The Patterns and Environmental Factors of Diversity, Co-Occurrence Networks, and Assembly Processes of Protistan Communities in Bulk Soils of Forests
Abstract
1. Introduction
2. Materials and Methods
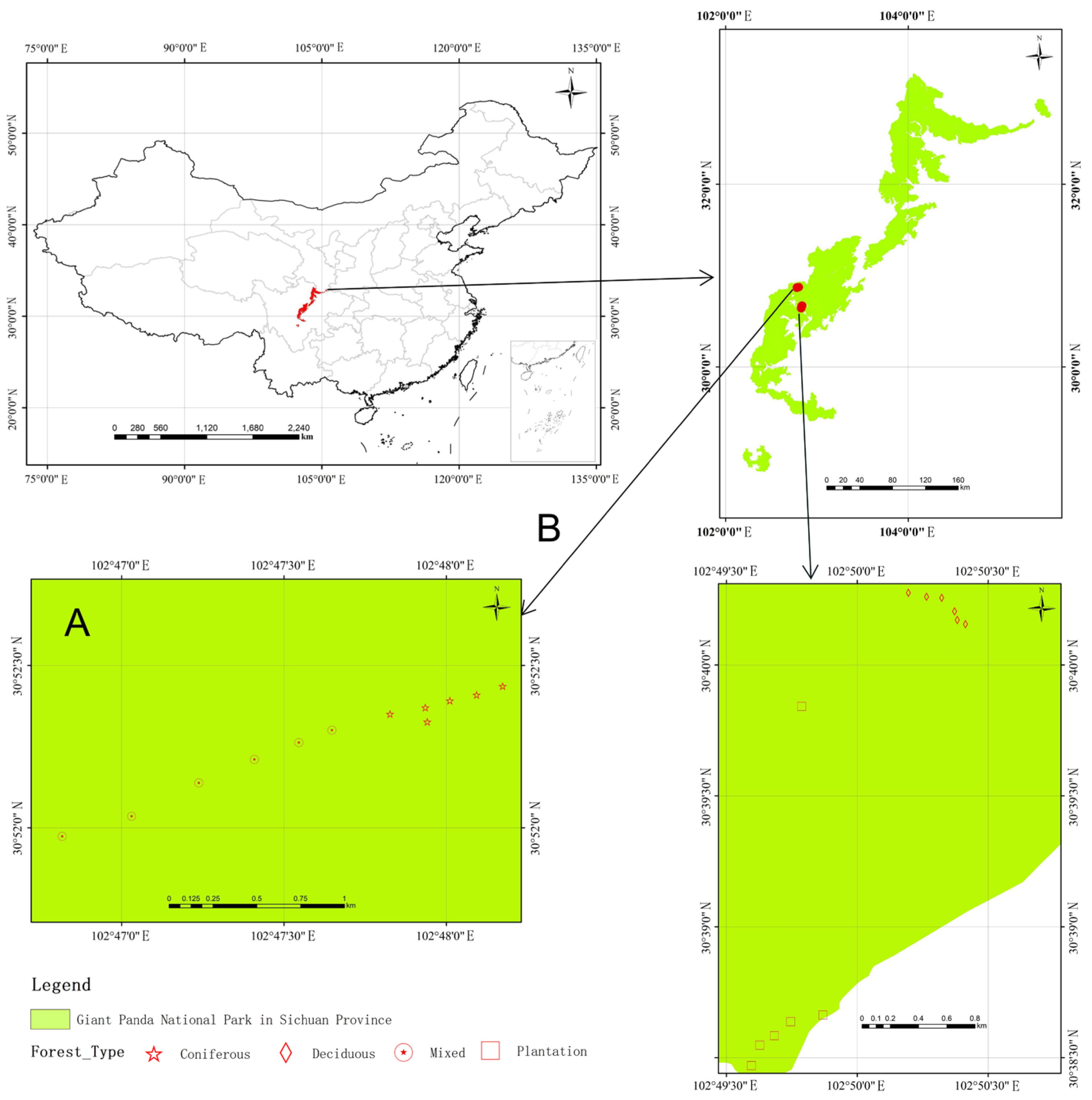
2.1. Sampling Sites
2.2. Soil Sampling
2.3. Soil Physicochemical Property Determination
2.4. DNA Extraction and Library Preparation
2.5. Bioinformatics Processing
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
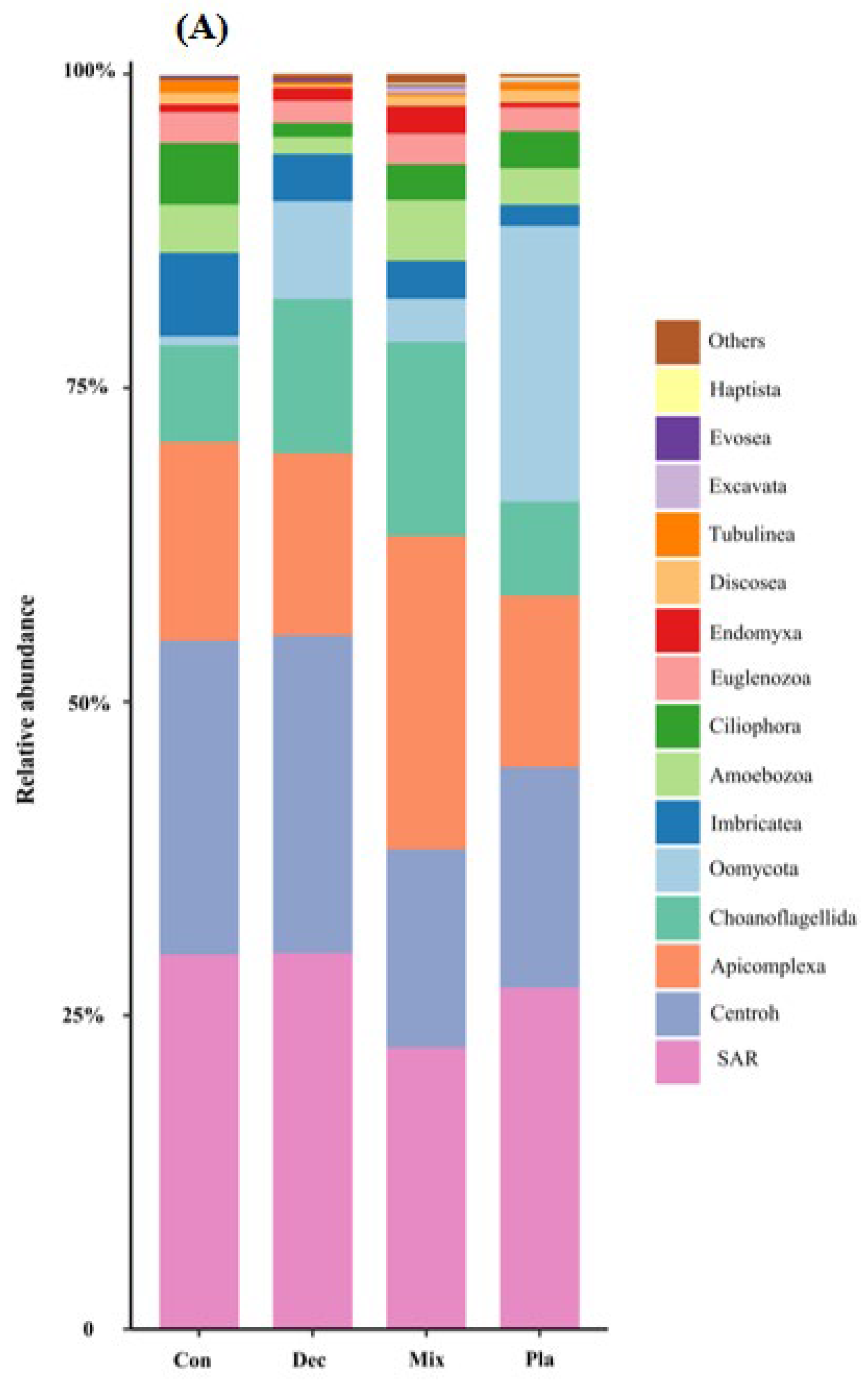
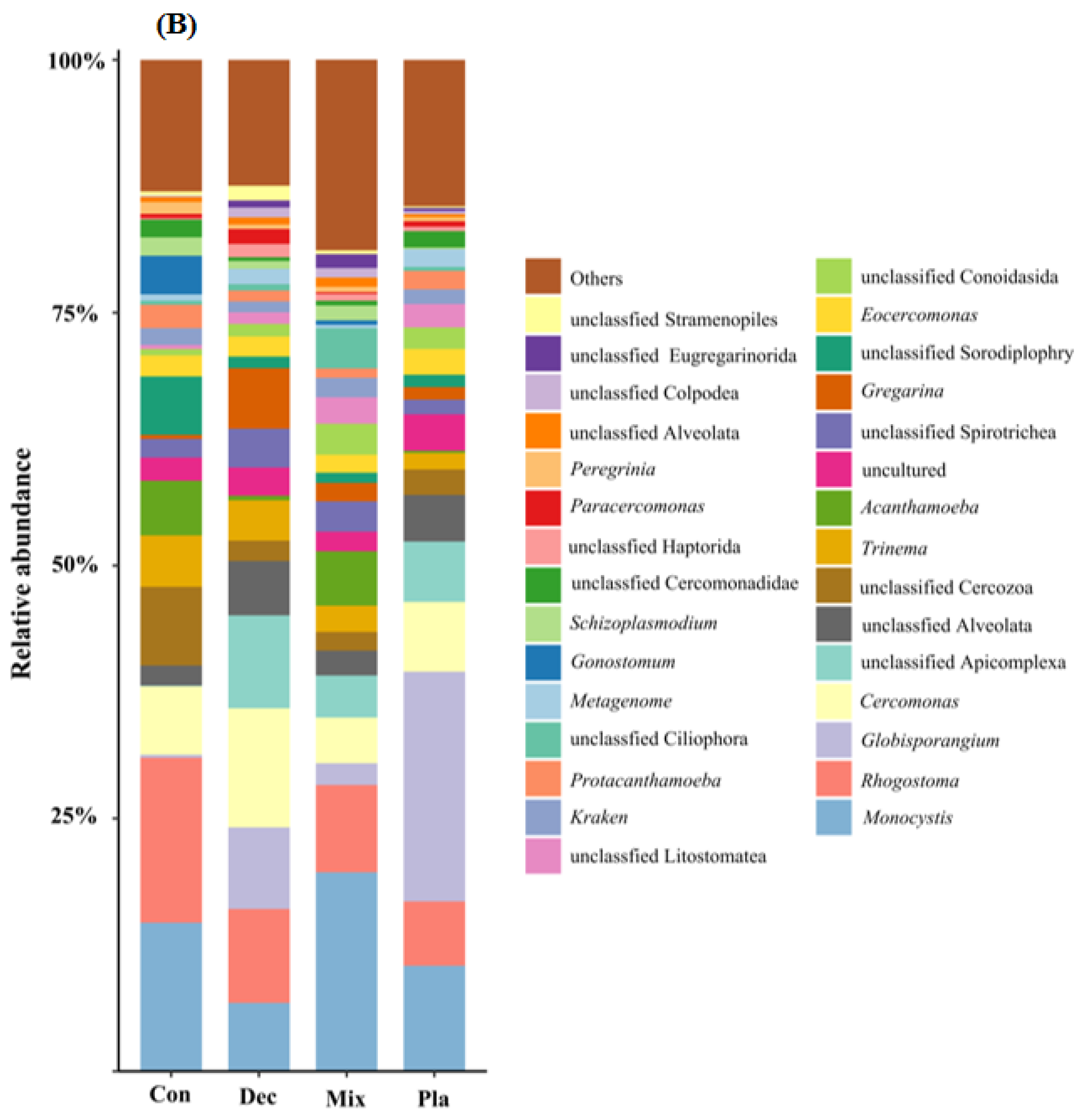
3.2. Taxonomic and Functional Group Composition
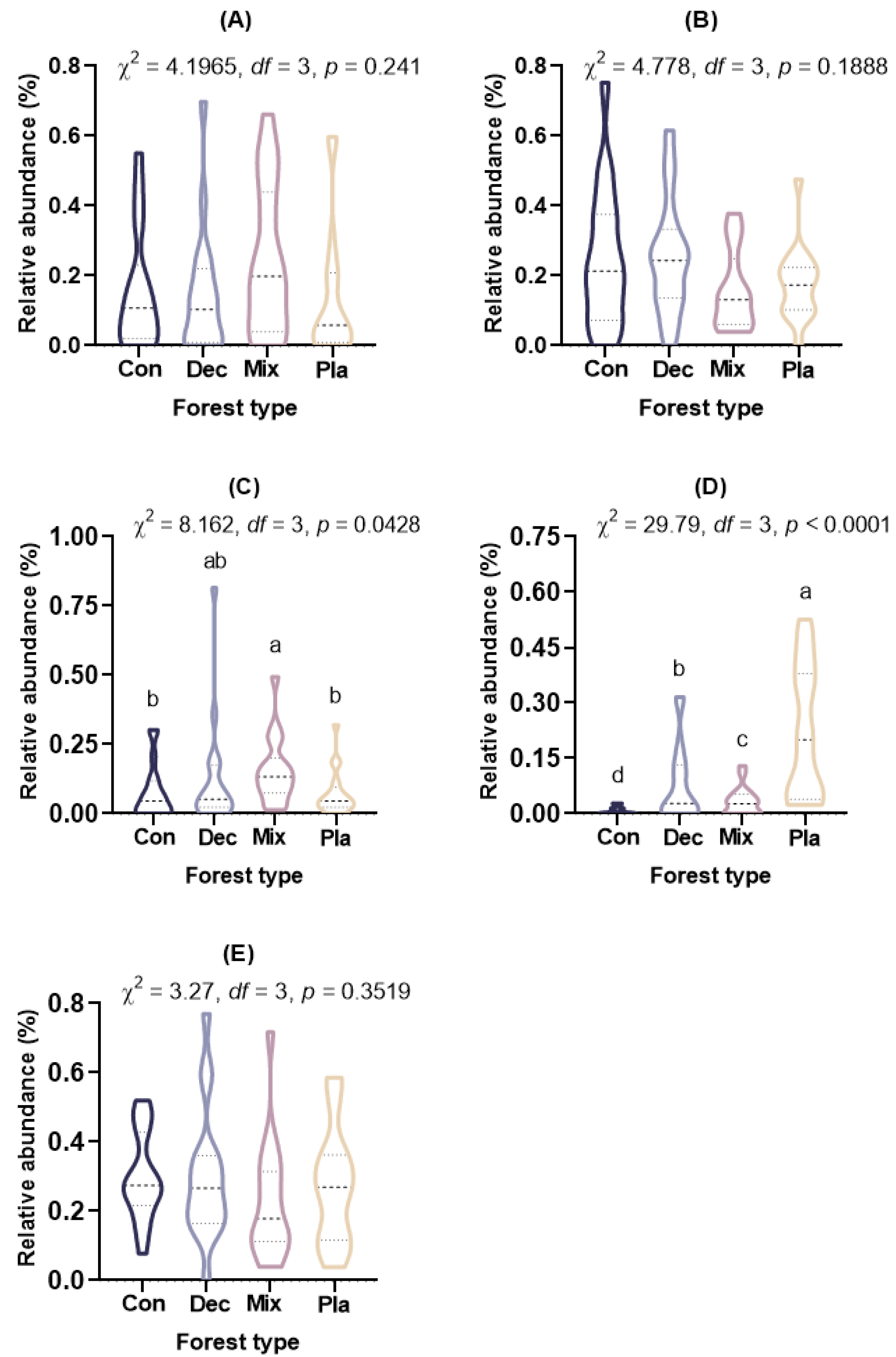
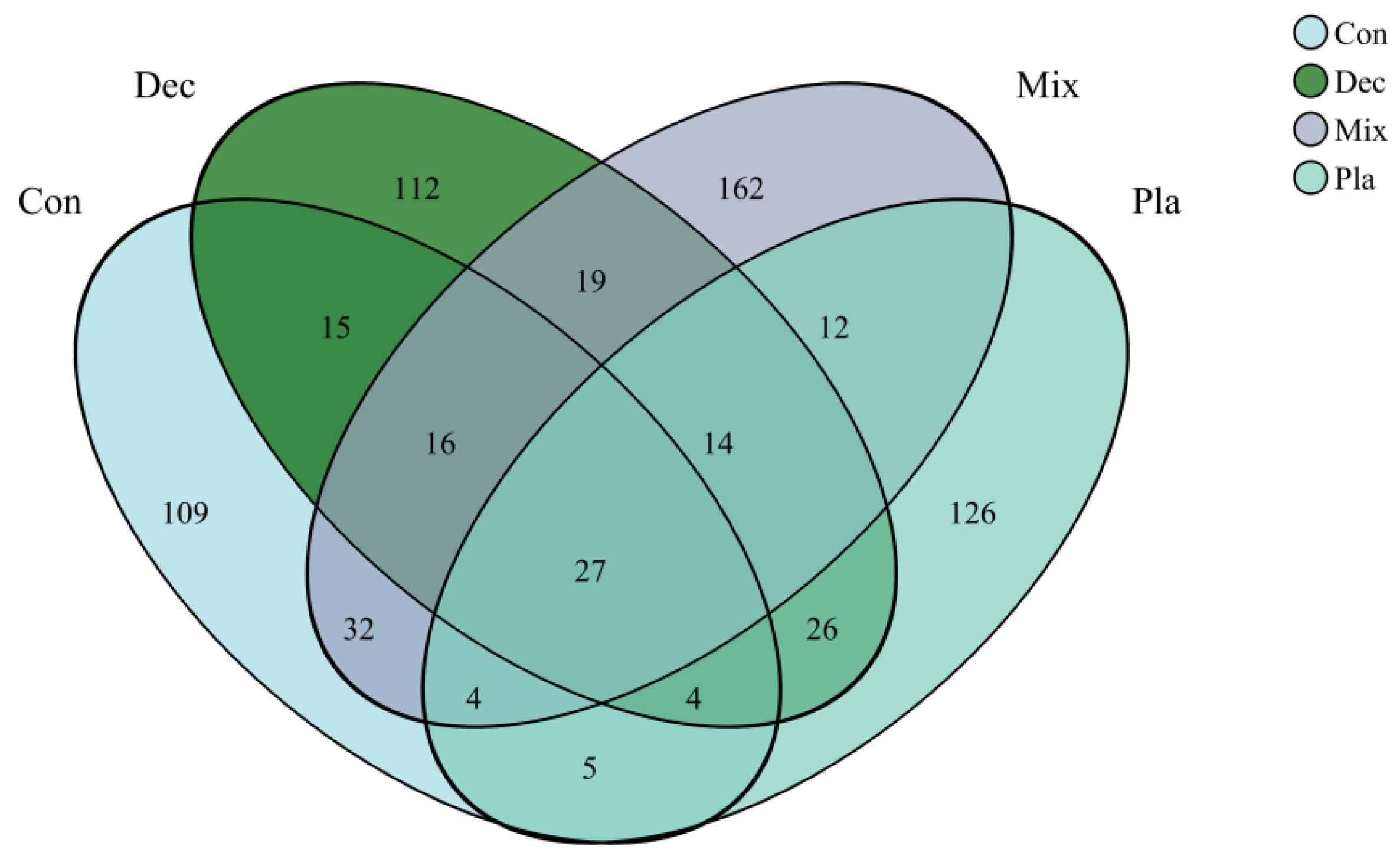
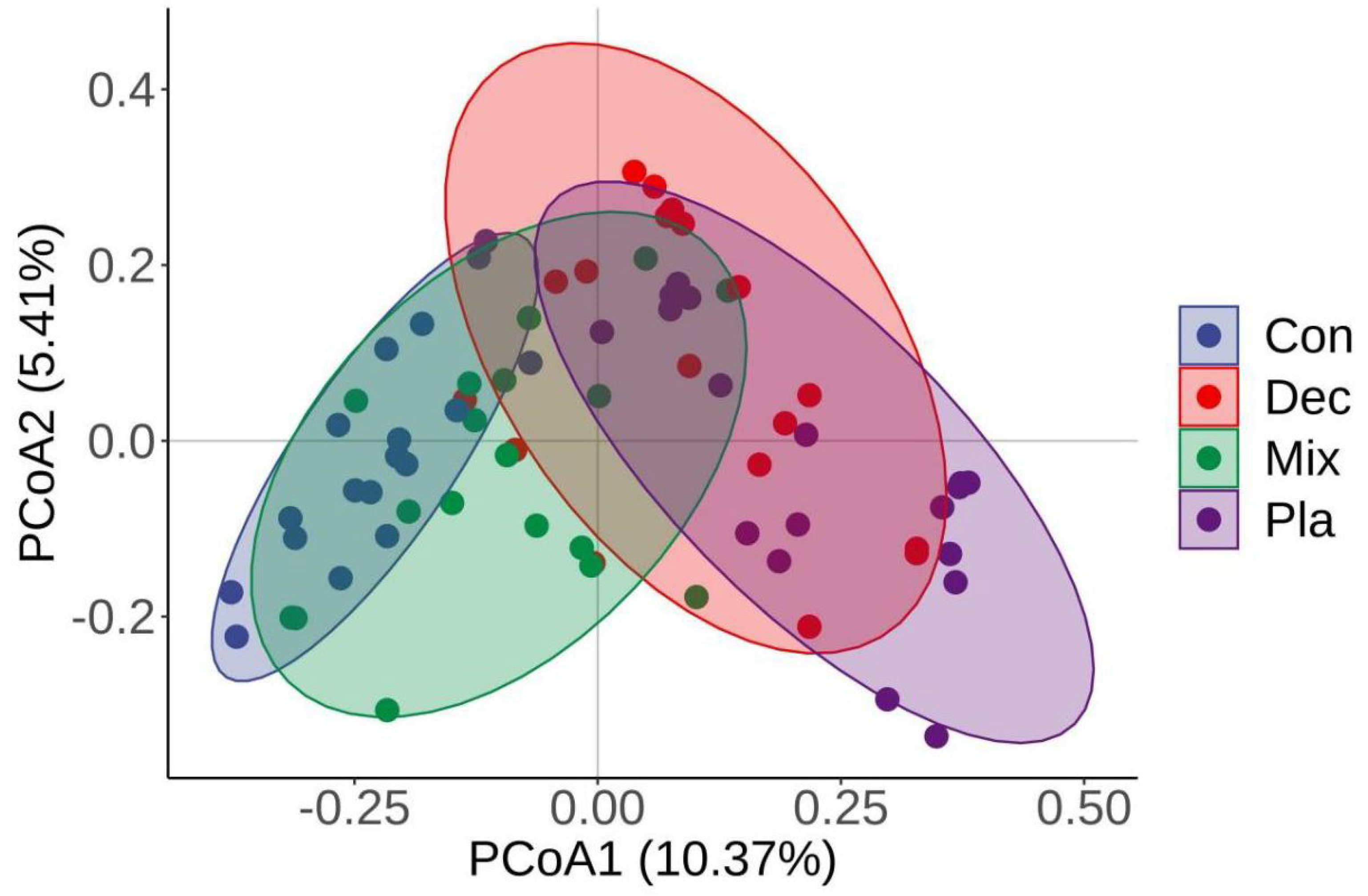
3.3. Diversity
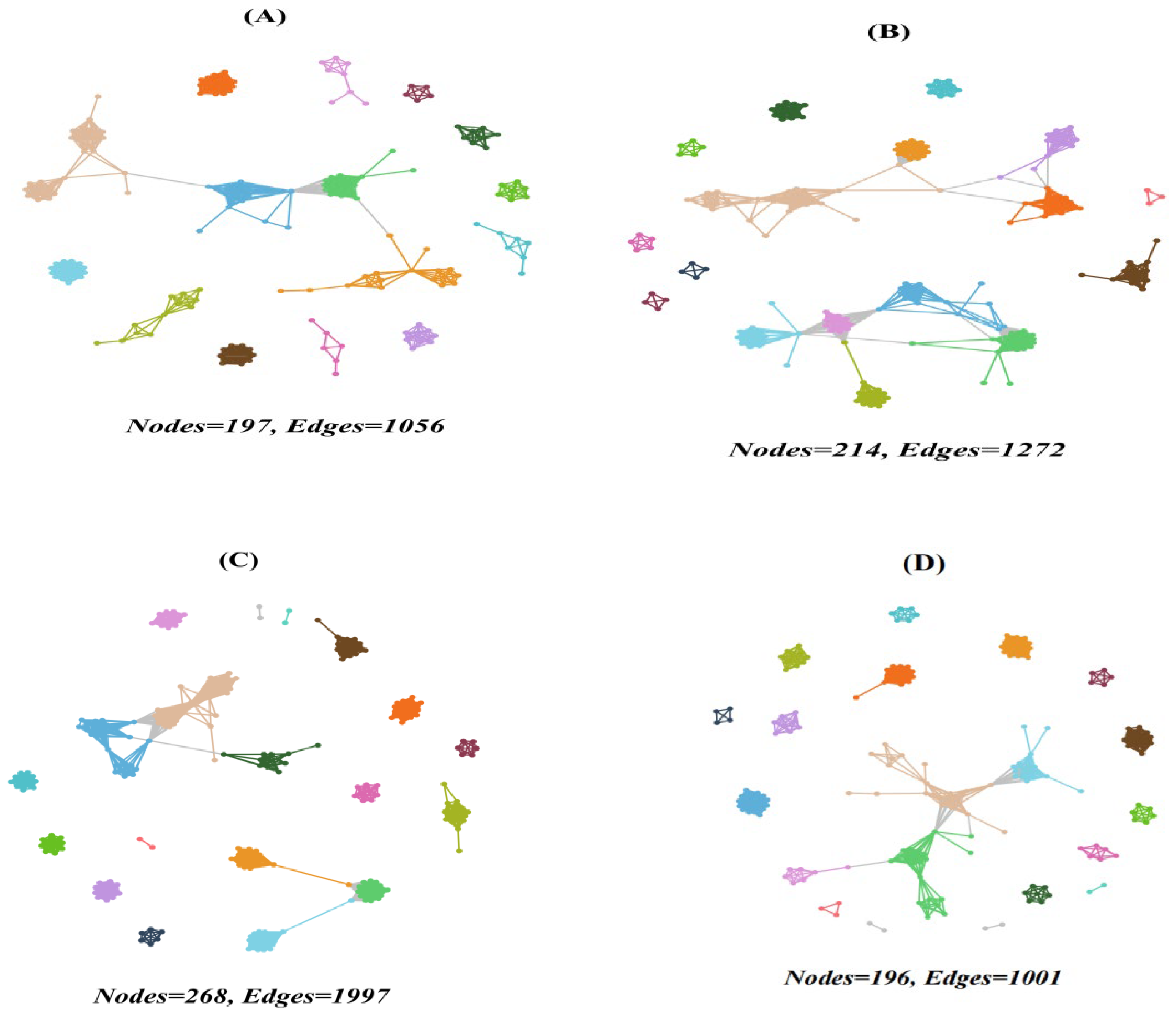
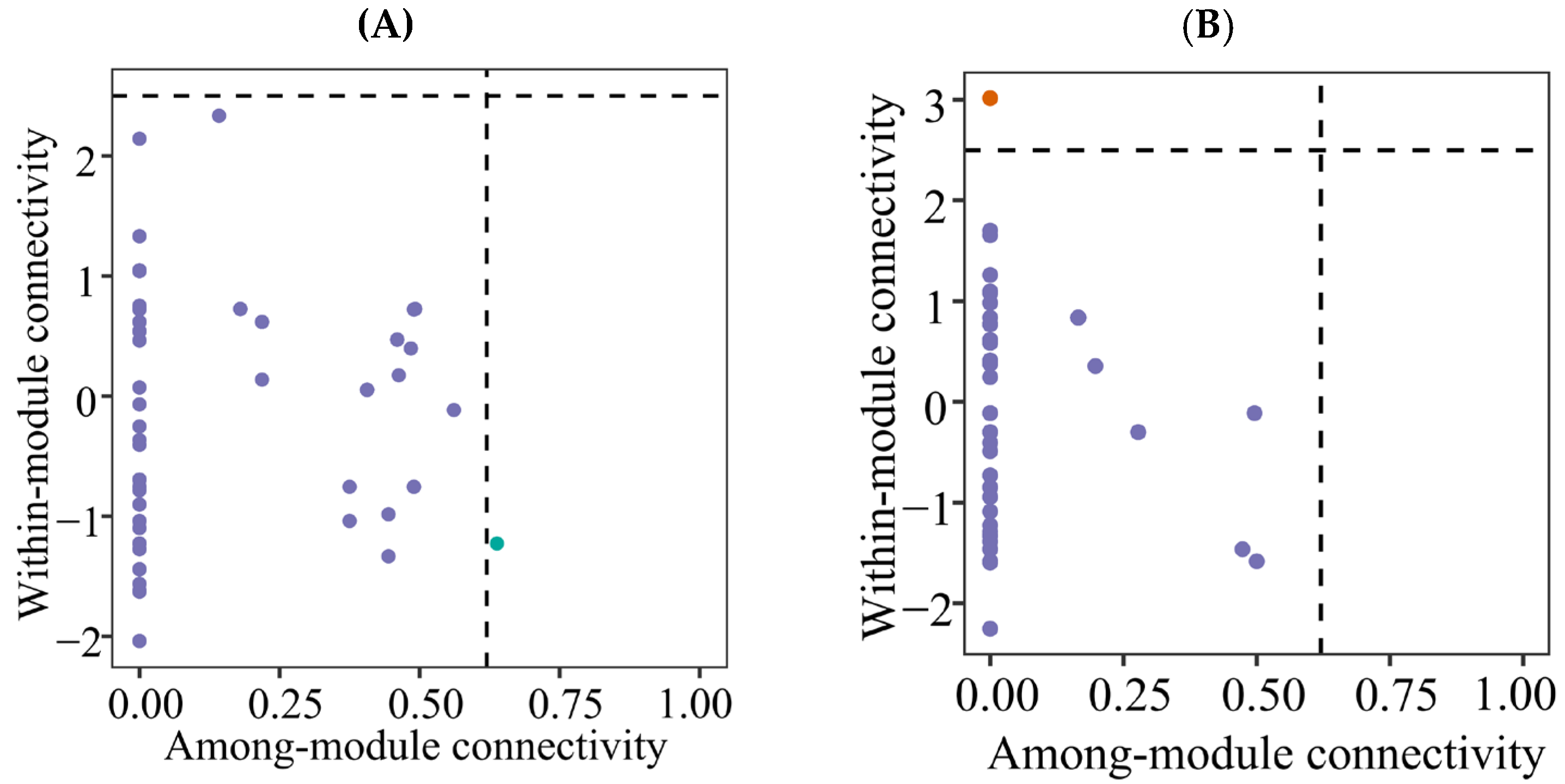
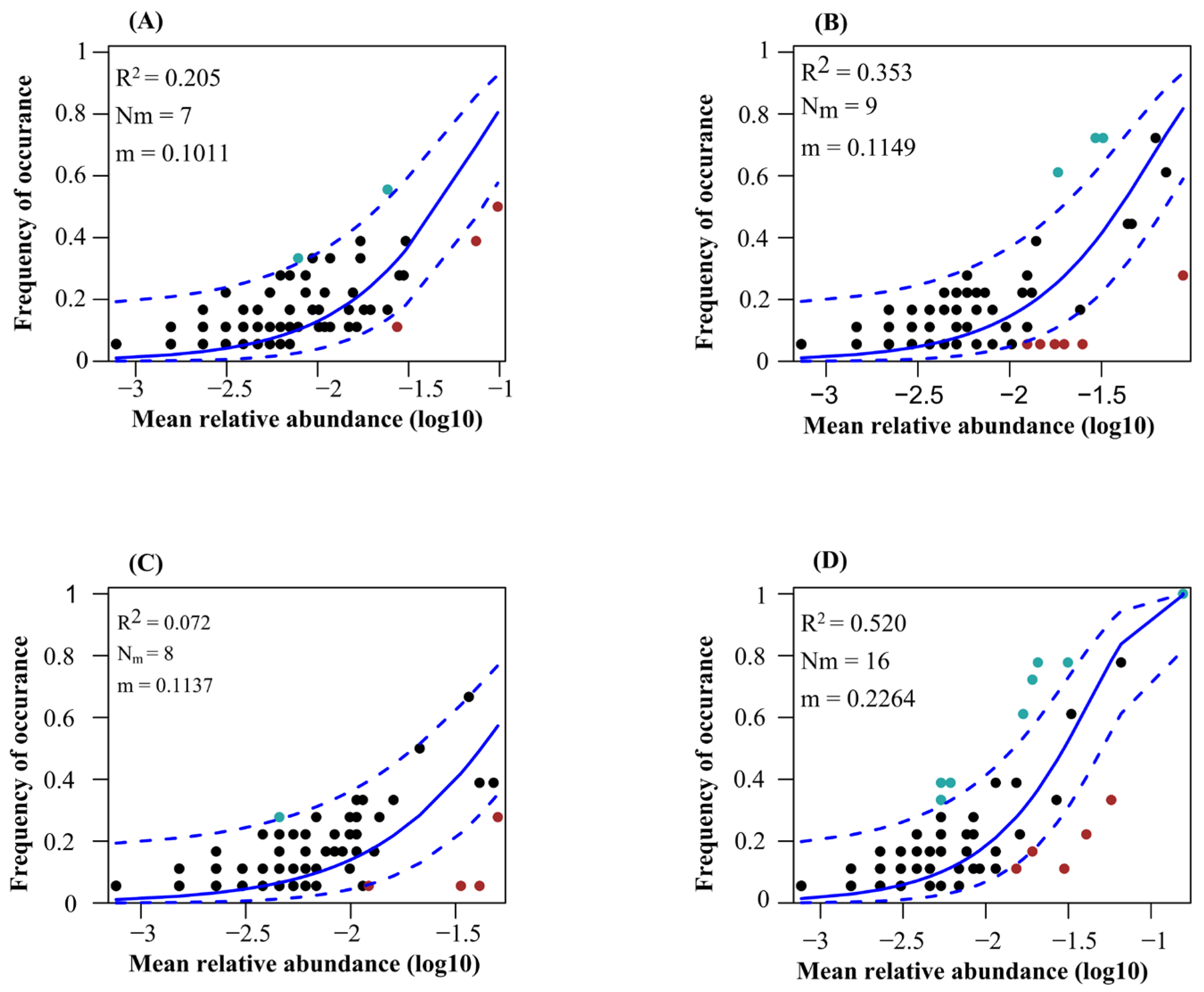
3.4. Co-Occurrence Networks
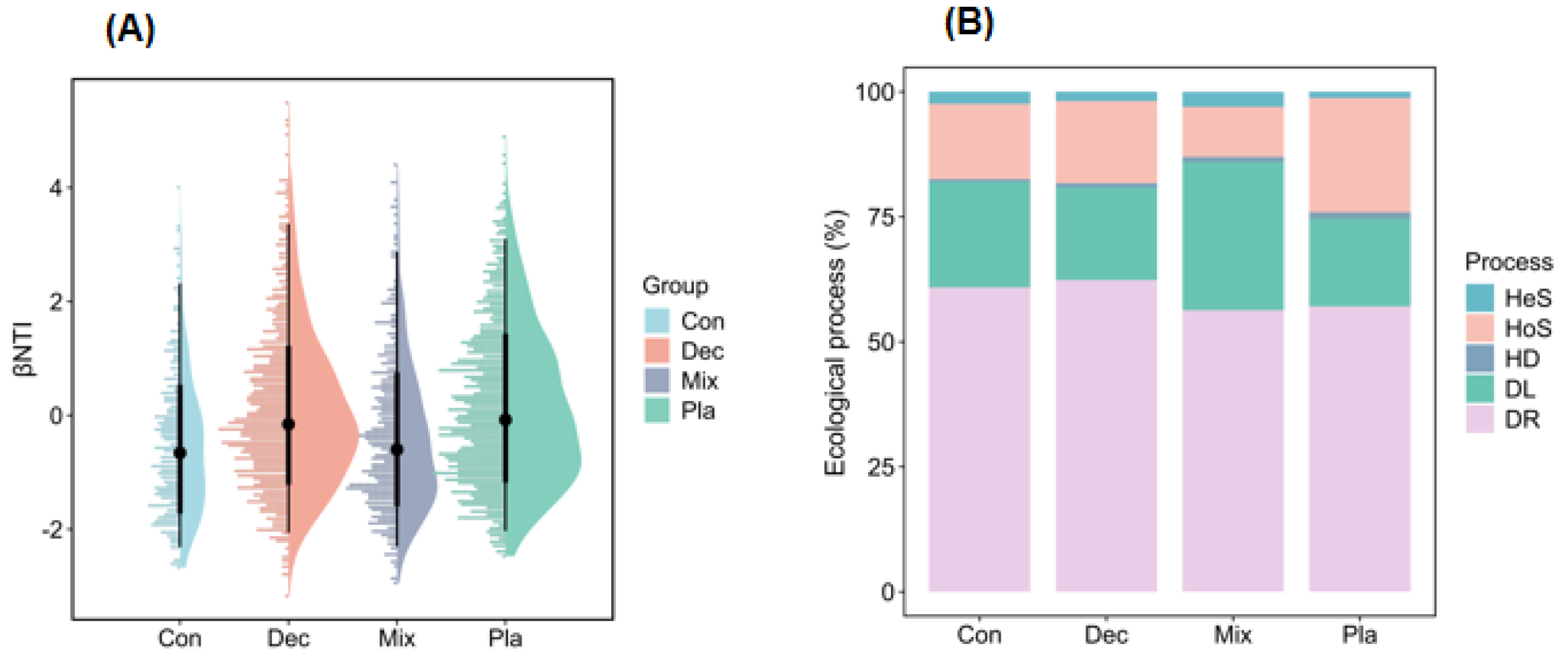
3.5. Community Assembly Processes
4. Discussion
4.1. Taxonomical and Functional Composition
4.2. Diversity Patterns
4.3. Co-Occurrence Networks
4.4. Community Assembly Processes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AN | Available nitrogen |
| AP | Available phosphorus |
| ASV | Amplicon sequence variant |
| C/N | Soil organic carbon/soil total nitrogen |
| C/P | Soil organic carbon/soil total phosphorus |
| EC | electrical conductivity |
| N/P | Soil total nitrogen/soil total phosphorus |
| SAR | Stramenopiles, Alveolates, Rhizaria |
| SOC | Soil organic carbon |
| SWC | Soil water content |
| TN | Total nitrogen |
| TP | Total phosphorus |
| PCoA | Principal coordinate analysis |
Appendix A

References
- Asiloglu, R.; Shiroishi, K.; Suzuki, K.; Turgay, O.C.; Harada, N. Soil properties have more significant effects on the community composition of protists than the rhizosphere effect of rice plants in alkaline paddy field soils. Soil Biol. Biochem. 2021, 161, 108397. [Google Scholar] [CrossRef]
- Bates, S.T.; Clemente, J.C.; Flores, G.E.; Walters, W.A.; Parfrey, L.W.; Knight, R.; Fierer, N. Global biogeography of highly diverse protistan communities in soil. ISME J. 2013, 7, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Mitchell, E.A.D.; Adl, S.; Bonkowski, M.; Dunthorn, M.; Ekelund, F.; Fernández, L.D.; Jousset, A.; Krashevska, V.; Singer, D.; et al. Soil protists: A fertile frontier in soil biology research. FEMS Microbiol. Rev. 2018, 42, 293–323. [Google Scholar] [CrossRef]
