Cinnamomum burmannii Essential Oil as a Promising Antimicrobial Agent Against Cutaneous Pathogens: Mechanistic Insights into Its Anti-Malassezia furfur Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strain and Culture Conditions
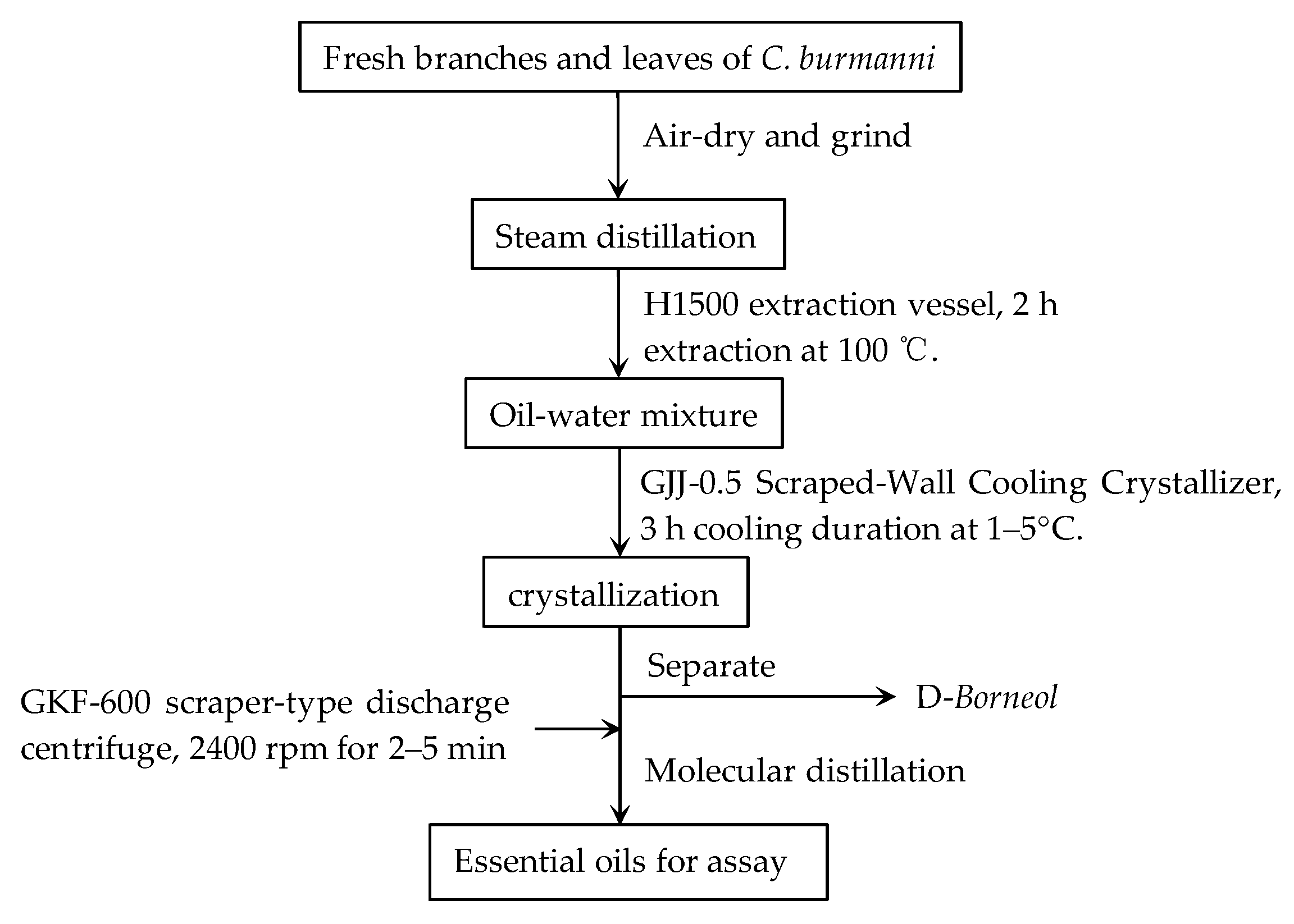
2.2. Preparation of Essential Oils Extract
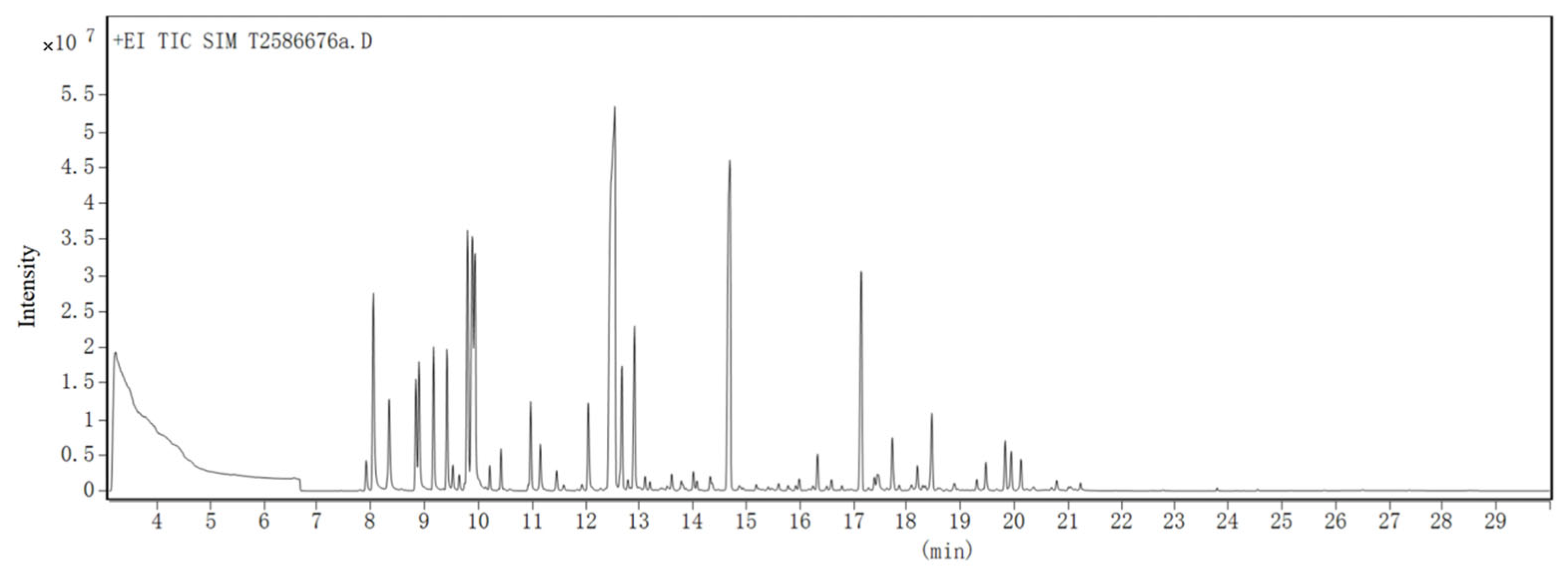
2.3. GC-MS Analysis of CBEO
2.4. Chemical Stability of CBEO
2.5. Antifungal Activity of CBEO
2.5.1. Determination of Minimum Inhibitory and Fungicidal Concentration Values
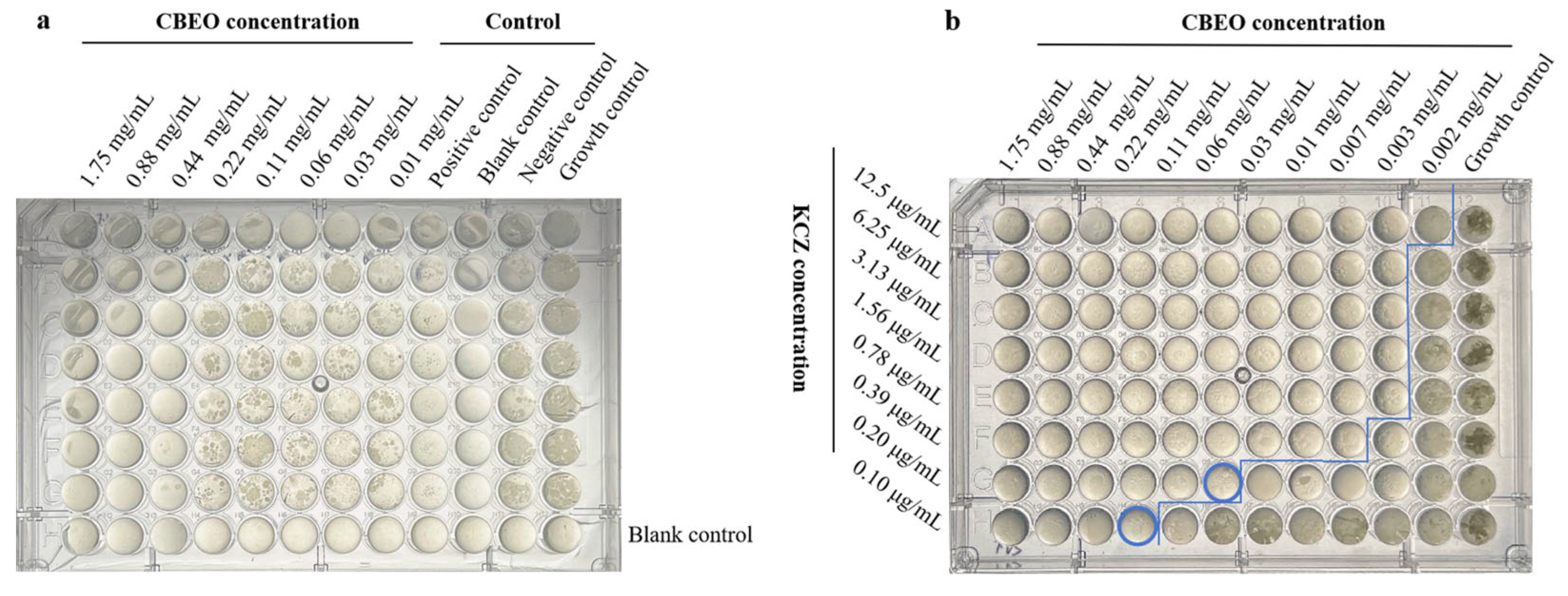
2.5.2. Checkerboard Assay
FICI = FICA + FICB
2.6. Time-Kill Kinetics Analysis of CBEO Against M. furfur
2.7. Biofilm Inhibition of CBEO Studies
2.8. Scanning Electron Microscopy (SEM)
2.9. Integrity of Cell Membrane Studies
2.10. Determination of Cellular Content Leakage
2.10.1. Leakage of Nucleotide and Protein
2.10.2. Leakage of Ion Measurement
2.11. Sorbitol Protection Assay
2.12. Ergosterol Binding Assay
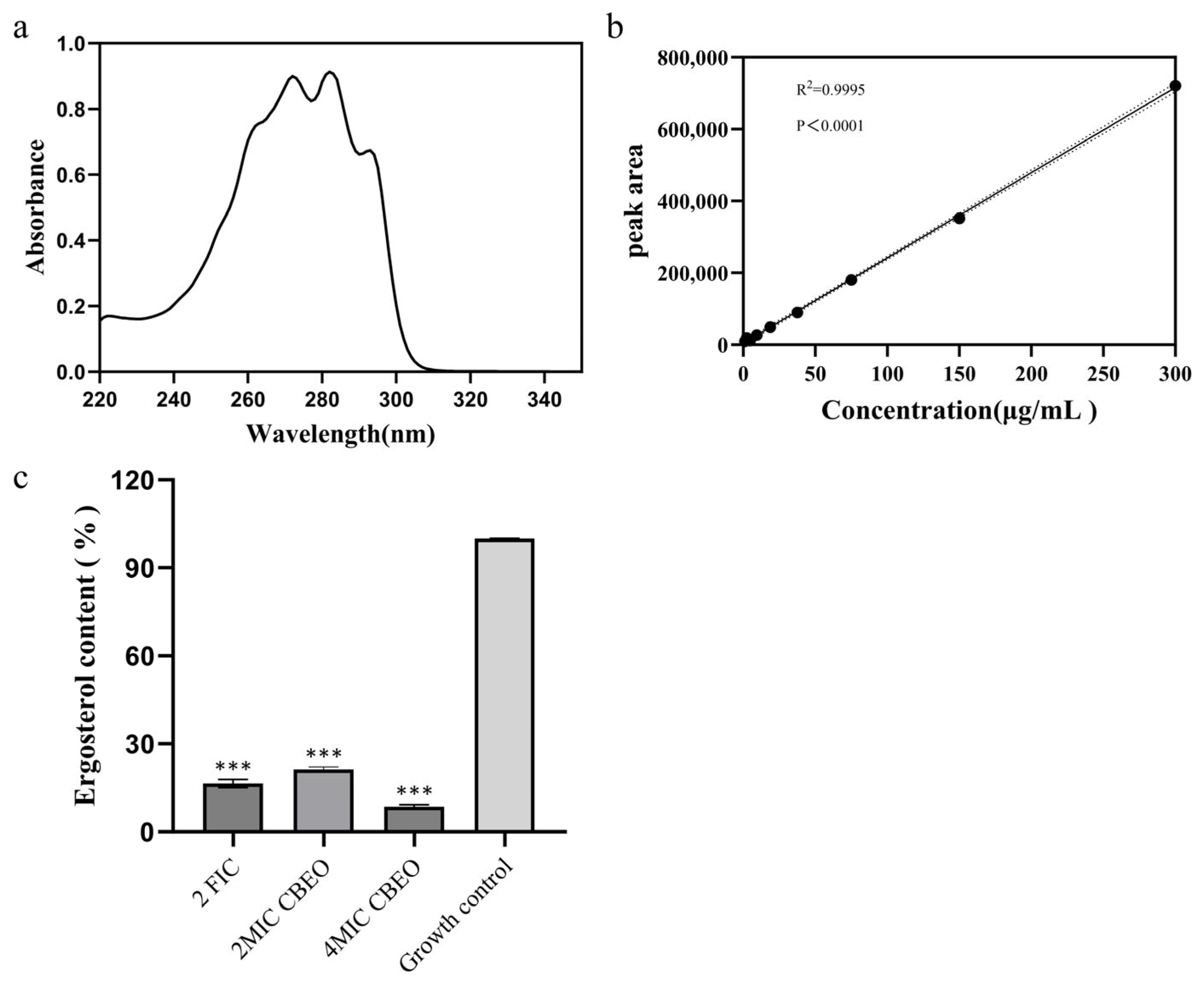
2.13. Determination of Ergosterol Content by Ultra-Performance Liquid Chromatography (UPLC)
2.13.1. Preparation of Standards and Determination of Wavelength
2.13.2. Sample Preparation for M. furfur
2.13.3. Instrumentation Conditions
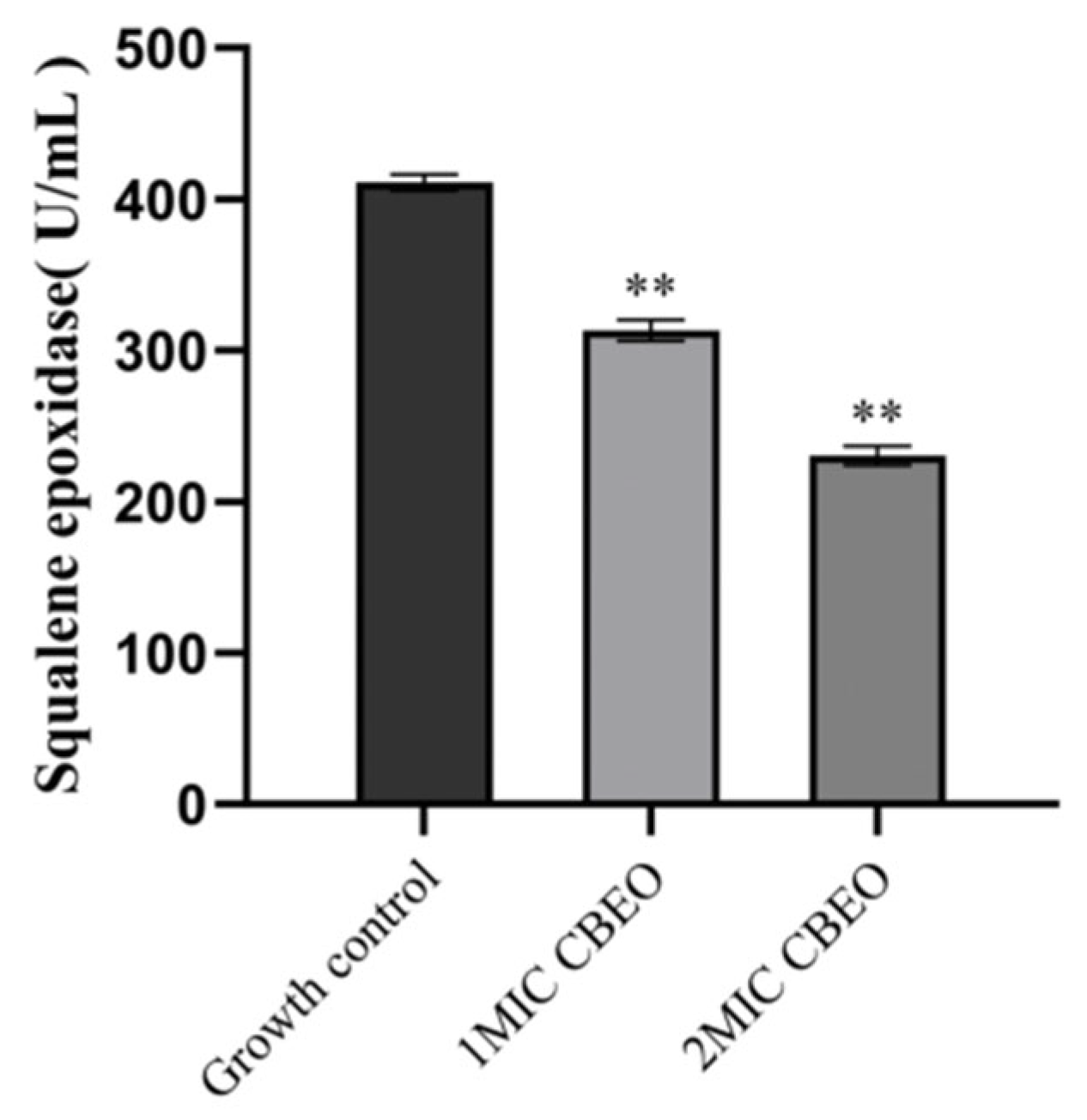
2.14. Assessment of Squalene Epoxidase (SE) Activity
2.15. Statistical Analysis
3. Results
3.1. Phytochemical Characterization by GC-MS Analysis
3.2. Thermal Stability Analysis of CBEO
3.3. Determination of MIC, MFC and FICI Values
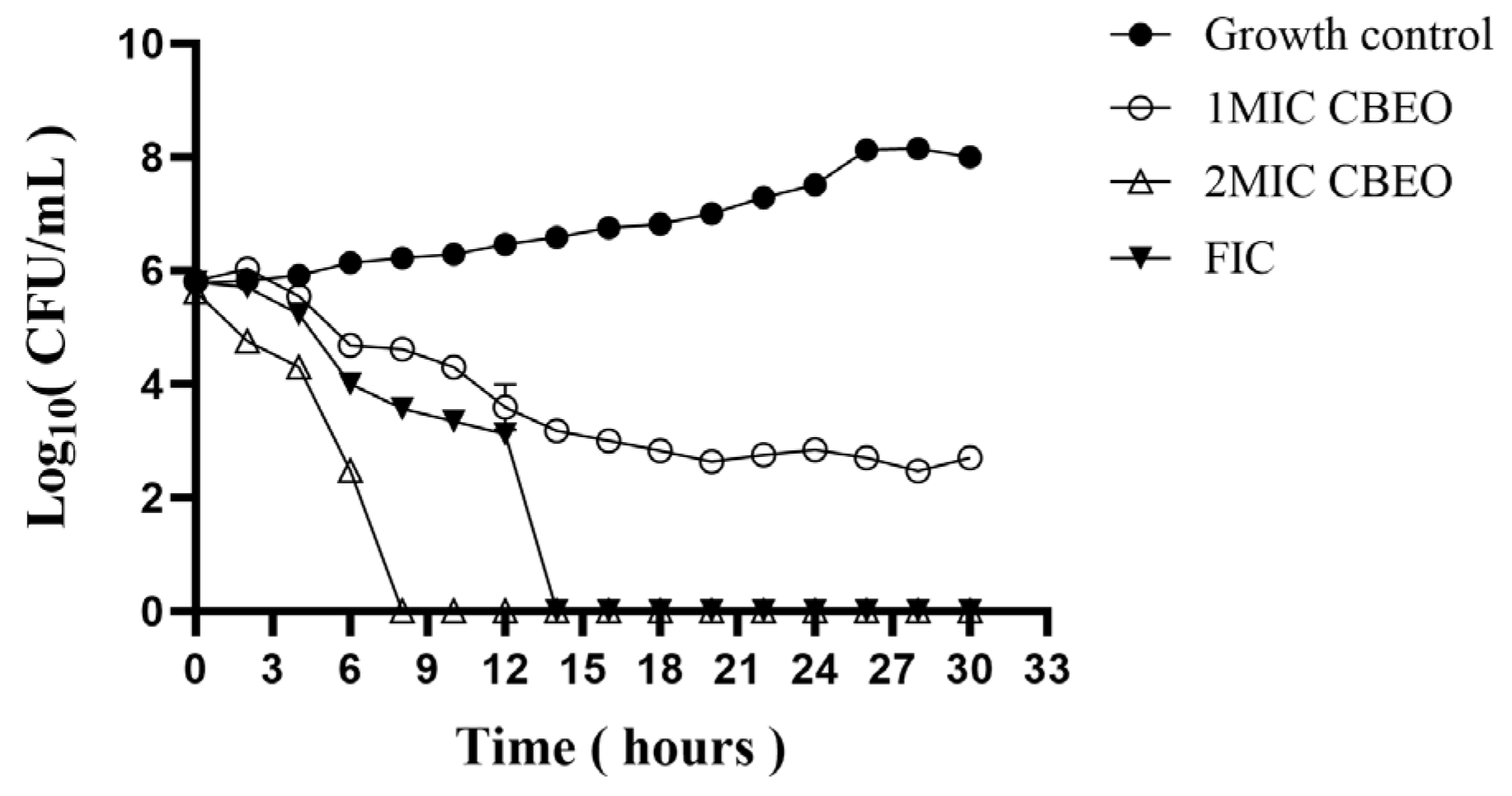
3.4. Time-Kill Kinetic Curve Analysis
3.5. Impact of CBEO on Biofilm Biomass of M. furfur
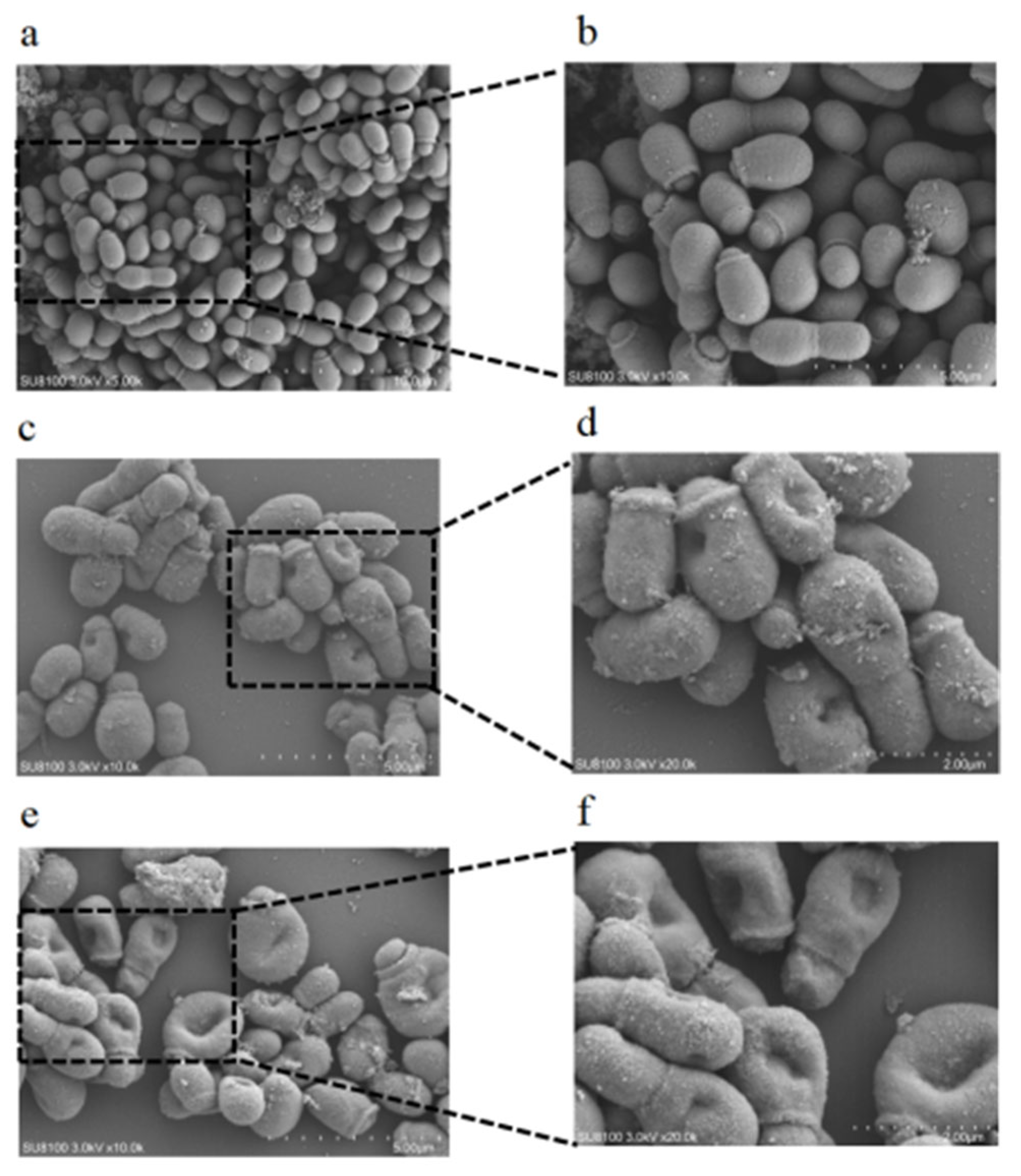
3.6. Scanning Electron Microscopy (SEM) Analysis
3.7. Permeability of Cell Membrane Studies
3.8. Membrane Integrity Analysis
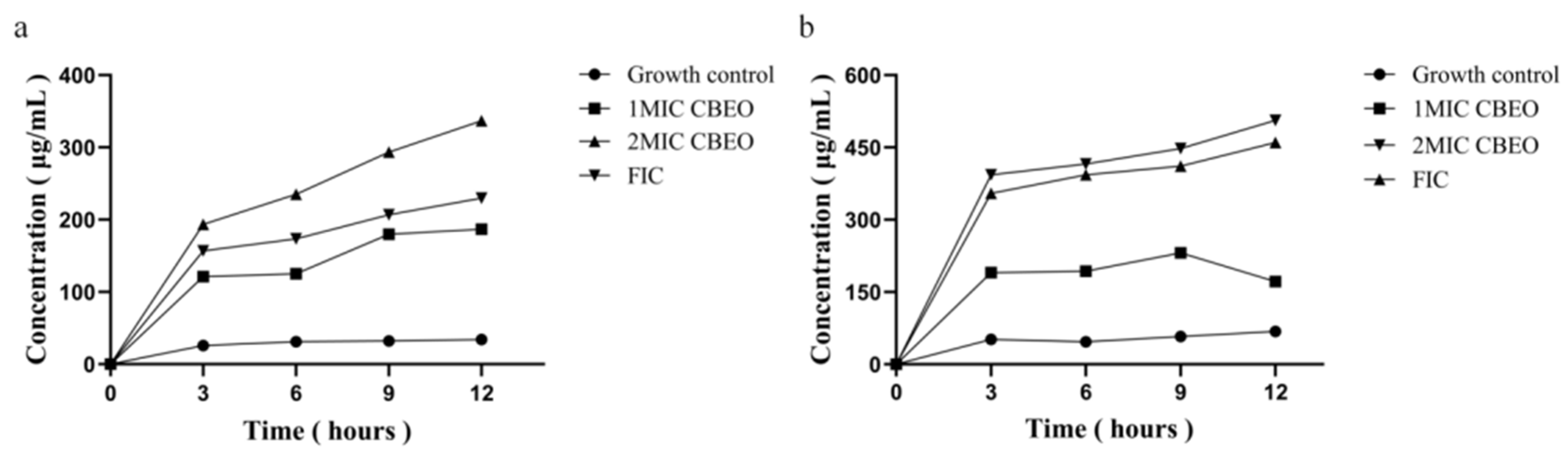
3.8.1. Analysis of Nucleic Acid and Protein Leakage
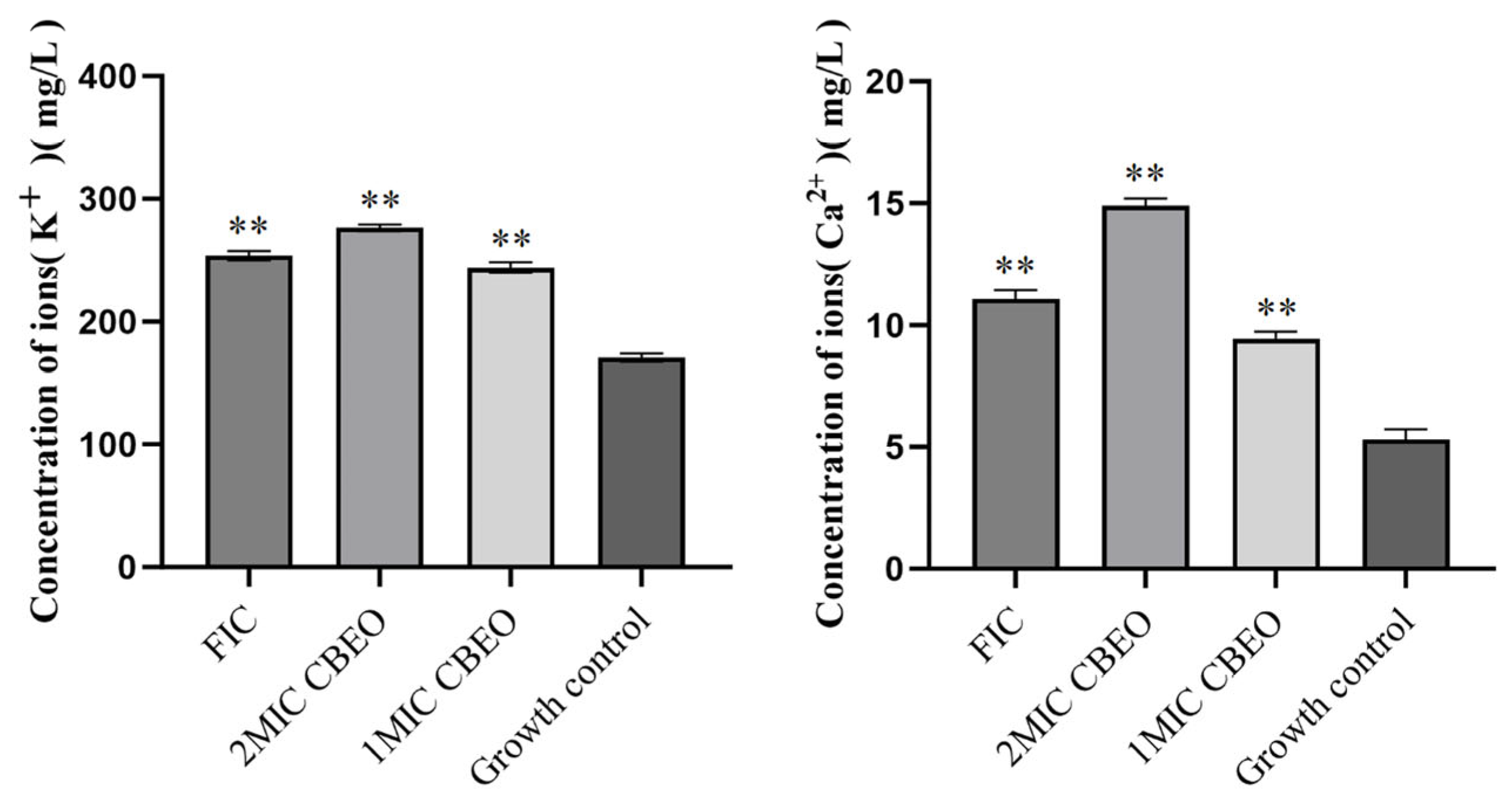
3.8.2. Quantification of Potassium and Calcium Ion Efflux
3.9. Effect of CBEO on the Cell Membrane/Wall Structure of M. furfur
3.10. Analytical Determination of Ergosterol Content
