1. Introduction
Potato (
Solanum tuberosum L.) ranks as the fourth most important staple crop worldwide, contributing significantly to global food security due to its high nutritional value and adaptability to diverse agroecological conditions [
1,
2]. Despite its agricultural importance, potato production faces substantial challenges from diseases, notably potato common scab (PCS), caused by several pathogenic
Streptomyces species, including
S. scabies,
S. acidiscabies, and
S. turgidiscabies [
3,
4]. PCS manifests as corky, pitted lesions on tuber surfaces, severely compromising their aesthetic quality and market value, leading to economic losses estimated at millions annually in major potato-producing regions [
5]. The disease’s widespread prevalence, cryptic nature, and persistence in soil make it a formidable challenge for growers, necessitating innovative and sustainable management strategies.
The pathogenicity of PCS is primarily driven by the phytotoxin Thaxtomin A, produced by pathogenic
Streptomyces spp., which disrupts plant cell wall integrity, induces necrosis, and facilitates disease symptom development [
6,
7]. Beyond potatoes, Thaxtomin A’s phytotoxicity affects other crops, such as sugar beets and radishes, amplifying its agricultural impact [
8]. Current PCS management strategies encompass breeding resistant cultivars, cultural practices, chemical treatments, and biological control, yet each approach has significant limitations. Developing resistant cultivars is a protracted and costly improvised breed of potatoes is time-consuming and often fails to provide complete immunity due to the genetic variability of
Streptomyces spp. [
9]. Cultural practices, such as crop rotation and soil pH adjustment, offer limited efficacy and are difficult to implement consistently across diverse farming systems [
10]. Chemical controls, including fumigants and antibiotics, risk environmental contamination and the emergence of resistant pathogen strains, while biological controls, such as antagonistic microorganisms, are slow-acting and context-dependent [
11,
12]. These challenges underscore the urgent need for safe, effective, and environmentally sustainable alternatives to manage PCS.
Copper (Cu) and zinc (Zn) are essential micronutrients with well-documented antimicrobial properties, offering a promising avenue for integrated disease management. Copper- and zinc-based compounds have been shown to inhibit a broad spectrum of plant pathogens by disrupting cellular structures, suppressing toxin production, and enhancing host resistance [
13,
14]. For instance, copper ions can destabilize bacterial membranes through oxidative stress, while zinc modulates enzymatic activities critical to pathogen virulence [
15,
16]. Recent studies have highlighted their potential in reducing Thaxtomin A synthesis and mitigating PCS severity in controlled settings [
17]. However, their precise mechanisms in
Streptomyces spp. suppression and plant resistance induction remain underexplored. This study investigated the efficacy of copper sulfate (CuSO
4) and zinc sulfate (ZnSO
4) in controlling PCS through a multifaceted approach, including in vitro antimicrobial assays, Thaxtomin A quantification, pot experiments, and molecular analyses of defense gene expression. By elucidating their roles in pathogen suppression and potato resistance enhancement, this research aims to provide actionable insights for developing novel, sustainable strategies to combat PCS and support global potato production [
18,
19].
2. Materials and Methods
2.1. Materials and Culture Media
The
Streptomyces strains used in this study included HRB-1 (
S. scabies), HL-12 (
S. rochei), JX-15 (
S. lavendulae), JMS-3 (
S. acidiscabies), and AC-15 (
S. bottropensis), which were isolated from potato-common-scab-infected soil and tuber samples using Gause’s No.1 medium, identified through 16S rRNA gene sequencing and morphological characterization, and preserved in 20% glycerol at −80 °C by our research team [
4,
20]. The test plant was the potato cultivar “Yujing 885”. The primary reagents included copper sulfate (CuSO
4), zinc sulfate (ZnSO
4), and widely used agricultural antibiotics (e.g., streptomycin sulfate and Zhongshengmycin), ensuring comparability of results. Other chemical reagents such as propidium iodide (PI) stain, Thaxtomin A, and chromatographic-grade acetonitrile were purchased from reputable suppliers and met analytical grade specifications. The culture media included ISP2, modified Gause’s No.1, oat bran liquid medium, and 1% water agar, all of which were sterilized at 120 °C for 20 min before use.
2.2. Antibacterial Effects of Copper and Zinc
The antibacterial activity of CuSO
4 and ZnSO
4 against
Streptomyces spp. was evaluated using the disk diffusion method. The CuSO
4 concentrations (0.04–0.16 g/mL) and ZnSO
4 concentrations (0.10–0.50 g/mL) were selected based on preliminary experiments and relevant literature to ensure optimal pathogen suppression without phytotoxicity [
13,
14]. A 100 μL aliquot of bacterial suspension was evenly spread onto ISP2 agar plates, and sterilized filter paper discs (0.6 cm in diameter) were placed at the center, each loaded with 15 μL of the corresponding concentrations of the reagents. Three biological replicates were performed for each concentration of CuSO
4 and ZnSO
4 to ensure reproducibility of the results. After incubation at 28 °C for 7 days, inhibition zone diameters were measured, and inhibition rates were calculated. Additionally, the inhibitory effects of agricultural streptomycin sulfate, Zhongshengmycin, Polyoxin, Kasugamycin, and Chunlei·Wangtong at gradient concentrations were tested. Half-maximal inhibitory concentrations (EC
50) were calculated using regression analysis to evaluate the toxicity of the reagents. Data were analyzed using SPSS 25.0 with ANOVA and Tukey’s test (
p < 0.05).
2.3. Mechanisms of Copper and Zinc Effects on Streptomyces Scabies
The impact of CuSO4 and ZnSO4 on bacterial cell membrane permeability and nuclear integrity was assessed using electrical conductivity and PI staining assays. HRB-1 suspensions (100 μL) were inoculated into ISP2 liquid media containing different concentrations of CuSO4 (0.001, 0.0015, 0.002 g/mL) and ZnSO4 (0.002, 0.0035, 0.005 g/mL) and cultured in a shaker. Changes in electrical conductivity of the fermentation broth were measured at 0, 1, 2, 3, and 4 h to evaluate cell membrane damage. Bacterial cells were collected by centrifugation, stained with PI, and observed under a fluorescence microscope to assess nuclear damage. Additionally, high-performance liquid chromatography (HPLC) was used to analyze the Thaxtomin A production by S. scabies under different treatments.