- Singer, D.; Seppey, C.V.W.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; de Groot, G.A.; de Vargas, C.; et al. Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems. Environ. Int. 2021, 146, 106262. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Geisen, S.; Delgado-Baquerizo, M.; Maestre, F.T.; Turner, B.L.; Fierer, N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 2020, 6, eaax8787. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brownk, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef]
- Nguyen, B.A.T.; Chen, Q.L.; Yan, Z.Z.; Li, C.; He, J.Z.; Hu, H.W. Distinct factors drive the diversity and composition of protistan consumers and phototrophs in natural soil ecosystems. Soil Biol. Biochem. 2021, 160, 108317. [Google Scholar] [CrossRef]
- Seppey, C.V.W.; Singer, D.; Dumack, K.; Fournier, B.; Belbahri, L.; Mitchell, E.A.D.; Lara, E. Distribution patterns of soil microbial eukaryotes suggests widespread algivory by phagotrophic protists as an alternative pathway for nutrient cycling. Soil Biol. Biochem. 2017, 112, 68–76. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, L.; Liu, S.; Liu, F.; Saleem, M.; Han, X.; Shu, L.; Yu, X.; Hu, R.; He, Z.; et al. Biogeography of soil protistan consumer and parasite is contrasting and linked to microbial nutrient mineralization in forest soils at a wide-scale. Soil Biol. Biochem. 2022, 165, 108513. [Google Scholar] [CrossRef]
- Chen, B.; Xiong, W.; Qi, J.; Pan, H.; Chen, S.; Peng, Z.; Gao, H.; Zhang, L.; Jiang, Y.; Wei, G.; et al. Trophic interrelationships drive the biogeography of protistan community in agricultural ecosystems. Soil Biol. Biochem. 2021, 163, 108445. [Google Scholar] [CrossRef]
- Gao, Y.; Song, H.; Zhou, F.; Chen, S.; He, G.; Yan, J.; Sun, Q.; Long, H.; Zhai, Z.; Hu, D.; et al. Community of soil-inhabiting myxomycetes shares similar assembly mechanisms with fungi, and is affected by bacterial community in subtropical forests of China. Soil Biol. Biochem. 2022, 175, 108854. [Google Scholar] [CrossRef]
- Nguyen, T.B.A.; Chen, Q.L.; Yan, Z.Z.; Li, C.; He, J.Z.; Hu, H.W. Trophic interrelationships of bacteria are important for shaping soil protist communities. Env. Microbiol. Rep. 2023, 15, 298–307. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, A.S.F.; Mendes, L.W.; Lemos, L.N.; Antunes, J.E.L.; Beserra, J.E.A.; de Lyra, M.d.C.C.P.; Figueiredo, M.d.V.B.; Lopes, Â.C.d.A.; Gomes, R.L.F.; Bezerra, W.M.; et al. Protist species richness and soil microbiome complexity increase towards climax vegetation in the Brazilian Cerrado. Commun. Biol. 2018, 1, 135. [Google Scholar] [CrossRef]