3.11. Analytical Determination of Squalene Epoxidase (SE) Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhao, H.; Li, C.; Rajapakse, M.P.; Wong, W.C.; Xu, J.; Saunders, C.W.; Reeder, N.L.; Reilman, R.A.; Scheynius, A.; et al. Genus-Wide Comparative Genomics of Malassezia Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin. PLoS Genet. 2015, 11, e1005614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Theelen, B.; Groenewald, M.; Bai, F.Y.; Boekhout, T. Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Persoonia 2014, 33, 41–47. [Google Scholar] [CrossRef]
- Naik, B.; Sasikumar, J.; Das, S.P. From Skin and Gut to the Brain: The Infectious Journey of the Human Commensal Fungus Malassezia and Its Neurological Consequences. Mol. Neurobiol. 2025, 62, 533–556. [Google Scholar] [CrossRef]
- Guglielmo, A.; Sechi, A.; Patrizi, A.; Gurioli, C.; Neri, I. Head and neck dermatitis, a subtype of atopic dermatitis induced by Malassezia spp.: Clinical aspects and treatment outcomes in adolescent and adult patients. Pediatr. Dermatol. 2021, 38, 109–114. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Mirbod, F.; Filler, S.G.; Banno, Y.; Cole, G.T.; Kitajima, Y.; Edwards, J.E., Jr.; Nozawa, Y.; Ghannoum, M.A. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect. Immun. 1995, 63, 1993–1998. [Google Scholar] [CrossRef]
- Chebil, W.; Rhimi, W.; Haouas, N.; Romano, V.; Belgacem, S.; Belhadj Ali, H.; Babba, H.; Cafarchia, C. Virulence factors of Malassezia strains isolated from pityriasis versicolor patients and healthy individuals. Med. Mycol. 2022, 60, myac060. [Google Scholar] [CrossRef]
- Ianiri, G.; LeibundGut-Landmann, S.; Dawson, T.L., Jr. Malassezia: A Commensal, Pathogen, and Mutualist of Human and Animal Skin. Annu. Rev. Microbiol. 2022, 76, 757–782. [Google Scholar] [CrossRef]
- Hamdino, M.; Saudy, A.A.; El-Shahed, L.H.; Taha, M. Identification of Malassezia species isolated from some Malassezia associated skin diseases. J. Mycol. Med. 2022, 32, 101301. [Google Scholar] [CrossRef]
