Co-Inoculating Burkholderia vietnamiensis B418 and Trichoderma harzianum T11W Reduced Meloidogyne incognita Infestation of Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Co-Culture Conditions
2.3. Preparation of Root-Knot Nematodes (RKNs)
2.4. Pot Experiment
2.5. Metagenomic Sequencing and Bioinmatics
2.6. Statistical Analysis
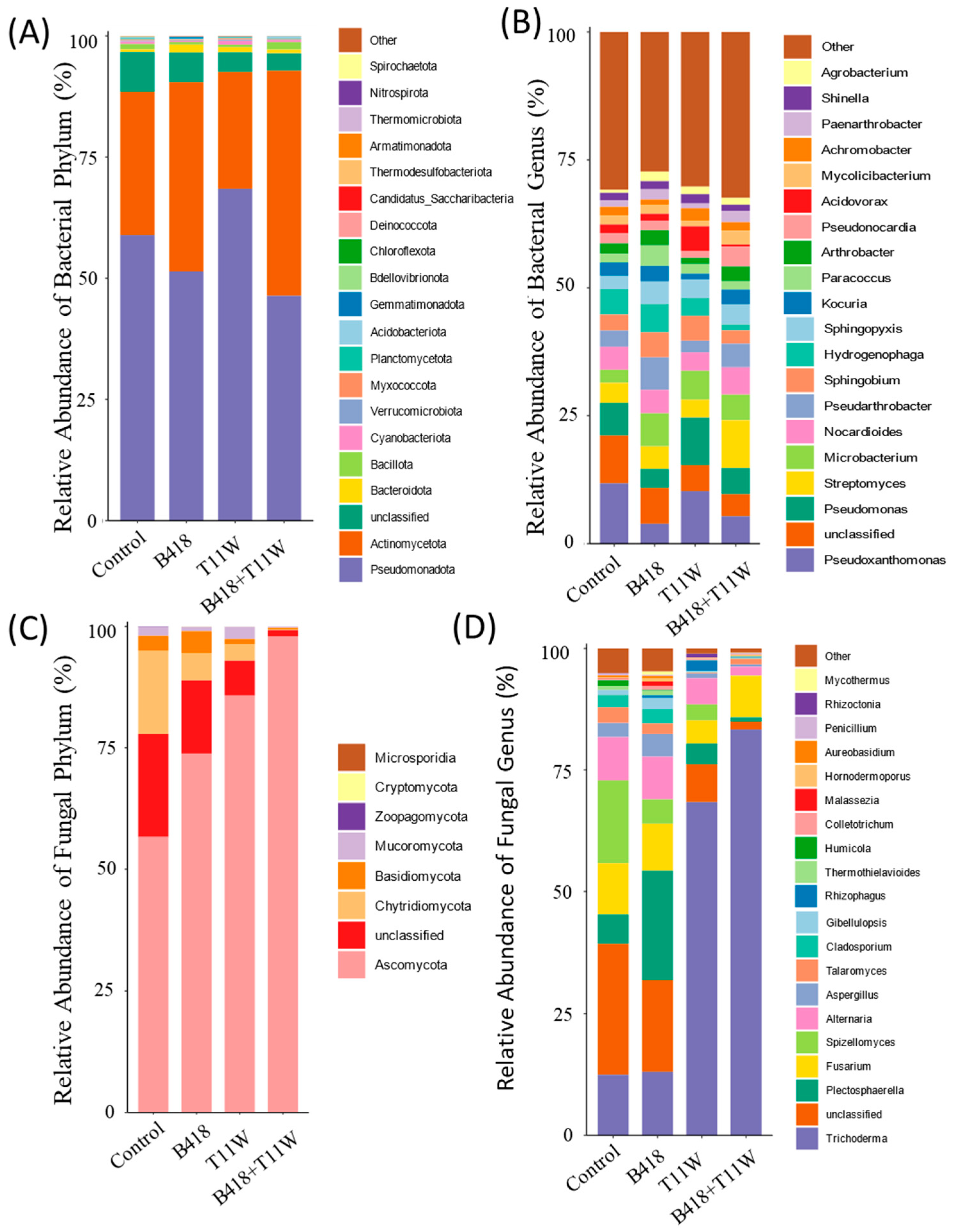
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralmi, N.H.A.A.; Khandaker, M.M.; Mat, N. Occurrence and control of root knot nematode in crops: A review. Aust. J. Crop Sci. 2016, 11, 1649–1654. [Google Scholar] [CrossRef]
- Sang, Y.H.; Ren, K.; Chen, Y.; Wang, B.; Meng, Y.F.; Zhou, W.B.; Jiang, Y.L.; Xu, J.J. Integration of soil microbiology and metabolomics to elucidate the mechanism of the accelerated infestation of tobacco by the root-knot nematode. Front. Microbiol. 2024, 15, 1455880. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Aioub, A.A.A.; Elesawy, A.E.; Ammar, E.E. Plant growth promoting rhizobacteria (PGPR) and their role in plant-parasitic nematodes control: A fresh look at an old issue. J. Plant Dis. Prot. 2022, 129, 1305–1321. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Chen, K.; Wu, Y.; Hu, J.; Wei, Y.; Li, J.; Yang, H.; Ryder, M.; Denton, M.D. Near-complete genomes of two Trichoderma species: A resource for biological control of plant pathogens. Mol. Plant-Microbe Interact. 2020, 33, 1036–1039. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, B.M.; Kang, M.K.; Park, D.J.; Choi, I.S.; Park, H.Y.; Lim, C.H.; Son, K.H. Assessment of nematicidal and plant growth-promoting effects of Burkholderia sp. JB-2 in root-knot nematode-infested soil. Front. Plant Sci. 2023, 14, 1216031. [Google Scholar] [CrossRef]
- Liu, M.M.; Philp, J.; Wang, Y.L.; Hu, J.D.; Wei, Y.L.; Li, J.S.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D.; et al. Plant growth-promoting rhizobacteria Burkholderia vietnamiensis B418 inhibits root-knot nematode on watermelon by modifying the rhizosphere microbial community. Sci. Rep. 2022, 12, 8381. [Google Scholar] [CrossRef]
- Xu, C.; Yu, H. Insights into constructing a stable and efficient microbial consortium. Chin. J. Chem. Eng. 2021, 30, 112–120. [Google Scholar] [CrossRef]
- Li, J.D.; Zhao, J.J.; Fan, H.Y.; Zhu, X.F.; Wang, Y.Y.; Liu, X.Y.; Duan, Y.X.; Chen, L.J. Optimization of fermentation conditions for co-culture nematicidal Bacillus amyloliquefaciens Sneb709 and Sinorhizobium fredii Sneb183. Chin. J. Biol. Control 2021, 37, 771–784. (In Chinese) [Google Scholar]
- Siddiqui, I.A.; Shaukat, S.S. Trichoderma harzianum enhances the production of nematicidal compounds in vitro and improves biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Lett. Appl. Microbiol. 2004, 38, 169–175. [Google Scholar] [CrossRef]
