphire, S.; Eutsey, R.A.; Prentice, J.A.; Yerneni, S.S.; Arun, A.; Bridges, A.A.; Rosch, J.W.; Conway, J.F.; et al. Pneumococcal extracellular vesicles mediate horizontal gene transfer via the transformation machinery. mSphere 2024, 9, e0072724. [Google Scholar] [CrossRef]
- Niu, C.; Huang, J.; Wei, L.; Wang, J.; Ran, S. Proinflammatory Effect of Membrane Vesicles Derived from Enterococcus faecalis at Neutral and Alkaline pH. J. Endod. 2024, 50, 1602–1611.e1610. [Google Scholar] [CrossRef]
- Mäkinen, P.L.; Clewell, D.B.; An, F.; Mäkinen, K.K. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain 0G1-10). J. Biol. Chem. 1989, 264, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, L.R.; Thomas, V.C.; Narayanan, S.; Olson, S.; Fleming, S.D.; Hancock, L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect. Immun. 2010, 78, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, J.; Hryniewicz, W.; Sadowy, E. Gelatinase-associated phenotypes and genotypes among clinical isolates of Enterococcus faecalis in Poland. Pol. J. Microbiol. 2011, 60, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Willett, J.L.E.; Robertson, E.B.; Dunny, G.M. The Phosphatase Bph and Peptidyl-Prolyl Isomerase PrsA Are Required for Gelatinase Expression and Activity in Enterococcus faecalis. J. Bacteriol. 2022, 204, e0012922. [Google Scholar] [CrossRef]
- Ma, Y.; Bryant, A.E.; Salmi, D.B.; Hayes-Schroer, S.M.; McIndoo, E.; Aldape, M.J.; Stevens, D.L. Identification and characterization of bicistronic speB and prsA gene expression in the group A Streptococcus. J. Bacteriol. 2006, 188, 7626–7634. [Google Scholar] [CrossRef]
- Guo, L.; Wu, T.; Hu, W.; He, X.; Sharma, S.; Webster, P.; Gimzewski, J.K.; Zhou, X.; Lux, R.; Shi, W. Phenotypic characterization of the foldase homologue PrsA in Streptococcus mutans. Mol. Oral Microbiol. 2013, 28, 154–165. [Google Scholar] [CrossRef]
- Li, X.; Wen, K.; Zhu, L.; Chen, C.; Yin, T.; Yang, X.; Zhao, K.; Zi, Y.; Zhang, H.; Luo, X.; et al. Genome-wide identification and expression analysis of the Eriobotrya japonica TIFY gene family reveals its functional diversity under abiotic stress conditions. BMC Genom. 2024, 25, 468. [Google Scholar] [CrossRef]
- Nelson, B.; Hong, S.H.; Lupoli, T.J. Protein Cofactor Mimics Disrupt Essential Chaperone Function in Stressed Mycobacteria. ACS Infect. Dis. 2022, 8, 901–910. [Google Scholar] [CrossRef]
- Peng, M.; Jaeger, K.E.; Lu, Y.; Fan, Z.; Zeng, W.; Sampathkumar, A.; Wigge, P.A. Activation and memory of the heat shock response is mediated by prion-like domains of sensory HSFs in Arabidopsis. Mol. Plant 2025, 18, 457–467. [Google Scholar] [CrossRef]
- Huang, H.; Yang, H.; Feng, S.; Zhang, X.; Chen, C.; Yan, H.; Li, R.; Liu, M.; Lin, J.; Wen, Y.; et al. High salt condition alters LPS synthesis and induces the emergence of drug resistance mutations in Helicobacter pylori. Antimicrob. Agents Chemother. 2024, 68, e0058724. [Google Scholar] [CrossRef]
- Majeed, T.; Lee, C.C.; Orts, W.J.; Tabassum, R.; Shah, T.A.; Jardan, Y.A.B.; Dawoud, T.M.; Bourhia, M. Characterization of a thermostable protease from Bacillus subtilis BSP strain. BMC Biotechnol. 2024, 24, 49. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Colmenares, I.; González, J.; Sánchez, N.S.; Ochoa-Gutiérrez, D.; Escobar-Sánchez, V.; Segal-Kischinevzky, C. Regulation of Catalase Expression and Activity by DhHog1 in the Halotolerant Yeast Debaryomyces hansenii Under Saline and Oxidative Conditions. J. Fungi 2024, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Y.; Liu, S.S.; Guo, X.H.; Wang, P.; Li, C.Y.; Liao, L.; Qin, Q.L. Increase of ATP synthesis and amino acids absorption contributes to cold adaptation in Antarctic bacterium Poseidonibacter antarcticus SM1702(T). Extrem. Life Under Extrem. Cond. 2024, 29, 3. [Google Scholar] [CrossRef]