- Turner, T.R.; Ramakrishnan, K.; Walshaw, J.; Heavens, D.; Alston, M.; Swarbreck, D.; Osbourn, A.; Grant, A.; Poole, P.S. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 2013, 7, 2248–2258. [Google Scholar] [CrossRef]
- Ledeganck, P.; Nijs, I.; Beyens, L. Plant functional group diversity promotes soil protist diversity. Protist 2003, 154, 239–249. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Wang, Y.; Ning, D.; Arellano, A.; Ramanculova, L.; Yuan, M.M.; Byer, A.; Craven, K.D.; Saha, M.C.; Brodie, E.L.; et al. Protist diversity and community complexity in the rhizosphere of switchgrass are dynamic as plants develop. Microbiome 2021, 9, 96. [Google Scholar] [CrossRef]
- Xiong, W.; Song, Y.; Yang, K.; Gu, Y.; Wei, Z.; Kowalchuk, G.A.; Xu, Y.; Jousset, A.; Shen, Q.; Geisen, S. Rhizosphere protists are key determinants of plant health. Microbiome 2020, 8, 27. [Google Scholar] [CrossRef]
- Fiore-Donno, A.M.; Human, Z.R.; Štursová, M.; Mundra, S.; Morgado, L.; Kauserud, H.; Baldrian, P.; Bonkowski, M. Soil compartments (bulk soil, litter, root and rhizosphere) as main drivers of soil protistan communities’ distribution in forests with different nitrogen deposition. Soil Biol. Biochem. 2022, 168, 108628. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Yang, P.; Hu, J.; Zhao, T.; Zhang, Y.; Ding, X.; Yan, X. A comparison of microbial composition under three tree ecosystems using the stochastic process and network complexity approaches. Front. Microbiol. 2022, 13, 1018077. [Google Scholar] [CrossRef]
- Fang, K.; Tang, N.; Liu, J.; Zhang, X.Y.; He, H.L.; Zhao, W.Q.; Kou, Y.P.; Liu, Q. The influence of soil factors on protist community dynamics during plant succession in subalpine natural and planted forests. Soil Biol. Biochem. 2024, 191, 109365. [Google Scholar] [CrossRef]
- Schulz, G.; Schneider, D.; Brinkmann, N.; Edy, N.; Daniel, R.; Polle, A.; Scheu, S.; Krashevska, V. Changes in trophic groups of protists with conversion of rainforest into rubber and oil palm plantations. Front Microbiol. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.B.; He, J.Z.; Quan, Z.; Wu, C.F.; Sheng, R.; Zhang, L.M.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Geisen, S.; Quist, C.W. Microbial-faunal interactions in the rhizosphere. In Rhizosphere Biology: Interactions Between Microbes and Plants; Springer: Singapore, 2021; pp. 237–253. [Google Scholar] [CrossRef]