2.4. Effects of Copper and Zinc on Potato Growth
Healthy, pre-sprouted potato tubers were cut into seed pieces and planted in pots containing equal amounts of soil and compound fertilizer, with a planting depth of 10 cm. After one month, roots were inoculated with a mixed suspension of Streptomyces spp. at a concentration of 1 × 108 cfu/mL, applied every 7 days for three consecutive treatments. Subsequently, different concentrations of CuSO4 (0.001, 0.0015, 0.002 g/mL) and ZnSO4 (0.002, 0.0035, 0.005 g/mL) were applied, while the control group received sterile water, and the positive control group was treated with 0.00035 g/mL agricultural streptomycin sulfate. At the end of the experiment, plant growth parameters, including height, stem diameter, fresh weight, and dry weight, were measured. Relative chlorophyll content was determined using a chlorophyll meter to evaluate the growth-promoting effects of copper and zinc treatments.
2.5. Analysis of Resistance Enzyme Activity and Gene Expression
After treatment with CuSO4 (0.002 g/mL) and ZnSO4 (0.005 g/mL), plant samples were collected at 4, 8, 12, 36, and 60 h to measure the activities of resistance-related enzymes, including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), polyphenol oxidase (PPO), and malondialdehyde (MDA). Total RNA was extracted from samples ground in liquid nitrogen and reverse-transcribed into cDNA. Quantitative real-time PCR (qPCR) was performed to analyze the expression of resistance-related genes (PR1, PR3, PR9, SOD1, HSF1). Gene expression levels were calculated using the 2−ΔΔCt method.
2.6. Soil Safety Assessment
To evaluate the environmental safety of CuSO
4 and ZnSO
4 applications, soil samples were collected from the pot experiments described in
Section 2.4 after the final treatment application. For each treatment (CK, CuSO
4 at 0.001 g/mL, 0.0015 g/mL, and 0.002 g/mL, and ZnSO
4 at 0.002 g/mL, 0.0035 g/mL, and 0.005 g/mL), three replicate soil samples were taken from the top 0–15 cm layer, air-dried, and sieved through a 2 mm mesh. The copper (Cu) and zinc (Zn) contents in the soil were determined using atomic absorption spectrophotometry (AAS, PerkinElmer Analyst 800,PerkinElmer, Inc., Waltham, MA, USA) following the Chinese standard method (HJ 491-2019) [
21]. Briefly, 0.5 g of each soil sample was digested with a mixture of nitric acid (HNO
3) and hydrochloric acid (HCl) (3:1 ratio) in a microwave digestion system, and the resulting solution was analyzed for Cu and Zn concentrations. Calibration curves were prepared using standard solutions of Cu and Zn, and all measurements were performed in triplicate to ensure accuracy. The results were compared against the risk screening values for agricultural land as per the Chinese Soil Environmental Quality Standard (GB15618-2018) [
22], which sets limits at 100 mg/kg for Cu and 300 mg/kg for Zn.
2.7. Statistical Analysis
All data were analyzed using SPSS 25.0 with one-way ANOVA followed by Tukey’s post hoc test (
p < 0.05), unless specified. Analyses aimed to evaluate treatment and time effects on biological responses. Antibacterial effects (
Supplementary Table S1) compared inhibition zones across concentrations within each Streptomyces strain. Thaxtomin A levels (
Supplementary Table S2) were compared across treatments. Growth parameters (