- Borda, L.J.; Perper, M.; Keri, J.E. Treatment of seborrheic dermatitis: A comprehensive review. J. Dermatol. Treat. 2019, 30, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, M.; Zhou, L.; Lu, F.; Bie, X.; Lu, Z. Bacillomycin D effectively controls growth of Malassezia globosa by disrupting the cell membrane. Appl. Microbiol. Biotechnol. 2020, 104, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R. Zinc Pyrithione: A Topical Antimicrobial with Complex Pharmaceutics. J. Drugs Dermatol. 2016, 15, 140–144. [Google Scholar]
- Huang, X.; Welsh, R.M.; Deming, C.; Proctor, D.M.; Thomas, P.J.; NISC Comparative Sequencing Program; Gussin, G.M.; Huang, S.S.; Kong, H.H.; Bentz, M.L.; et al. Skin Metagenomic Sequence Analysis of Early Candida auris Outbreaks in U.S. Nursing Homes. mSphere 2021, 6, e0028721. [Google Scholar] [CrossRef]
- Liu, Y.T.; Lee, M.H.; Lin, Y.S.; Lai, W.L. The inhibitory activity of Citral against Malassezia furfur. Processes 2022, 10, 802. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Koga-Ito, C.Y. Effects of Acetone Fraction from Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors. Front. Microbiol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef]
- HelmyAbdou, K.A.; Ahmed, R.R.; Ibrahim, M.A.; Abdel-Gawad, D.R.I. The anti-inflammatory influence of Cinnamomum burmannii against multi-walled carbon nanotube-induced liver injury in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 36063–36072. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef]
- Shi, L.; Lin, W.; Cai, Y.; Chen, F.; Zhang, Q.; Liang, D.; He, B. Oxidative Stress-Mediated Repression of Virulence Gene Transcription and Biofilm Formation as Antibacterial Action of Cinnamomum burmannii Essential Oil on Staphylococcus aureus. Int. J. Mol. Sci. 2024, 25, 3078. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lv, F.; Xian, M.; Luo, C.; Zhang, L.; Yang, M.; Li, Q.; Zhao, X. Inhibition Mechanism of Cinnamomum burmannii Leaf Essential Oil Against Aspergillus flavus and Aflatoxins. Foods 2025, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Far, F.E.; Al-Obaidi, M.M.J.; Desa, M.N.M. Efficacy of modified Leeming-Notman media in a resazurin microtiter assay in the evaluation of in-vitro activity of fluconazole against Malassezia furfur ATCC 14521. J. Mycol. Med. 2018, 28, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Nayeri, D.; Asgarpanah, J.; Shams-Ghahfarokhi, M.; Seyedjavadi, S.S.; Saremi, G.; Eslamifar, A.; Razzaghi-Abyaneh, M. Antifungal activity, mechanistic insights, and combinatorial effects of Pycnocycla bashagardiana essential oil against Aspergillus fumigatus. S. Afr. J. Bot. 2025, 181, 272–280. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Chowdhary, A.; Dannaoui, E. In Vitro antifungal combination of terbinafine with itraconazole against isolates of Trichophyton species. Antimicrob. Agents Chemother. 2022, 66, e01449-21. [Google Scholar] [CrossRef]
- Blais, J.; Dean, C.R.; Lapointe, G.; Leeds, J.A.; Ma, S.; Morris, L.; Moser, H.E.; Osborne, C.S.; Prosen, K.R.; Richie, D.; et al. In Vitro and In Vivo Properties of CUO246, a Novel Bacterial DNA Gyrase/Topoisomerase IV Inhibitor. Antimicrob. Agents Chemother. 2022, 66, e0092122. [Google Scholar] [CrossRef]
- Aiemsaard, J.; Kamollerd, C.; Uopasai, S.; Singh, R.; Thongkham, E. Efficiency of clove essential oil against planktonic cells and biofilms of Malassezia pachydermatis isolated from canine dermatitis. Thai J. Vet. Med. 2019, 49, 415–420. [Google Scholar] [CrossRef]
- Liu, M.M.; Zhao, Y.J.; Boekhout, T.; Wang, Q.M. Exploring the antibiofilm efficacy of cinnamaldehyde against Malassezia globosa associated pityriasis versicolor. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 130, 155542. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.K.; Bai, Y.B.; Cao, M.Z.; Wang, W.W.; Shang, Z.X.; Li, J.J.; Xu, M.L.; Wu, L.F.; Zhu, Z.; et al. Antibacterial activity and mechanism of Stevia extract against antibiotic-resistant Escherichia coli by interfering with the permeability of the cell wall and the membrane. Front. Microbiol. 2024, 15, 1397906. [Google Scholar] [CrossRef]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.B.F.C.; de Oliveira Bento, A.; Lourenço, E.M.G.; Ferreira, M.R.A.; Oliveira, W.N.; Soares, L.A.L.; GBarbosa, E.; Rocha, H.A.O.; Chaves, G.M. Mechanism of action and synergistic effect of Eugenia uniflora extract in Candida spp. PLoS ONE 2024, 19, e0303878. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Wang, J.H.; Liu, X.; Kuang, H.C.; Huang, X.N. Determination of ergosterol in ganoderma spore lipid from the germinating spores of Ganoderma lucidum by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 6172–6176. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, H.; Poluri, K.M. Geraniol eradicates Candida glabrata biofilm by targeting multiple cellular pathways. Appl. Microbiol. Biotechnol. 2021, 105, 5589–5605. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Shen, Z.; Chen, Y.; Chen, C.; SiTu, Y.; Tang, C.; Jiang, T. Isoflavaspidic Acid PB Extracted from Dryopteris fragrans (L.) Schott Inhibits Trichophyton rubrum Growth via Membrane Permeability Alternation and Ergosterol Biosynthesis Disruption. BioMed Res. Int. 2022, 2022, 6230193. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O. European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, & European Confederation of Medical Mycology ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar]
- Iatta, R.; Cafarchia, C.; Cuna, T.; Montagna, O.; Laforgia, N.; Gentile, O.; Rizzo, A.; Boekhout, T.; Otranto, D.; Montagna, M.T. Bloodstream infections by Malassezia and Candida species in critical care patients. Med. Mycol. 2014, 52, 264–269. [Google Scholar] [CrossRef]
- Rojas, F.D.; Sosa, M.d.L.A.; Fernández, M.S.; Cattana, M.E.; Córdoba, S.B.; Giusiano, G.E. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med. Mycol. 2014, 52, 641–646. [Google Scholar] [CrossRef]
- Cafarchia, C.; Iatta, R.; Immediato, D.; Puttilli, M.R.; Otranto, D. Azole susceptibility of Malassezia pachydermatis and Malassezia furfur and tentative epidemiological cut-off values. Med. Mycol. 2015, 53, 743–748. [Google Scholar] [CrossRef]
- Guan, X.L.; Souza, C.M.; Pichler, H.; Dewhurst, G.; Schaad, O.; Kajiwara, K.; Wakabayashi, H.; Ivanova, T.; Castillon, G.A.; Piccolis, M.; et al. Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol. Biol. Cell 2009, 20, 2083–2095. [Google Scholar] [CrossRef]
- Wang, W.; Nie, Y.; Liu, X.Y.; Huang, B. The genome and transcriptome of Sarocladium terricola provide insight into ergosterol biosynthesis. Front. Cell. Infect. Microbiol. 2023, 13, 1181287. [Google Scholar] [CrossRef]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]



| Compounds | NIST_RI | CAS | Relative Content (%) |
|---|---|---|---|
| endo-Borneol | 1170 | 507-70-0 | 27.84 |