- Thakur, M.P.; Geisen, S. Trophic Regulations of the Soil Microbiome. Trends Microbiol. 2019, 27, 771–780. [Google Scholar] [CrossRef]
- Xiong, W.; Jousset, A.; Guo, S.; Karlsson, I.; Zhao, Q.; Wu, H.; Kowalchuk, G.A.; Shen, Q.; Li, R.; Geisen, S. Soil protist communities form a dynamic hub in the soil microbiome. ISME J. 2018, 12, 634–638. [Google Scholar] [CrossRef]
- Green, S.; Şerban, M.; Scholl, R.; Jones, N.; Brigandt, I.; Bechtel, W. Network analyses in systems biology: New strategies for dealing with biological complexity. Synthese 2017, 195, 1751–1777. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Lin, D.; Hu, H.W.; He, J.Z. Divergent changes in diversity and network complexity across different trophic-level organisms drive soil multifunctionality of fire-impacted subtropical forests. For. Ecosys. 2024, 11, 100227. [Google Scholar] [CrossRef]
- Qu, S.; Shen, C.; Zhang, L.; Wang, J.; Zhang, L.M.; Chen, B.; Sun, G.X.; Ge, Y. Dispersal limitation and host selection drive geo-specific and plant-specific differentiation of soil bacterial communities in the Tibetan alpine ecosystem. Sci. Total Environ. 2023, 863, 160944. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Martiny, J.B.H. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Telford, R.J.; Vandvik, V.; Birks, H.J.B. Dispersal limitations matter for microbial morphospecies. Science 2006, 312, 1015. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Nat. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Eisen, J.A.; Penn, K.; Allison, S.D.; Horner-Devine, M.C. Drivers of bacterial β-diversity depend on spatial scale. Proc. Nat. Acad. Sci. USA 2011, 108, 7850–7854. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Yang, J.; Li, M.; Wei, F. Food habits and space-use of red pandas Ailurus fulgens in the Fengtongzhai Nature Reserve, China: Food effects and behavioural responses. Acta Theriol. 2009, 54, 225–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Han, Z.; Wei, F. Activity patterns of wild red pandas in Fengtongzhai Nature Reserve, China. Ital. J. Zool. 2011, 78, 398–404. [Google Scholar] [CrossRef]
- Yang, B.; Feng, W.; Zhou, W.; He, K.; Yang, Z. Association between soil physicochemical properties and bacterial community structure in diverse forest ecosystems. Microorganisms 2024, 12, 728. [Google Scholar] [CrossRef]