Supplementary Table S3) were compared across treatments. Enzyme activities and MDA levels were compared among treatments and time points (4, 8, 12, 36, 60 h). Gene expression was compared among time points within treatments and among treatments (CK, 0.002 g/mL CuSO
4, 0.005 g/mL ZnSO
4) at each time point. Soil Cu and Zn contents (
Supplementary Table S4) were compared across concentrations within treatments. EC
50 values (
Table 1) were calculated via regression analysis. Data are mean ± SE from three replicates. Detailed results are available from the corresponding author upon request.
Table 1.
Determination of the in vitro virulence of copper sulfate and zinc sulfate against five Streptomyces spp.
Table 1.
Determination of the in vitro virulence of copper sulfate and zinc sulfate against five Streptomyces spp.
| Medicament | Strains | Regression Equation | R2 | EC50 (g/mL) |
|---|
| CuSO4 | HRB-1 (S. scabies) | Y = 0.7256x + 5.2297 | 0.9709 | 0.4824 |
| HL-12 (S. rochei) | Y = 1.8788x + 5.7666 | 0.9708 | 0.3908 |
| JX-15 (S. lavendulae) | Y = 1.4410x + 6.1002 | 0.9841 | 0.1724 |
| JMS-3 (S. acidiscabies) | Y = 2.0650x + 6.3676 | 0.9997 | 0.2176 |
| AC-15 (S. bottropensis) | Y = 0.7739x + 5.2670 | 0.9426 | 0.4518 |
| ZnSO4 | HRB-1 (S. scabies) | Y = 0.5828x + 5.0079 | 0.9872 | 0.9693 |
| HL-12 (S. rochei) | Y = 1.2072x + 5.0065 | 0.9988 | 0.9877 |
| JX-15 (S. lavendulae) | Y = 0.8400x + 4.9510 | 0.9775 | 1.1438 |
| JMS-3 (S. acidiscabies) | Y = 1.0692x + 4.8013 | 0.8255 | 1.5341 |
| AC-15 (S. bottropensis) | Y = 0.9122x + 4.8199 | 0.9917 | 1.5756 |
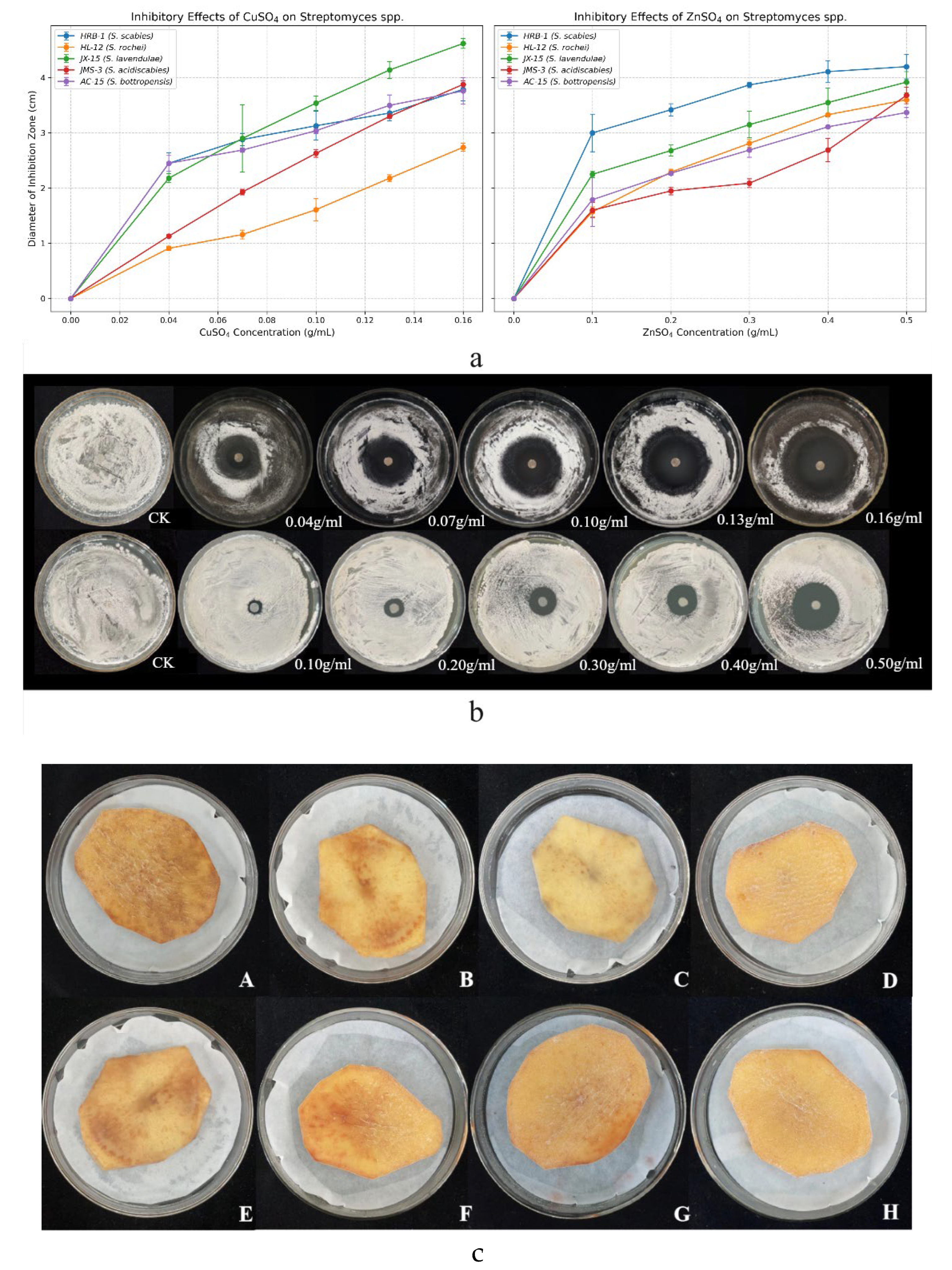
Figure 1.
(a) Inhibitory effects of copper sulfate and zinc sulfate on five Streptomyces spp. at varying concentrations. (b) Specific inhibitory effects on selected strains. (first row) Inhibitory effects of copper sulfate on strain AC-15 at different concentrations. (second row) Inhibitory effects of zinc sulfate on strain JMS-3 at different concentrations. (c) Infection effects of copper sulfate (CuSO4) and zinc sulfate (ZnSO4) on Streptomyces scabies in potato chips in vitro. Note: A, E: CK; B: 0.001 g/mL CuSO4; C: 0.0015 g/mL CuSO4; D: 0.002 g/mL CuSO4; F: 0.002 g/mL ZnSO4; G: 0.0035 g/mL ZnSO4; H: 0.005 g/mL ZnSO4.
Figure 1.
(a) Inhibitory effects of copper sulfate and zinc sulfate on five Streptomyces spp. at varying concentrations. (b) Specific inhibitory effects on selected strains. (first row) Inhibitory effects of copper sulfate on strain AC-15 at different concentrations. (second row) Inhibitory effects of zinc sulfate on strain JMS-3 at different concentrations. (c) Infection effects of copper sulfate (CuSO4) and zinc sulfate (ZnSO4) on Streptomyces scabies in potato chips in vitro. Note: A, E: CK; B: 0.001 g/mL CuSO4; C: 0.0015 g/mL CuSO4; D: 0.002 g/mL CuSO4; F: 0.002 g/mL ZnSO4; G: 0.0035 g/mL ZnSO4; H: 0.005 g/mL ZnSO4.
4. Discussion
This study provides a comprehensive evaluation of copper sulfate (CuSO4) and zinc sulfate (ZnSO4) as sustainable agents for managing potato common scab (PCS), caused by Streptomyces spp., through pathogen suppression, toxin inhibition, and enhancement of plant resistance. By integrating in vitro assays, pot experiments, field trials, and molecular analyses, our results highlight the multifaceted benefits of these micronutrients in improving potato health and yield while addressing environmental safety concerns.