- Scott, M.A.; Fagernes, C.E.; Nilsson, G.E.; Stensløkken, K.O. Maintained mitochondrial integrity without oxygen in the anoxia-tolerant crucian carp. J. Exp. Biol. 2024, 227, jeb247409. [Google Scholar] [CrossRef]
- Deebani, A.; Mary, J.; Dhinoja, S.; Al Qaryoute, A.; Fallatah, W.; Jagadeeswaran, P. Knockdown of zebrafish tmem242 enhances the production of ROS that signals to increase f9a expression resulting in DIC-like condition. Sci. Rep. 2025, 15, 3058. [Google Scholar] [CrossRef]
- Kinoshita, N.; Unemoto, T.; Kobayashi, H. Proton motive force is not obligatory for growth of Escherichia coli. J. Bacteriol. 1984, 160, 1074–1077. [Google Scholar] [CrossRef]
- Das, B.; Bhadra, R.K. (p)ppGpp Metabolism and Antimicrobial Resistance in Bacterial Pathogens. Front. Microbiol. 2020, 11, 563944. [Google Scholar] [CrossRef]
- Wendrich, T.M.; Blaha, G.; Wilson, D.N.; Marahiel, M.A.; Nierhaus, K.H. Dissection of the mechanism for the stringent factor RelA. Mol. Cell 2002, 10, 779–788. [Google Scholar] [CrossRef]
- Srivatsan, A.; Wang, J.D. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 2008, 11, 100–105. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Y.; Zhang, W.; Lu, Q.; Wen, G.; Luo, Q.; Shao, H.; Pan, Z.; Zhang, T. (p)ppGpp synthetases are required for the pathogenicity of Salmonella Pullorum in chickens. Microbiol. Res. 2021, 245, 126685. [Google Scholar] [CrossRef]
- Aedo, S.; Tomasz, A. Role of the Stringent Stress Response in the Antibiotic Resistance Phenotype of Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Strugeon, E.; Tilloy, V.; Ploy, M.C.; Da Re, S. The Stringent Response Promotes Antibiotic Resistance Dissemination by Regulating Integron Integrase Expression in Biofilms. mBio 2016, 7, e00868-16. [Google Scholar] [CrossRef] [PubMed]
- Arioli, S.; Roncada, P.; Salzano, A.M.; Deriu, F.; Corona, S.; Guglielmetti, S.; Bonizzi, L.; Scaloni, A.; Mora, D. The relevance of carbon dioxide metabolism in Streptococcus thermophilus. Microbiology 2009, 155, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jiang, X.; Wang, Y.; Huang, Q.; Chen, D.; Hou, C.; Mu, Y.; Shen, J. Enhanced anaerobic reduction of nitrobenzene at high salinity by betaine acting as osmoprotectant and regulator of metabolism. Water Res. 2022, 223, 118982. [Google Scholar] [CrossRef]
- Yang, T.; Nian, Y.; Lin, H.; Li, J.; Lin, X.; Li, T.; Wang, R.; Wang, L.; Beattie, G.A.; Zhang, J.; et al. Structure and mechanism of the osmoregulated choline transporter BetT. Sci. Adv. 2024, 10, eado6229. [Google Scholar] [CrossRef]
- Zhu, F.; Ren, L.; Cheng, W.; Zhou, H.; Li, Y.; Liu, N.; Rong, G.; Liu, Y.; Yu, P.; Lv, J.; et al. A Dynamic Deferoxamine Polymer with Exceptional Performance in Mitochondrial Iron Depletion and Cytosolic Protein Delivery. Small 2025, 21, e2412093. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Wang, T.; Li, H.; He, J.; Xu, A. Role of iron homeostasis in the mutagenicity of disinfection by-products in mammalian cells. Ecotoxicol. Environ. Saf. 2024, 285, 117122. [Google Scholar] [CrossRef]