- Mazel, F.; Malard, L.; Niculita-Hirzel, H.; Yashiro, E.; Mod, H.K.; Mitchell, E.A.D.; Singer, D.; Buri, A.; Pinto, E.; Guex, N.; et al. Soil protist function varies with elevation in the Swiss Alps. Environ. Microbiol. 2021, 24, 1689–1702. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.; Jiang, Y.; Zeng, R.J.; Yao, M.; Li, X. A guide for comparing microbial co-occurrence networks. iMeta 2023, 2, e71. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Li, B.; Li, Y.; Du, X.; Sun, Y.; Li, Q.; Martijn Bezemer, T. Soil addition improves multifunctionality of degraded grasslands through increasing fungal richness and network complexity. Geoderma 2023, 437, 116607. [Google Scholar] [CrossRef]
- Costa, L.D.F.; Rodrigues, F.A.; Travieso, G.; Villas Boas, P.R. Characterization of complex networks: A survey of measurements. Adv. Phys. 2007, 56, 167–242. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Wang, M.; Masoudi, A.; Wang, C.; Zhao, L.; Yang, J.; Yu, Z.; Liu, J. Seasonal variations affect the ecosystem functioning and microbial assembly processes in plantation forest soils. Front. Microbiol. 2024, 15, 1391193. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.B.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The Revised Classification of Eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–514. [Google Scholar] [CrossRef]
- Geisen, S.; Laros, I.; Vizcaíno, A.; Bonkowski, M.; de Groot, G.A. Not all are free-living: High-throughput DNA metabarcoding reveals a diverse community of protists parasitizing soil metazoa. Mol. Ecol. 2015, 24, 4556–4569. [Google Scholar] [CrossRef] [PubMed]
- Lahr, D.J.G.; Lara, E.; Mitchell, E.A.D. Time to regulate microbial eukaryote nomenclature. Biol. J. Linn. Soc. 2012, 107, 469–476. [Google Scholar] [CrossRef]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The New Tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; He, G.; Shchepin, O.N.; Yan, S.; Chen, S. Influence of forest type on dark-spored myxomycete community in subtropical forest soil, China. Soil Biol. Biochem. 2019, 138, 107606. [Google Scholar] [CrossRef]
- Brasier, C.M.; Jung, T. Progress in understanding Phytophthora diseases of trees in Europe. In Phytophthora in Forests and Natural Ecosystems, Proceedings of the 2nd International IUFRO Working Party 7.02.09 Meeting, Albany, Australia, 30 September–5 October 2001; McComb, J.A., Hardy, G.E.S.J., Eds.; Murdoch University Print: Perth, Australia, 2003; pp. 4–18. ISBN 0-86905-825-5. [Google Scholar]