CuSO
4 and ZnSO
4 exhibited potent dose-dependent antibacterial activity against five
Streptomyces strains, with inhibition zone diameters reaching 3.79 cm at 0.002 g/mL CuSO
4 and 4.20 cm at 0.005 g/mL ZnSO
4, compared to 0.00 cm in the control (
Figure 1a). These translated into inhibition rates of 22.02% and 42.86%, respectively, surpassing the efficacy of streptomycin in some strains (
Figure 1a). Detached tuber assays further confirmed their effectiveness, reducing lesion expansion and browning in potato slices (
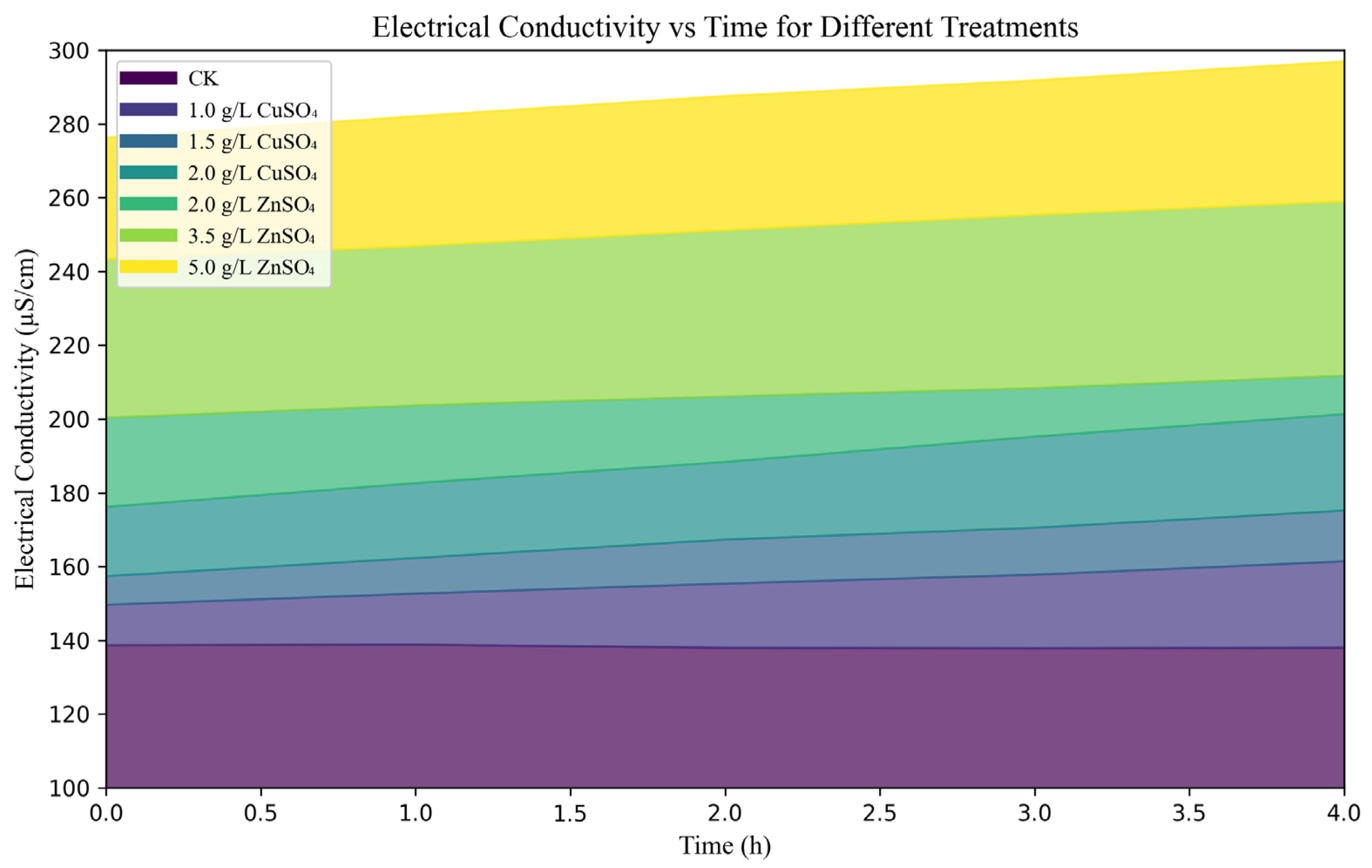
Figure 1b), indicating safety for tuber application at tested concentrations. Mechanistically, electrical conductivity assays showed that 0.002 g/mL CuSO
4 and 0.005 g/mL ZnSO
4 increased bacterial membrane permeability to 201.39 µS/cm and 297.05 µS/cm, respectively (
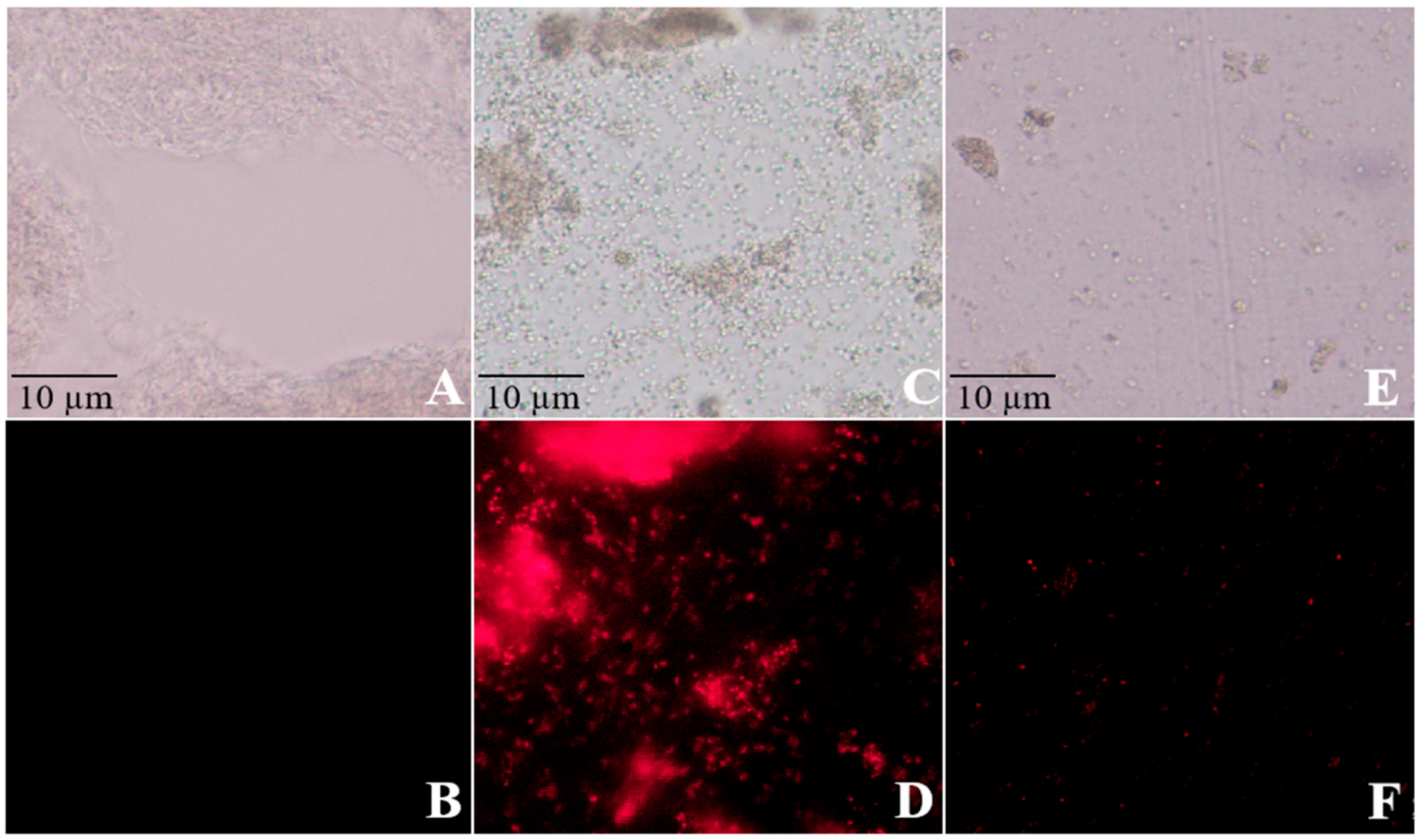
Figure 2), while PI staining revealed nucleic acid leakage (
Figure 3). These findings suggest that Cu
2+ and Zn
2+ ions induce oxidative stress, disrupting bacterial cell structures, consistent with Quaglia et al. (2021), who reported similar membrane-destabilizing effects on
Pseudomonas syringae [
13]. Compared to streptomycin, which risks resistance development [
12], CuSO
4 and ZnSO
4 offer a more sustainable approach due to their natural occurrence and lower resistance potential, making them promising alternatives for PCS management.