- Verneuil, N.; Mazé, A.; Sanguinetti, M.; Laplace, J.M.; Benachour, A.; Auffray, Y.; Giard, J.C.; Hartke, A. Implication of (Mn)superoxide dismutase of Enterococcus faecalis in oxidative stress responses and survival inside macrophages. Microbiology 2006, 152, 2579–2589. [Google Scholar] [CrossRef]
- Li, H.; Du, X.; Chen, C.; Qi, J.; Wang, Y. Integrating transcriptomics and metabolomics analysis on kojic acid combating Acinetobacter baumannii biofilm and its potential roles. Microbiol. Res. 2022, 254, 126911. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Y.G.; Zeng, L.Y.; Pan, Y.; Huang, X.Y.; Bian, L.Q.; Zhu, Y.J.; Zhang, R.R.; Zhang, J. Evaluation of antibacterial and anti-biofilm properties of kojic acid against five food-related bacteria and related subcellular mechanisms of bacterial inactivation. Food Sci. Technol. Int. Cienc. Y Tecnol. De Los Aliment. Int. 2019, 25, 3–15. [Google Scholar] [CrossRef]
- Shibata, K.; Yasui, M.; Sano, M.; Fukuwatari, T. Fluorometric determination of 2-oxoadipic acid, a common metabolite of tryptophan and lysine, by high-performance liquid chromatography with pre-chemical derivatization. Biosci. Biotechnol. Biochem. 2011, 75, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Wang, A.J.; Wang, X.Y. Chemokine receptor-2 deficiency induced mild experimental periapical lesion in mice. J. Dent. Sci. 2025, 20, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, O.; Xia, J.; Chiu, I.M.; Gibbs, J.L. Dynamics of Innate Immune Response in Bacteria-Induced Mouse Model of Pulpitis. J. Endod. 2023, 49, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, J.; Yue, J.; Zhou, L.; Mao, H.; Li, J.; Sun, Z.; Chen, Z.; Zhang, L. Nlrp3 inflammasome drives regulatory T cell depletion to accelerate periapical bone erosion. Int. Endod. J. 2024, 57, 1110–1123. [Google Scholar] [CrossRef]
- Dai, X.; Ma, R.; Jiang, W.; Deng, Z.; Chen, L.; Liang, Y.; Shao, L.; Zhao, W. Enterococcus faecalis-Induced Macrophage Necroptosis Promotes Refractory Apical Periodontitis. Microbiol. Spectr. 2022, 10, e0104522. [Google Scholar] [CrossRef]
- Chi, D.; Lin, X.; Meng, Q.; Tan, J.; Gong, Q.; Tong, Z. Real-Time Induction of Macrophage Apoptosis, Pyroptosis, and Necroptosis by Enterococcus faecalis OG1RF and Two Root Canal Isolated Strains. Front. Cell. Infect. Microbiol. 2021, 11, 720147. [Google Scholar] [CrossRef]
- Cecil, J.D.; O’Brien-Simpson, N.M.; Lenzo, J.C.; Holden, J.A.; Singleton, W.; Perez-Gonzalez, A.; Mansell, A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef]
- Deo, P.; Chow, S.H.; Han, M.L.; Speir, M.; Huang, C.; Schittenhelm, R.B.; Dhital, S.; Emery, J.; Li, J.; Kile, B.T.; et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 2020, 5, 1418–1427. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lee, M.K.S.; Singleton, W.; Achuthan, A.; Lee, M.C.; O’Brien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C.; et al. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front. Cell. Infect. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, X.; Li, M.; Luo, H. Rhodococcus equi-Derived Extracellular Vesicles Promoting Inflammatory Response in Macrophage through TLR2-NF-κB/MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 9742. [Google Scholar] [CrossRef]