- Creamer, R.E.; Barel, J.M.; Bongiorno, G.; Zwetsloot, M. The life of soils: Integrating the who and how of multifunctionality. Soil Biol. Biochem. 2022, 166, 108561. [Google Scholar] [CrossRef]
- Jagadesh, M.; Dash, M.; Kumari, A.; Singh, S.K.; Verma, K.K.; Kumar, P.; Bhatt, R.; Sharma, S.K. Revealing the hidden world of soil microbes: Metagenomic insights into plant, bacteria, and fungi interactions for sustainable agriculture and ecosystem restoration. Microbiol. Res. 2024, 285, 127764. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chowdhury, D.; Zhang, Z.M.; Cheung, W.K.; Lu, A.P.; Bian, Z.X.; Zhang, L. A review of computational tools for generating metagenome-assembled genomes from metagenomic sequencing data. Comput. Struct. Biotechnol. J. 2021, 19, 6301–6314. [Google Scholar] [CrossRef]
- Yue, H.; Yue, W.J.; Jiao, S.; Kim, H.; Lee, Y.; Wei, G.H.; Song, W.N.; Shu, D.T. Plant domestication shapes rhizosphere microbiome assembly and metabolic functions. Microbiome 2023, 11, 70. [Google Scholar] [CrossRef]
- Wani, A.K.; Rahayu, F.; Alkahtani, A.M.; Alreshidi, M.A.; Yadav, K.K.; Parnidi; Fauziah, L.; Murianingrum, M.; Akhtar, N.; Mufidah, E.; et al. Metagenomic profiling of rhizosphere microbiota: Unraveling the plant-soil dynamics. Physiol. Mol. Plant Pathol. 2024, 133, 102381. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Fadiji, A.E.; Babalola, O.O. Plant health status affects the functional diversity of the rhizosphere microbiome associated with Solanum lycopersicum. Front. Sustain. Food Syst. 2022, 6, 894312. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; Enshasy, H.E.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Li, Y.; Lei, S.N.; Cheng, Z.Q.; Jin, L.Y.; Zhang, T.; Liang, L.M.; Cheng, L.J.; Zhang, Q.Y.; Xu, X.H.; Lan, C.H.; et al. Microbiota and functional analyses of nitrogen-fixing bacteria in root-knot nematode parasitism of plants. Microbiome 2023, 11, 48. [Google Scholar] [CrossRef]
- Lu, P.; Shi, H.L.; Tao, J.M.; Jin, J.J.; Wang, S.J.; Zheng, Q.X.; Liu, P.P.; Xiang, B.K.; Chen, Q.S.; Xu, Y.L.; et al. Metagenomic insights into the changes in the rhizosphere microbial community caused by the root-knot nematode Meloidogyne incognita in tobacco. Environ. Res. 2023, 216, 114848. [Google Scholar] [CrossRef]
- Yu, C.; Lv, J.; Xu, H.Y. Plant growth-promoting fungi and rhizobacteria control Fusarium damping-off in Mason pine seedlings by impacting rhizosphere microbes and altering plant physiological pathways. Plant Soil 2024, 499, 503–519. [Google Scholar] [CrossRef]