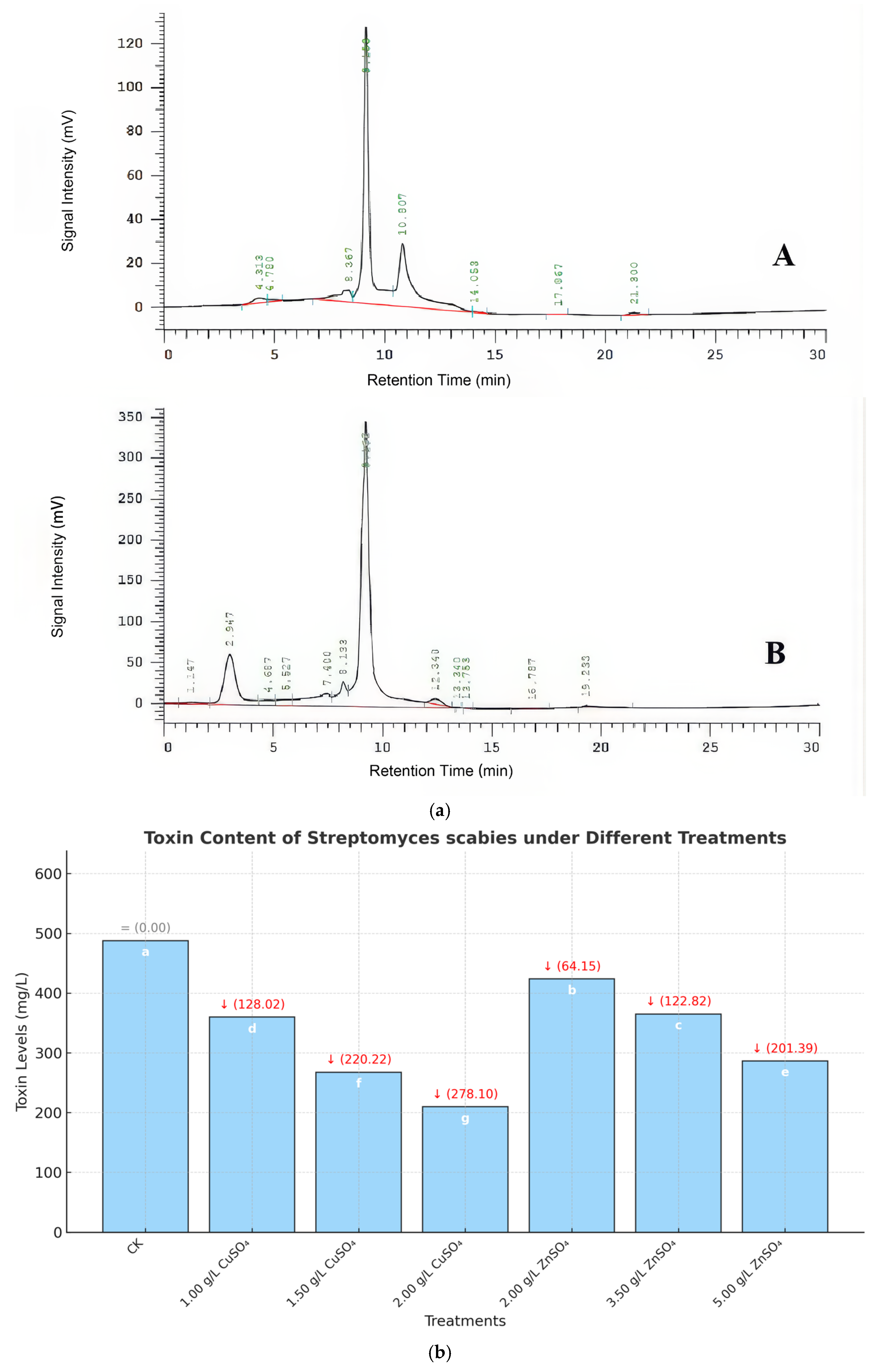
A critical aspect of PCS pathogenicity is the production of Thaxtomin A, which disrupts plant cell wall integrity and induces necrosis [
7]. CuSO
4 and ZnSO
4 significantly inhibited Thaxtomin A production, achieving maximum inhibition rates of 57.02% (0.002 g/mL CuSO
4) and 41.29% (0.005 g/mL ZnSO
4), corresponding to reductions of 278.10 mg/L and 201.39 mg/L, respectively (
Figure 4b). This aligns with Hassan et al. (2021), who noted metal ions’ role in toxin suppression [
17], but our study extends these findings by linking Thaxtomin A reduction to enhanced plant resistance. The decrease in Thaxtomin A likely reduces disease severity by limiting pathogen virulence, creating a favorable environment for subsequent plant defense responses.