- Hui, Q.; Asadi, A.; Park, Y.J.; Kieffer, T.J.; Ao, Z.; Warnock, G.L.; Marzban, L. Amyloid formation disrupts the balance between interleukin-1β and interleukin-1 receptor antagonist in human islets. Mol. Metab. 2017, 6, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, M.; Chamlati, J.M.; Hennenlotter, J.; Todenhöfer, T.; Lütfrenk, T.; Jersinovic, S.; Tsaur, I.; Stenzl, A.; Rausch, S. Interleukin-1β/Interleukin (IL)-1-Receptor-Antagonist (IL1-RA) Axis in Invasive Bladder Cancer-An Exploratory Analysis of Clinical and Tumor Biological Significance. Int. J. Mol. Sci. 2024, 25, 2447. [Google Scholar] [CrossRef] [PubMed]
- Gallentine, W.B.; Shinnar, S.; Hesdorffer, D.C.; Epstein, L.; Nordli, D.R., Jr.; Lewis, D.V.; Frank, L.M.; Seinfeld, S.; Shinnar, R.C.; Cornett, K.; et al. Plasma cytokines associated with febrile status epilepticus in children: A potential biomarker for acute hippocampal injury. Epilepsia 2017, 58, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.; Wang, W.; Yin, Q.; He, X.; Tao, D.; Wang, H.; Liu, W.; Wang, Y.; Dong, Z.; et al. CD80 Antibody and MTX Co-Engineered Extracellular Vesicles Targets CD80(+) Macrophages to Suppress Inflammation and Alleviate Chronic Inflammatory Diseases. Int. J. Nanomed. 2025, 20, 6379–6398. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Yue, D.; Cao, L.; Li, L.; Wang, D.; Ping, Y.; Shen, Z.; Zheng, Y.; Wang, L.; et al. Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett. 2019, 452, 244–253. [Google Scholar] [CrossRef]
- Muppala, S.; Xiao, R.; Gajeton, J.; Krukovets, I.; Verbovetskiy, D.; Stenina-Adognravi, O. Thrombospondin-4 mediates hyperglycemia- and TGF-beta-induced inflammation in breast cancer. Int. J. Cancer 2021, 148, 2010–2022. [Google Scholar] [CrossRef]
- Geurts, J.; Jurić, D.; Müller, M.; Schären, S.; Netzer, C. Novel Ex Vivo Human Osteochondral Explant Model of Knee and Spine Osteoarthritis Enables Assessment of Inflammatory and Drug Treatment Responses. Int. J. Mol. Sci. 2018, 19, 1314. [Google Scholar] [CrossRef]
- Chen, B.; Huang, S.; Su, Y.; Wu, Y.J.; Hanna, A.; Brickshawana, A.; Graff, J.; Frangogiannis, N.G. Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circ. Res. 2019, 125, 55–70. [Google Scholar] [CrossRef]
- Carlos, C.P.; de Carvalho, E.P.; Angeli Junior, E.V.; Garcia Filho, G.F.; Doná, J.P.L.; Batanero, R.P.O.; Guena, R.O.; Agren, C.; Baptista, M.; Bizotto, T.S.G.; et al. Angiotensin involvement in kidney injury induced by rheumatoid arthritis in rat. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1271–1279. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Huang, W.; Chan, P.; Zhou, J.; Liang, J.; Guo, L. Membrane Vesicles of Enterococcus faecalis: In Vitro Composition Analysis and Macrophage Inflammatory Response Under Different pH Conditions. Microorganisms 2025, 13, 1344. https://doi.org/10.3390/microorganisms13061344
Yuan Z, Huang W, Chan P, Zhou J, Liang J, Guo L. Membrane Vesicles of Enterococcus faecalis: In Vitro Composition Analysis and Macrophage Inflammatory Response Under Different pH Conditions. Microorganisms. 2025; 13(6):1344. https://doi.org/10.3390/microorganisms13061344
Chicago/Turabian StyleYuan, Zijian, Wenling Huang, Poukei Chan, Jiani Zhou, Jingheng Liang, and Lihong Guo. 2025. "Membrane Vesicles of Enterococcus faecalis: In Vitro Composition Analysis and Macrophage Inflammatory Response Under Different pH Conditions" Microorganisms 13, no. 6: 1344. https://doi.org/10.3390/microorganisms13061344
APA StyleYuan, Z., Huang, W., Chan, P., Zhou, J., Liang, J., & Guo, L. (2025). Membrane Vesicles of Enterococcus faecalis: In Vitro Composition Analysis and Macrophage Inflammatory Response Under Different pH Conditions. Microorganisms, 13(6), 1344. https://doi.org/10.3390/microorganisms13061344