- Kamalanathan, V.; Sevugapperumal, N.; Nallusamy, S.; Ashraf, S.; Kailasam, K.; Afzal, M. Metagenomic approach deciphers the role of community composition of mycobiome structured by Bacillus velezensis VB7 and Trichoderma koningiopsis TK in tomato rhizosphere to suppress root-knot nematode infecting tomato. Microorganisms 2023, 11, 2467. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, J.S.; Yang, H.T. Identification of Trichoderma strain T11-W and its parasitism to southern root-knot nematode eggs. Shandong Sci. 2014, 27, 38–42. (In Chinese) [Google Scholar]
- Hu, J.D.; Zhou, Y.; Chen, K.; Li, J.S.; Wei, Y.L.; Wang, Y.L.; Wu, Y.Z.; Ryder, M.H.; Yang, H.T.; Denton, M.D. Large-scale Trichoderma diversity was associated with ecosystem, climate and geographic location. Environ. Microbiol. 2020, 22, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Zuhair, R.; Moustafa, Y.T.A.; Mustafa, N.S.A.; El-Dahshouri, M.F.; Zhang, L.X.; Ageba, M.F. Efficacy of amended vermicompost for bio-control of root knot nematode (RKN) Meloidogyne incognita infesting tomato in Egypt. Environ. Technol. Innov. 2022, 27, 102397. [Google Scholar] [CrossRef]
- Fan, H.Y.; Yao, M.L.; Wang, H.M.; Zhao, D.; Zhu, X.F.; Wang, Y.Y.; Liu, X.Y.; Duan, Y.X.; Chen, L.J. Isolation and effect of Trichoderma citrinoviride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiol. 2020, 20, 299. [Google Scholar] [CrossRef]
- Ye, S.; Yan, R.; Li, X.W.; Lin, Y.F.; Yang, Z.H.; Ma, Y.H.; Ding, Z. Biocontrol potential of Pseudomonas rhodesiae GC-7 against the root-knot nematode Meloidogyne graminicola through both antagonistic effects and induced plant resistance. Front. Microbiol. 2022, 13, 1025727. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2016, 3, e104. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.P.; Sharma, A.K. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis 2017, 71, 175–183. [Google Scholar] [CrossRef]
- Sharma, N.; Khanna, K.; Manhas, R.K.; Bhardwaj, R.; Ohri, P.; Alkahtani, J.; Alwahibi, M.S.; Ahmad, P. Insights into the role of Streptomyces hydrogenans as the plant growth promoter, photosynthetic pigment enhancer and biocontrol agent against Meloidogyne incognita in Solanum lycopersicum seedlings. Plants 2020, 9, 1109. [Google Scholar] [CrossRef]
- Kaur, T.; Jasrotia, S.; Ohri, P.; Manhas, R.K. Evaluation of in vitro and in vivo nematicidal potential of a multifunctional streptomycete, Streptomyces hydrogenans strain DH16 against Meloidogyne incognita. Microbiol. Res. 2016, 192, 247–252. [Google Scholar] [CrossRef]