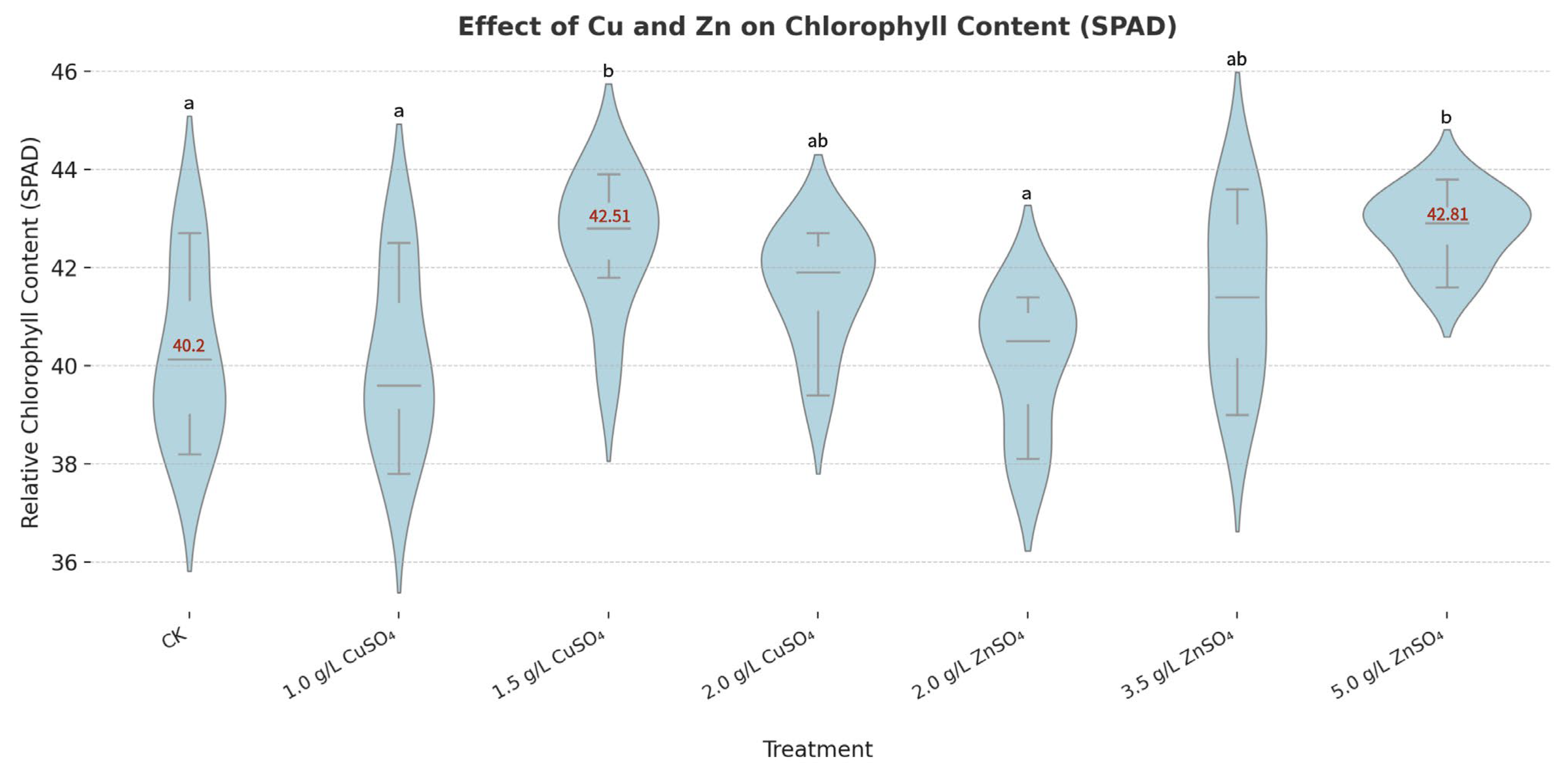
At the plant level, CuSO
4 and ZnSO
4 significantly enhanced growth parameters and chlorophyll content, supporting their dual role in disease resistance and growth promotion (
Figure 5 and
Figure 6). Specifically, 0.005 g/mL ZnSO
4 significantly increased plant height to 43.48 ± 3.22 cm (vs. 40.24 ± 1.36 cm in CK), fresh weight to 126.14 ± 9.74 g, and dry weight to 42.25 ± 3.94 g, while 0.002 g/mL CuSO
4 significantly raised stem diameter to 1.13 ± 0.03 cm (vs. 0.98 ± 0.07 cm in CK) and chlorophyll content to 42.51 ± 0.80 SPAD (vs. 40.20 ± 0.75 SPAD in CK) (
p < 0.05,
Supplementary Table S3). These improvements are attributable to Cu and Zn’s roles as essential micronutrients in chlorophyll synthesis and enzymatic functions [
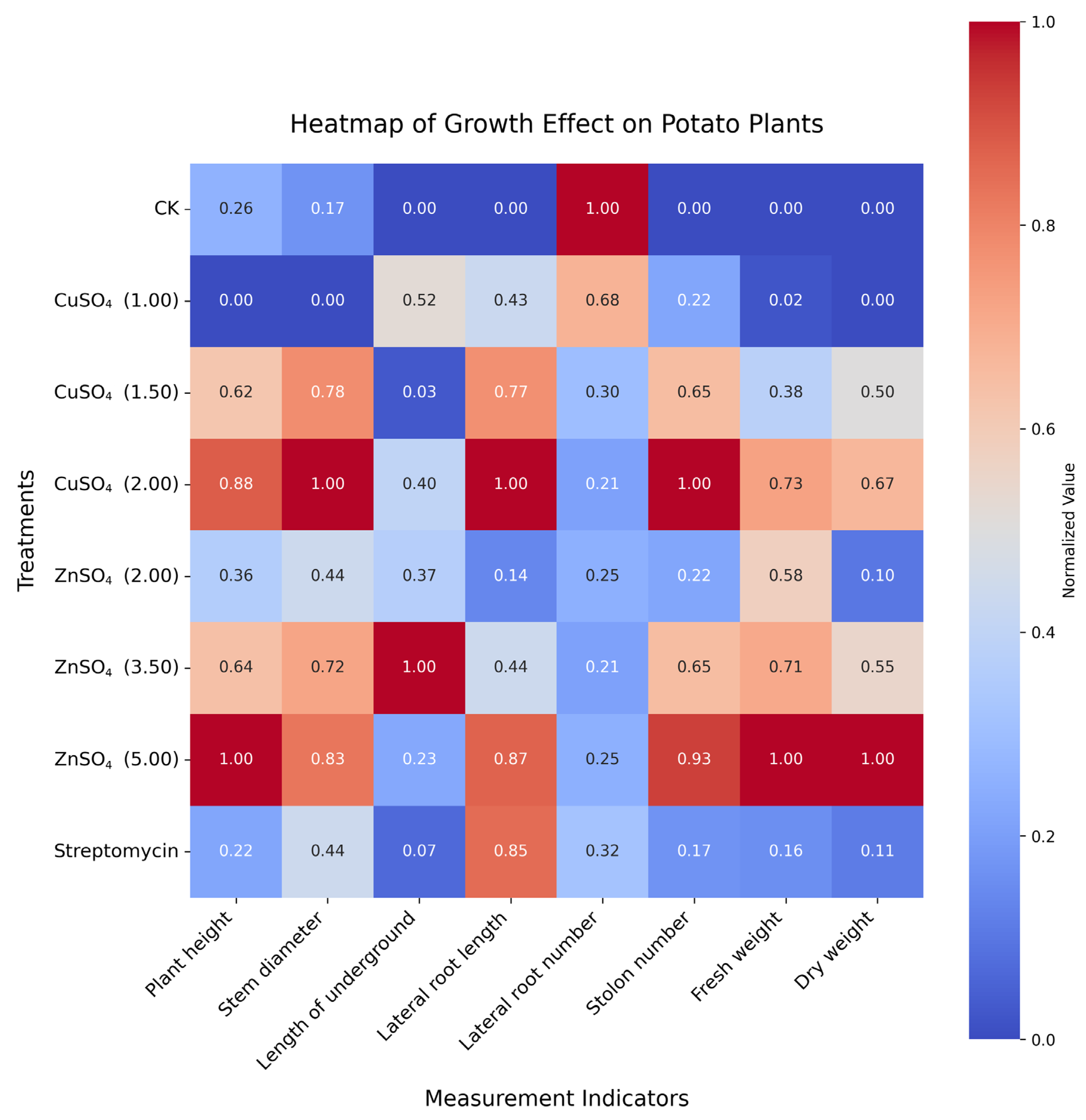
23]. The heatmap analysis (
Figure 5) showed consistent enhancements across multiple growth indicators, with normalized values reaching up to 1.0, indicating maximum improvement relative to CK. Similar growth-promoting effects of Zn have been reported in maize and rice [
24], but our study uniquely demonstrates these benefits in the context of PCS management, linking growth enhancement to improved disease resistance.