| Benzene, 1-methyl-3-(1-methylethyl)- | 1022 | 535-77-3 | 8.90 |
| .alpha.-Terpineol | 1196 | 98-55-5 | 7.23 |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | 1287 | 5655-61-8 | 7.16 |
| .alpha.-Pinene | 936 | 80-56-8 | 4.60 |
| D-Limonene | 1031 | 5989-27-5 | 3.59 |
| Dihydrocarvyl acetate | 1306 | 20,777-49-5 | 3.59 |
| .beta.-Ocimene | 1037 | 13,877-91-3 | 3.27 |
| .alpha.-Phellandrene | 1005 | 99-83-2 | 3.14 |
| 2-Pentanol | 699 | 6032-29-7 | 2.09 |
| Cedrene | 1422 | 11,028-42-5 | 1.73 |
| .beta.-Myrcene | 992 | 123-35-3 | 1.40 |
| Pentanoic acid, 2-hydroxy-3-methyl-, methyl ester | 989 | 41,654-19-7 | 1.38 |
| Eucalyptol | 1034 | 470-82-6 | 1.37 |
| Levomenthol | 1177 | 2216-51-5 | 1.37 |
| Nonane, 4,5-dimethyl- | 1046 | 17,302-23-7 | 1.35 |
| d-Menthol | 1167 | 15,356-60-2 | 1.32 |
| Pantolactone | 1037 | 599-04-2 | 1.16 |
| 4-Nonanone | 1030 | 4485-09-0 | 1.16 |
| 1H-Cyclopropa[a]naphthalene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,7,7a-tetramethyl-, [1aR-(1a.alpha.,7.alpha.,7a.alpha.,7b.alpha.)]- | 1432 | 17,334-55-3 | 0.87 |
| (3R,3aR,7R,8aS)-3,8,8-Trimethyl-6-methyleneoctahydro-1H-3a,7-methanoazulene | 1414 | 79,120-98-2 | 0.82 |
| Cyclohexene, 1-methyl-4-(1-methylethylidene)- | 1091 | 586-62-9 | 0.79 |
| Butanoic acid, 3-methyl-, 1-ethenyl-1,5-dimethyl-4-hexenyl ester | 1464 | 1118-27-0 | 0.77 |
| Butanoic acid, 3-methyl-3-nitroso-, methyl ester | 1019 | 49,680-44-6 | 0.58 |
| Camphor | 1151 | 76-22-2 | 0.54 |
| Geranyl formate | 1301 | 105-86-2 | 0.54 |
| cis-Chrysanthenol | 1162 | 55,722-60-6 | 0.51 |
| 1-Tridecene | 1292 | 2437-56-1 | 0.42 |
| 6-Undecanol | 1281 | 23,708-56-7 | 0.42 |
| Camphenone, 6- | 1095 | 55,659-42-2 | 0.38 |
| 5-Nonenal, (E)- | 1107 | 2277-18-1 | 0.38 |
| .beta.-Bisabolene | 1509 | 495-61-4 | 0.38 |
| Bicyclo[4.1.0]hept-2-ene, 3,7,7-trimethyl-, (1S-cis)- | 985 | 4497-92-1 | 0.37 |
| Camphene | 952 | 79-92-5 | 0.36 |
| Cyclopentanone, 2-methyl-3-(1-methylethyl)- | 1174 | 54,549-81-4 | 0.35 |
| Cyclohexanepropanoic acid, 2-propenyl ester | 1435 | 2705-87-5 | 0.33 |
| trans-.beta.-Ocimene | 1049 | 3779-61-1 | 0.31 |
| Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1.alpha.,2.beta.,5.alpha.)- | 1070 | 15,537-55-0 | 0.31 |