- Chaurasia, A.; Meena, B.R.; Tripathi, A.N.; Pandey, K.K.; Rai, A.B.; Singh, B. Actinomycetes: An unexplored microorganisms for plant growth promotion and biocontrol in vegetable crops. World J. Microbiol. Biotechnol. 2018, 34, 132. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Ying, J.; Zhang, K.; Hu, Z.; Liu, Z.; Chen, S. Integrated metagenomics and metabolomics analysis reveals changes in the microbiome and metabolites in the rhizosphere soil of Fritillaria unibracteata. Front. Plant Sci. 2023, 14, 1223720. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Lyu, H.; Chen, X. Effects of a compound Trichoderma agent on Coptis chinensis growth, nutrients, enzyme activity, and microbial community of rhizosphere soil. PeerJ 2023, 11, e15652. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma biofertilizer links to altered soil chemistry, altered microbial communities, and improved grassland biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of two Trichoderma strains on plant growth, rhizosphere soil nutrients, and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Gu, X.; Gao, A.; Liu, L.; Wu, X.; Pan, H.; Zhang, H. Pantoea jilinensis D25 enhances tomato salt tolerance via altering antioxidant responses and soil microbial community structure. Environ. Res. 2024, 243, 117846. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Yao, Y.; Guo, N.; Zhang, Y.; Qiao, W. Effects of Rhizophagus intraradices on plant growth and the composition of microbial communities in the roots of continuous cropping soybean at maturity. Sustainability 2021, 13, 6623. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability ofagricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Sui, L.; Li, J.; Philp, J.; Yang, K.; Wei, Y.; Li, H.; Li, J.; Li, L.; Ryder, M.; Toh, R.; et al. Trichoderma atroviride seed dressing influenced the fungal community and pathogenic fungi in the wheat rhizosphere. Sci. Rep. 2022, 12, 9677. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- She, S.; Niu, J.; Zhang, C.; Xiao, Y.; Chen, W.; Dai, L.; Liu, X.; Yin, H. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 2017, 199, 267–275. [Google Scholar] [CrossRef]
- Bergstrand, L.H.; Cardenas, E.; Holert, J.; VanHamme, J.D.; Mohn, W.W. Delineation of steroid-degrading microorganisms through comparative genomic analysis. mBio 2016, 7, e00166. [Google Scholar] [CrossRef]
- Bouhss, A.; Trunkfield, A.E.; Bugg, T.D.H.; Mengin-Lecreulx, D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev. 2008, 32, 208–233. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Wong-Ng, J.; Celani, A.; Vergassola, M. Exploring the function of bacterial chemotaxis. Curr. Opin. Microbiol. 2018, 45, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Núñez, M.A.; Orozco-Ramírez, Q. Characterizing bacterial communities in agroecosystems of the UNESCO global geopark Mixteca Alta, Oaxaca. Agriculture 2024, 14, 2180. [Google Scholar] [CrossRef]