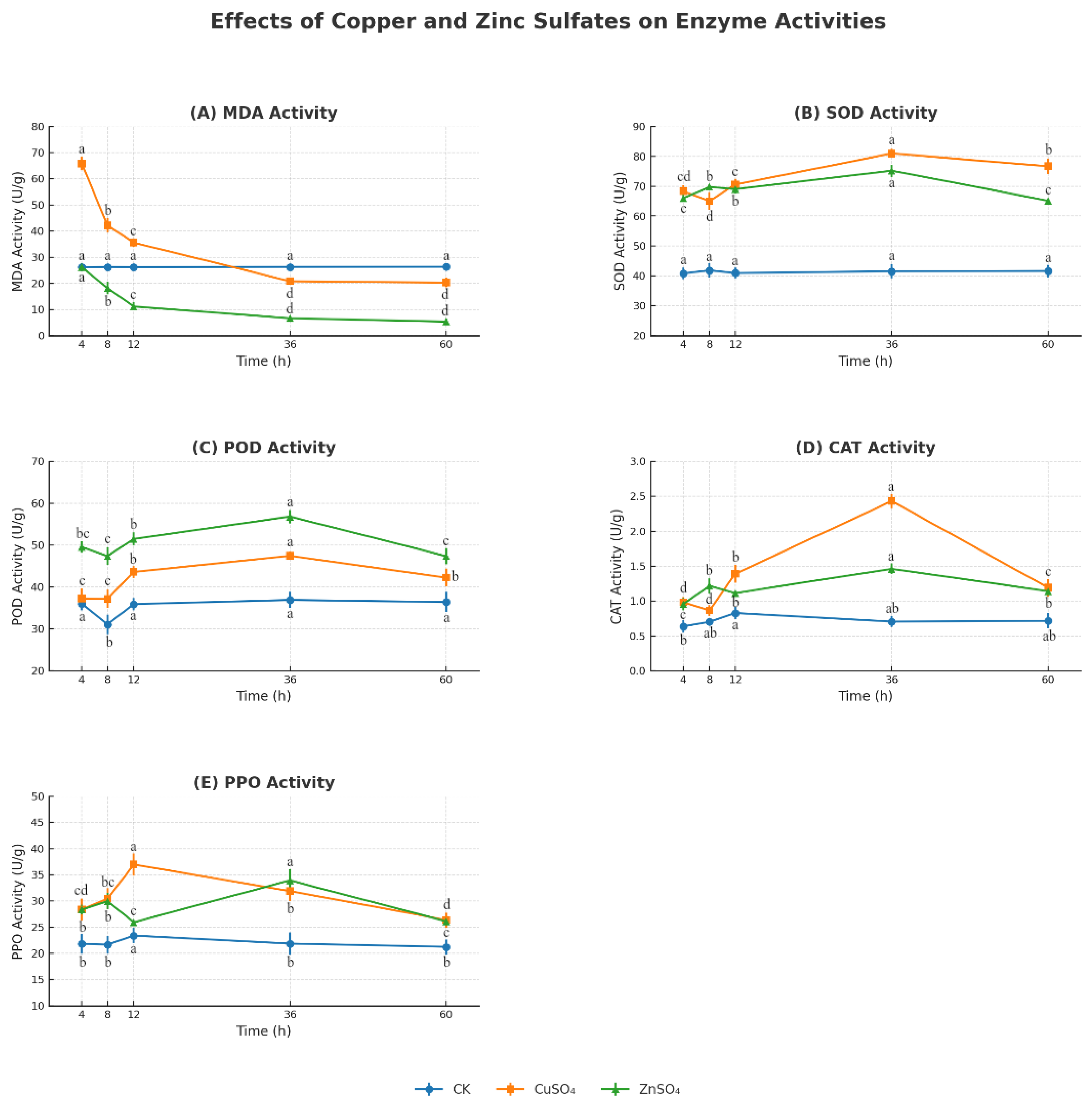
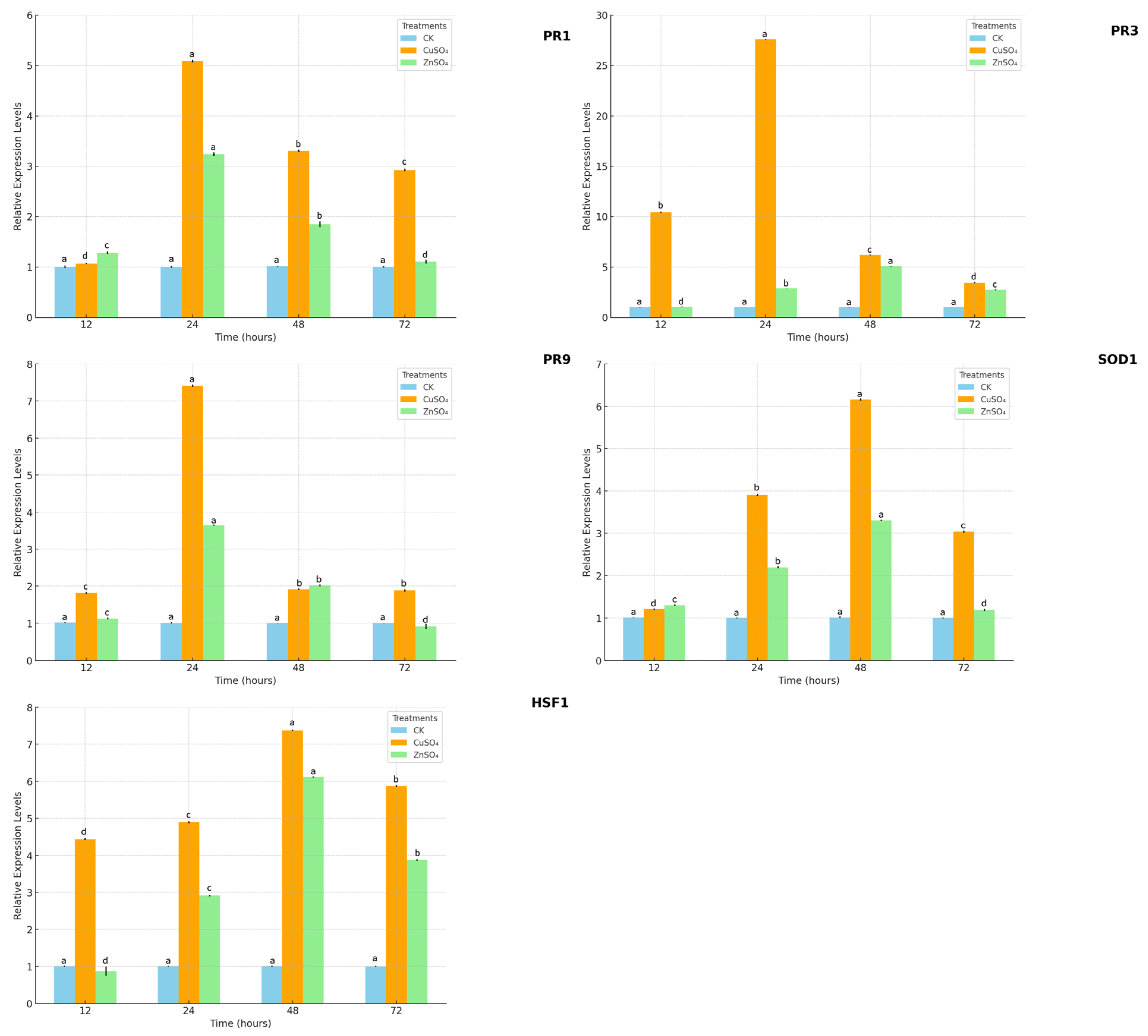
The treatments also bolstered potato resistance by modulating oxidative stress and defense gene expression. CuSO
4 and ZnSO
4 significantly increased the activities of SOD, POD, CAT, and PPO, while reducing MDA levels (
Figure 7), indicating a reduction in lipid peroxidation and oxidative damage caused by pathogen-induced ROS. This aligns with Kopecky et al. (2021), who found Zn supplementation enhanced SOD and CAT activities under biotic stress [
14]. qPCR analysis revealed significant upregulation of defense-related genes (PR1, PR3, PR9, SOD1, HSF1), with PR3 expression peaking at 5.09-fold after ZnSO
4 treatment (
Figure 8). PR3, encoding a chitinase, likely degrades pathogen cell walls, while SOD1 mitigates oxidative stress, and HSF1 enhances stress signaling [
25,
26]. The interplay between Thaxtomin A reduction, antioxidant enzyme activation, and defense gene upregulation forms a synergistic defense mechanism, reducing disease severity while promoting plant vigor. This integrated response highlights the potential of CuSO
4 and ZnSO
4 to enhance systemic acquired resistance (SAR), a mechanism previously noted in other crops under stress [
26].
Field trials validated these findings, showing control efficiencies of 56.58% for CuSO
4 and 59.06% for ZnSO
4, with ZnSO
4 increasing yield by 19.29%. These efficiencies are comparable to or exceed those of chemical controls like streptomycin [
19], but with added sustainability benefits. However, regular use of CuSO
4 and ZnSO
4 raises concerns about potential soil contamination with heavy metals (Cu and Zn). Soil safety assessments indicated that at the tested concentrations (up to 0.002 g/mL CuSO
4 and 0.005 g/mL ZnSO
4), soil Cu and Zn levels remained below the risk screening values of the Chinese Soil Environmental Quality Standard (GB15618-2018), at 74.09 mg/kg for Cu (limit: 100 mg/kg) and 94.67 mg/kg for Zn (limit: 300 mg/kg) (