| 2H-2a,7-Methanoazuleno[5,6-b]oxirene, octahydro-3,6,6,7a-tetramethyl- | 1585 | 29,597-36-2 | 0.30 |
| Methacrylamide | 1149 | 79-39-0 | 0.30 |
| Citral | 1273 | 5392-40-5 | 0.26 |
| 2-Decanone | 1193 | 693-54-9 | 0.26 |
| 2H-Pyran-2-one, tetrahydro-6-propyl- | 1288 | 698-76-0 | 0.26 |
| Humulene | 1467 | 6753-98-6 | 0.25 |
| 7-Oxabicyclo[4.1.0]heptane, 1-methyl-4-(2-methyloxiranyl)- | 1294 | 96-08-2 | 0.25 |
| 5-Methylhexanoic acid | 1043 | 628-46-6 | 0.25 |
| 5-Octen-1-ol, (Z)- | 1074 | 64,275-73-6 | 0.25 |
| N,N′-Methylenebismethacrylamide | 1566 | 2359-15-1 | 0.23 |
| Isobornyl formate | 1233 | 1200-67-5 | 0.23 |
| Acetic acid, cinnamyl ester | 1446 | 103-54-8 | 0.21 |
| Nerolidol | 1544 | 142-50-7 | 0.21 |
| Bicyclo[3.1.1]hept-3-en-2-one, 4,6,6-trimethyl-, (1S)- | 1204 | 1196-01-6 | 0.20 |
| .beta.-Pinene | 980 | 127-91-3 | 0.20 |
| 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene-, [1ar-(1a.alpha.,4a.alpha.,7.beta.,7a.beta.,7b.alpha.)]- | 1576 | 6750-60-3 | 0.18 |
| Geranyl acetate | 1384 | 105-87-3 | 0.18 |
| Cinnamaldehyde, (E)- | 1270 | 14,371-10-9 | 0.18 |
| Niacinamide | 1419 | 98-92-0 | 0.17 |
| Aromandendrene | 1440 | 489-39-4 | 0.17 |
| p-Mentha-1,8-dien-7-ol | 1297 | 536-59-4 | 0.16 |
| D-Fenchone | 1103 | 4695-62-9 | 0.15 |
| 3,3-Dimethyl-6-methylenecyclohexene | 1001 | 20,185-16-4 | 0.15 |
| .gamma.-Elemene | 1433 | 29,873-99-2 | 0.15 |
| Alloaromadendrene | 1461 | 25,246-27-9 | 0.15 |
| Cedrol | 1600 | 77-53-2 | 0.13 |
| Succinic anhydride | 1023 | 108-30-5 | 0.13 |
| 3-Buten-2-ol, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 1428 | 22,029-76-1 | 0.13 |
| 2-Ethylhexyl methacrylate | 1296 | 688-84-6 | 0.12 |
| 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | 1495 | 495-60-3 | 0.11 |
| 1-Butanamine, 3-methyl-N-(3-methylbutylidene)- | 1047 | 35,448-31-8 | 0.11 |
| .beta.-Guaiene | 1490 | 88-84-6 | 0.11 |
| 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | 1147 | 1125-21-9 | 0.10 |
| Thymol | 1291 | 89-83-8 | 0.10 |
| 4aH-Cycloprop[e]azulen-4a-ol, decahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,4.beta.,4a.beta.,7.alpha.,7a.beta.,7b.alpha.)]- | 1568 | 5986-49-2 | 0.10 |
| (+)-4-Carene | 1009 | 29,050-33-7 | 0.10 |