- Wittlinger, J.P.; Castejón, N.; Hausmann, B.; Berry, D.; Schnorr, S.L. Shewanella is a putative producer of polyunsaturated fatty acids in the gut soil of the composting earthworm Eisenia fetida. Appl. Environ. Microbiol. 2025, 91, e0206924. [Google Scholar] [CrossRef] [PubMed]






| No. | Name | Description |
|---|---|---|
| 1 | RKNs | 20 mL nematode suspension (200 eggs/mL) |
| 2 | B418 | 20 mL nematode suspension (200 eggs/mL), 10 mL 14-day B418 fermentation broth (fermentation broth with bacteria cells) |
| 3 | T11W | 20 mL nematode suspension (200 eggs/mL), 10 mL 14-day T11W fermentation broth (fermentation broth with mycelia cells) |
| 4 | B418 + T11W | 20 mL nematode suspension (200 eggs/mL), 10 mL 14-day B418 + T11W fermentation broth (fermentation broth with bacteria and mycelia cells) |
| Treatments | Plant Height (cm) | Fresh Weight (g) | Disease Index |
|---|---|---|---|
| RKNs | 17.53 ± 0.54 c | 4.88 ± 0.24 c | 93.33 ± 1.56 a |
| B418 | 22.14 ± 0.59 b | 6.80 ± 0.37 b | 38.33 ± 1.56 b |
| T11W | 23.31 ± 0.58 ab | 8.09 ± 0.19 a | 42.50 ± 1.02 b |
| B418 + T11W | 24.28 ± 0.52 a | 8.59 ± 0.26 a | 26.67 ± 1.56 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, W.; Li, J.; Hu, J.; Wei, Y.; Wang, Y.; Yang, H.; Zhou, Y.; Wu, Y.; Zhang, S. Co-Inoculating Burkholderia vietnamiensis B418 and Trichoderma harzianum T11W Reduced Meloidogyne incognita Infestation of Tomato Plants. Microorganisms 2025, 13, 1337. https://doi.org/10.3390/microorganisms13061337
Jiang Y, Li W, Li J, Hu J, Wei Y, Wang Y, Yang H, Zhou Y, Wu Y, Zhang S. Co-Inoculating Burkholderia vietnamiensis B418 and Trichoderma harzianum T11W Reduced Meloidogyne incognita Infestation of Tomato Plants. Microorganisms. 2025; 13(6):1337. https://doi.org/10.3390/microorganisms13061337
Chicago/Turabian StyleJiang, Yanqing, Wenzhe Li, Jishun Li, Jindong Hu, Yanli Wei, Yilian Wang, Hetong Yang, Yi Zhou, Yuanzheng Wu, and Shanshan Zhang. 2025. "Co-Inoculating Burkholderia vietnamiensis B418 and Trichoderma harzianum T11W Reduced Meloidogyne incognita Infestation of Tomato Plants" Microorganisms 13, no. 6: 1337. https://doi.org/10.3390/microorganisms13061337
APA StyleJiang, Y., Li, W., Li, J., Hu, J., Wei, Y., Wang, Y., Yang, H., Zhou, Y., Wu, Y., & Zhang, S. (2025). Co-Inoculating Burkholderia vietnamiensis B418 and Trichoderma harzianum T11W Reduced Meloidogyne incognita Infestation of Tomato Plants. Microorganisms, 13(6), 1337. https://doi.org/10.3390/microorganisms13061337