- Linde, C.; Kemp, G.H.J.; Wingfield, M.J. Diseases of pines and eucalypts in South Africa associated with Pythium and Phytophthora species. S. Afr. Forestry J. 1994, 169, 25–32. [Google Scholar] [CrossRef]
- Geisen, S.; Tveit, A.T.; Clark, I.M.; Richter, A.; Svenning, M.M.; Bonkowski, M.; Urich, T. Metatranscriptomic census of active protists in soils. ISME J. 2015, 9, 2178–2190. [Google Scholar] [CrossRef]
- Adl, M.S.; Gupta, V.S. Protists in soil ecology and forest nutrient cycling. Can. J. Forest Res. 2006, 36, 1805–1817. [Google Scholar] [CrossRef]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Foissner, W. Soil protozoa as bioindicators: Pros and cons, methods, diversity, representative examples. Agric. Ecosyst. Environ. 1999, 77, 95–112. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.; Lucas-Borja, M.E.; Shi, X. Soil protist functional composition shifts with atmospheric nitrogen deposition in subtropical forests. J. Appl. Ecol. 2023, 60, 1161–1169. [Google Scholar] [CrossRef]
- Li, F.; Sun, A.; Liu, X.; Ren, P.; Wu, B.X.; Shen, J.P.; Bi, L.; He, J.Z.; Yang, Y.; Hu, H.W. Seasonality regulates the taxonomic and functional compositions of protists responding to climate warming in forest ecosystems. J. Sustain. Agric. Environ. 2023, 2, 529–540. [Google Scholar] [CrossRef]
- Hartmann, M.; Howes, C.G.; VanInsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Henrik Nilsson, R.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.J. Global dispersal of free-living microbial eukaryote species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E. Nitrogen Fixation in Tropical Cropping Systems, 2nd ed.; CABI Publishing: Wallingford, UK, 2001. [Google Scholar]
- Sun, A.; Jiao, X.Y.; Chen, Q.; Trivedi, P.; Li, Z.; Li, F.; Zheng, Y.; Lin, Y.; Hu, H.W.; He, J.Z. Fertilization alters protistan consumers and parasites in crop-associated microbiomes. Environ. Microbiol. 2021, 23, 2169–2183. [Google Scholar] [CrossRef]
- Singer, D.; Kosakyan, A.; Seppey, C.V.; Pillonel, A.; Fernández, L.D.; Fontaneto, D.; Mitchell, E.A.D.; Lara, E. Environmental filtering and phylogenetic clustering correlate with the distribution patterns of cryptic protist species. Ecology 2018, 99, 904–914. [Google Scholar] [CrossRef]