Supplementary Table S4). While these levels are safe for agricultural use in the short term, repeated applications over time could lead to accumulation, potentially exceeding safe thresholds and affecting soil microbial communities and long-term soil health [
13]. For instance, excessive Cu accumulation has been linked to reduced microbial diversity in agricultural soils, while high Zn levels may impact soil pH and nutrient availability [
18]. To mitigate these risks, we recommend regular monitoring of soil Cu and Zn levels and exploring integrated management strategies, such as combining CuSO
4 and ZnSO
4 with biological controls to reduce application frequency.
This study provides actionable insights for PCS management, with CuSO
4 and ZnSO
4 offering a sustainable alternative to chemical controls. We recommend field application at 0.002 g/mL CuSO
4 and 0.005 g/mL ZnSO
4, applied as foliar sprays or soil drenches, to balance efficacy and safety. Future research should focus on optimizing application strategies, evaluating synergistic effects with biological controls, and assessing long-term impacts on soil health and microbial ecology [
27]. Additionally, molecular studies targeting the downstream regulatory networks of PR3 and SOD1 could further elucidate the pathways through which CuSO
4 and ZnSO
4 enhance resistance, paving the way for precision agriculture applications.
In conclusion, CuSO4 and ZnSO4 offer a sustainable strategy for PCS management by suppressing Streptomyces spp., reducing Thaxtomin A production, and enhancing potato growth and resistance through physiological and molecular mechanisms. While their short-term soil safety is confirmed, careful management is required to prevent long-term heavy metal accumulation, ensuring their viability in sustainable potato production.