| Eugenol | 1362 | 97-53-0 | 0.10 |
| δ-cadinol | 1610 | 36,564-42-8 | 0.10 |
| Salvial-4(14)-en-1-one | 1595 | 73,809-82-2 | 0.09 |
| 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate, (Z)- | 1365 | 141-12-8 | 0.09 |
| Total | 100 |
| Strain | Concentration of CBEO | Positive Control | Negative Control | Growth Control | Blank Control | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1.75 | 0.88 | 0.44 | 0.22 | 0.11 | |||||
| M. furfur ATCC44344 | − | − | + | + | + | − | + | + | − |
| ) | |||||
|---|---|---|---|---|---|
| Growth Control | 1/2 × MIC CBEO | 1 × MIC CBEO | 2 × MIC CBEO | 4 × MIC CBEO | 8 × MIC CBEO |
| 1.00 | 0.68 ± 0.15 | 0.52 ± 0.07 | 0.13 ± 0.04 | 0.04 | 0.03 |
| Groups | Effect of CBEO on MIC Value of M. furfur (mg/mL) | |||
|---|---|---|---|---|
| Sorbitol (0.8 M) | Ergosterol | |||
| − | + | − | + | |
| CBEO | 0.875 | 0.875 | 0.875 | 7.000 ** |
| AMB | − | − | 0.008 | 0.128 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Cai, S.; Wang, Y.; Tan, Y.; Xu, J.; Xiong, P. Cinnamomum burmannii Essential Oil as a Promising Antimicrobial Agent Against Cutaneous Pathogens: Mechanistic Insights into Its Anti-Malassezia furfur Activity. Microorganisms 2025, 13, 1241. https://doi.org/10.3390/microorganisms13061241
Wang W, Cai S, Wang Y, Tan Y, Xu J, Xiong P. Cinnamomum burmannii Essential Oil as a Promising Antimicrobial Agent Against Cutaneous Pathogens: Mechanistic Insights into Its Anti-Malassezia furfur Activity. Microorganisms. 2025; 13(6):1241. https://doi.org/10.3390/microorganisms13061241
Chicago/Turabian StyleWang, Wenwen, Shuizhu Cai, Ying Wang, Yanhui Tan, Jing Xu, and Ping Xiong. 2025. "Cinnamomum burmannii Essential Oil as a Promising Antimicrobial Agent Against Cutaneous Pathogens: Mechanistic Insights into Its Anti-Malassezia furfur Activity" Microorganisms 13, no. 6: 1241. https://doi.org/10.3390/microorganisms13061241
APA StyleWang, W., Cai, S., Wang, Y., Tan, Y., Xu, J., & Xiong, P. (2025). Cinnamomum burmannii Essential Oil as a Promising Antimicrobial Agent Against Cutaneous Pathogens: Mechanistic Insights into Its Anti-Malassezia furfur Activity. Microorganisms, 13(6), 1241. https://doi.org/10.3390/microorganisms13061241