- Dupont, A.Ö.C.; Griffiths, R.I.; Bell, T.; Bass, D. Differences in soil micro-eukaryotic communities over soil pH gradients are strongly driven by parasites and saprotrophs. Environ. Microbiol. 2016, 18, 2010–2024. [Google Scholar] [CrossRef]
- Clarholm, M. Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol. Biochem. 1985, 17, 181–187. [Google Scholar] [CrossRef]
- Chenu, C.; Stotzky, G. Interactions between microorganisms and soil particles: An overview. In Interactions Between Soil Particles and Microorganisms: Impact on the Terrestrial Ecosystem; Huang, P., Bollag, J., Senesi, N., Eds.; John Wiley and Sons, Ltd.: Manchester, UK, 2002; pp. 1–40. [Google Scholar]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M.; Lussenhop, J. Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol. Biochem. 1998, 30, 905–916. [Google Scholar] [CrossRef]
- Adl, S.M. The Ecology of Soil Decomposition; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Hugerth, L.W.; Andersson, A.F. Analysing microbial community composition through amplicon sequencing: From sampling to hypothesis testing. Front. Microbiol. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef]
- Chen, Q.L.; Hu, H.W.; Sun, A.Q.; Zhu, Y.G.; He, J.Z. Aridity decreases soil protistan network complexity and stability. Soil Biol. Biochem. 2022, 166, 108575. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- HilleRisLambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking Community Assembly through the Lens of Coexistence Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Gravel, D.; Canham, C.D.; Beaudet, M.; Messier, C. Reconciling niche and neutrality: The continuum hypothesis. Ecol. Lett. 2006, 9, 399–409. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Nat. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef]
- Fukami, T.; Dickie, I.A.; Paula Wilkie, J.; Paulus, B.C.; Park, D.; Roberts, A.; Buchanan, P.K.; Allen, R.B. Assembly history dictates ecosystem functioning: Evidence from wood decomposer communities. Ecol. Lett. 2010, 13, 675–684. [Google Scholar] [CrossRef]
- Leibold, M.A.; Chase, J.M. Metacommunity Ecology; Princeton University Press: Princeton, NJ, USA, 2017; Volume 51. [Google Scholar]


| Object | Adonis | ANOSIM | MRPP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | R2 | Adjusted-p | R | Adjusted-p | A | Observed-δ | Expected-δ | Adjusted-p | |
| Among group | 3.3321 | 0.1282 | 0.001 | 0.3586 | 0.001 | 0.0488 | 0.8766 | 0.9216 | 0.001 |
| Con vs. Dec | 3.8148 | 01009 | 0.001 | 0.4608 | 0.001 | 0.0393 | 0.9245 | 0.8881 | 0.001 |
| Con vs. Mix | 1.9036 | 0.0530 | 0.001 | 0.1855 | 0.001 | 0.0131 | 0.9286 | 0.9164 | 0.002 |
| Con vs. Pla | 5.7874 | 0.1455 | 0.001 | 0.7138 | 0.001 | 0.0647 | 0.9121 | 0.8531 | 0.001 |
| Dec vs. Mix | 2.1576 | 0.0597 | 0.001 | 0.1876 | 0.001 | 0.0167 | 0.9153 | 0.9001 | 0.001 |
| Dec vs. Pla | 2.7590 | 0.0751 | 0.001 | 0.2155 | 0.001 | 0.0264 | 0.8595 | 0.8368 | 0.001 |
| Mix vs. Pla | 3.8226 | 0.1011 | 0.001 | 0.4169 | 0.001 | 0.0411 | 0.9021 | 0.8650 | 0.001 |
| Variable | PCoA1 | PCoA2 | r2 | p |
|---|---|---|---|---|
| pH | 0.9975 | −0.0709 | 0.3928 | 0.001 |
| SOC | −0.9965 | 0.0836 | 0.1142 | 0.018 |
| TN | −0.9283 | −0.3718 | 0.0472 | 0.195 |
| TP | 0.8339 | 0.5519 | 0.2767 | 0.001 |
| SWC | −0.9964 | −0.0847 | 0.3125 | 0.001 |
| AN | 0.2809 | −0.9597 | 0.0086 | 0.758 |
| AP | 0.9260 | −0.3775 | 0.0618 | 0.102 |
| EC | 0.9888 | −0.1493 | 0.2829 | 0.001 |
| C/N | −0.7462 | 0.6658 | 0.1684 | 0.001 |
| C/P | −0.8050 | −0.5932 | 0.1020 | 0.005 |
| N/P | −0.8142 | −0.5806 | 0.1014 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Wu, L.; Yang, Z.; Zhang, Z.; Feng, W.; Zheng, W.; Xu, C. The Patterns and Environmental Factors of Diversity, Co-Occurrence Networks, and Assembly Processes of Protistan Communities in Bulk Soils of Forests. Microorganisms 2025, 13, 1249. https://doi.org/10.3390/microorganisms13061249
Yang B, Wu L, Yang Z, Zhang Z, Feng W, Zheng W, Xu C. The Patterns and Environmental Factors of Diversity, Co-Occurrence Networks, and Assembly Processes of Protistan Communities in Bulk Soils of Forests. Microorganisms. 2025; 13(6):1249. https://doi.org/10.3390/microorganisms13061249
Chicago/Turabian StyleYang, Bing, Lin Wu, Zhisong Yang, Zhihe Zhang, Wanju Feng, Weichao Zheng, and Chi Xu. 2025. "The Patterns and Environmental Factors of Diversity, Co-Occurrence Networks, and Assembly Processes of Protistan Communities in Bulk Soils of Forests" Microorganisms 13, no. 6: 1249. https://doi.org/10.3390/microorganisms13061249
APA StyleYang, B., Wu, L., Yang, Z., Zhang, Z., Feng, W., Zheng, W., & Xu, C. (2025). The Patterns and Environmental Factors of Diversity, Co-Occurrence Networks, and Assembly Processes of Protistan Communities in Bulk Soils of Forests. Microorganisms, 13(6), 1249. https://doi.org/10.3390/microorganisms13061249






