Can Vaginal Seeding at Birth Improve Health Outcomes of Cesarean Section-Delivered Infants? A Scoping Review
Abstract
1. Background
2. Importance of Infant Microbiome Development in Immune and Metabolic Education and Regulation
2.1. The Impact of the Developing Gut Microbiome on Immune System Education
2.2. Microbiome Impact on Metabolic Regulation
3. C-Section Delivery Disruption of Infant Microbiome Colonization
4. C-Section-Associated Adverse Health Outcomes
4.1. C-Section Association with Obesity and Metabolic Regulation
4.2. C-Section Association with Immune Disorders
4.3. C-Section Association with Neurodevelopmental Disorders
4.4. Rationale for Restoring the Microbiome of C-Section Infants by Vaginal Seeding
5. Objectives
6. Methods
7. Evidence of the Effect of Vaginal Seeding and Other Maternal–Infant Microbial Seeding Interventions on the Infant Microbiome
| Author Location Date Published | Title | Study Patients | Sample Collection | Sequencing Type | Diversity | Enriched Bacteria in Seeded Infants | Reduced Bacteria in Seeded Infants | Key Finding and Contributions |
|---|---|---|---|---|---|---|---|---|
| Dominguez-Bello MG et al. USA, Puerto Rico March 2016 | Partial restoration of the microbiota of Cesarean-born infants via vaginal microbial transfer [10] | 4 VS 7 CS 7 VD | Birth, 3 d, 7 d, 14 d, 21 d, 30 d (oral, skin, and anal swabs) | 16S RNA | No difference between groups | Anal: Lactobacillus (7 d), Bacteroides (14 d) Skin: Lactobacillus (overall), Bacteroidales family S24–7 (overall) | Not Reported | -VS infant microbiome more similar to VD than CS |
| Mueller NT et al. USA June 2023 | Maternal Bacterial Engraftment in Multiple Body Sites of Cesarean Section Born Neonates after Vaginal Seeding—a Randomized Controlled Trial [100] | 10 VS 10 placebo-CS | 1 d (stool and skin), 30 d (stool) | 16S RNA | α: VS reduced diversity in stool (1 d and 30 d) and skin (1 d) β: significant variance in stool and skin driven by treatment (1 d) | Gut: Aeromonas (1 d), Stenotrophomonas (30 d), Ruminobacter (30 d) Skin: Lactobacillus, Parvimonas, Ruminococcaceae (1 d) | Gut: Enterobacter (1 d), Enterobacteriaceae (1 d), Clostridium sensu stricto (30 d) Skin: Streptobacillus, Lactococcus, Acidibacter (1 d) | -First double-blinded, randomized, placebo-controlled trial of VS |
| Song SJ et al. USA, Chile, Bolivia, Spain August 2021 | Naturalization of the microbiota developmental trajectory of Cesarean-born babys after vaginal seeding [101] | 30 VS 49 CS 98 VD | Birth, 1 d, 2 d, 7 d, 14 d, 21 d, and monthly up to 1 yr (oral, skin, and stool swabs) | 16S RNA | α: no difference β: significant difference between groups | Gut: Bacteroides, Streptococcus, Clostridium (up to 6 m) Skin: Streptococcus, Neisseria, Thermus, Neisseriaceae (up to 6 m) Oral: Gemellaceae, Haemophilus, and Streptococcus (up to 6 m) | VS not effective in reducing CS-associated taxa | -VS developed more similarly to VD for gut and skin microbiome, not oral -VS microbiome was more stable than CS during first year of life |
| Zhou L et al. China July 2023 | Effects of vaginal microbiota transfer on the neurodevelopment and microbiome of Cesarean born infants: A blinded randomized controlled trial [104] | 32 VS 36 placebo-CS 33 VD | 3 d, 7 d, 30 d, 6 wk (stool) | 16S RNA | β: VS was significantly different from placebo-CS group but not VD group (42 d) | Gut: Lactobacillus (3 d to 6 wk) | Gut: Klebsiella (decreasing trajectory at 30 d and 6 wk), Bifidobacterium (increasing trajectory, after 7 d), Escherichia (increasing trajectory, after 30 d) | -VS accelerated gut microbiome maturation compared to placebo |
| Wilson BC et al. New Zealand June 2021 | Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial [106] | 12 oral-VS 13 placebo-CS 22 VD | Birth, 1 m, 3 m (stool) | Shotgun Metagenomic Sequencing1 | No significant difference between oral-VS and placebo-CS groups | No significant difference between oral-VS and placebo-CS groups | No significant difference between oral-VS and placebo-CS groups | -Maternal vaginal strain transmission was present in 4/12 oral-VS at 1 m and only 1/12 at 3 m -Oral-VS ineffective at influencing early life microbiome |
| Korpela K et al. Finland October 2020 | Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study [108] | 7 FMT 18 CS 29 VD | Birth, 2 d, 7 d, 14 d, 21 d, 28 d, 3 m (stool) | 16S RNA | No significant difference between groups | Gut: Bacteroides (up to 21 d), Bifidobacteriales (21 d to 3 m) | Gut: potentially pathogens including Enterococcus faecium, Enterococcus faecalis, Klebsiella pneumoniae, Salmonella enterica (7 d, 3 m) | -FMT gut microbiome was more similar to VD -FMT overcame lack of Bacteroides and delayed Bifidobacteria characteristic of CS |
| Liu Y et al. China January 2023 | Effects of vaginal seeding on gut microbiota, body mass index, and allergy risks in infants born through Cesarean delivery: a randomized clinical trial [105] | 57 VS 60 placebo-CS | Birth, 6 m, 12 m, 18 m, 24 m (stool) | 16S RNA | No significant difference between groups | Gut: Lactobacillus, Bacteroides (not significant trend, birth and 6 m) | No significant difference between groups | -Gut microbiome composition did not vary significantly between VS and CS infants |
8. Evidence of the Effect of Vaginal Seeding and Other Maternal–Infant Microbial Seeding Interventions on the Infant Microbiome
9. Safety and Challenges
10. Regulatory and Ethical Issues Around Vaginal Seeding
11. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C-section | Cesarean section |
| TNF | Tumor necrosis factor |
| AMP | Anti-microbial peptide |
| SCFA | Short chain fatty acid |
| Th | T-helper |
| GF | Germ-free |
| IgG | Immunoglobulin G |
| IL | Interleukin |
| IFN | Interferon |
| LPS | Lipopolysaccharide |
| BMI | Body mass index |
| RCT | Randomized controlled trial |
| ASQ-3 | Ages and Stages Questionnaire |
| ACOG | American College of Obstetricians and Gynecologists |
| IRB | Institutional review board |
| STI | Sexually transmitted infection |
| HSV | Herpes Simplex Virus |
| FDA | US Food and Drug Administration |
| IND | Investigation New Drug Application |
| FMT | Fecal microbiota transplantation |
References
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; van Beveren, G.J.; de Koff, E.M.; Lusarreta Parga, P.; Balcazar Lopez, C.E.; Koppensteiner, L.; Clerc, M.; Hasrat, R.; Arp, K.; Chu, M.L.J.N.; et al. Mother-to-infant microbiota transmission and infant microbiota development across multiple body sites. Cell Host Microbe 2023, 31, 447–460. [Google Scholar] [CrossRef]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 197–205. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Derilus, D.; Godoy-Vitorino, F.; Rosado, H.; Agosto, E.; Dominguez-Bello, M.G.; Cavallin, H. An in-depth survey of the microbial landscape of the walls of a neonatal operating room. PLoS ONE 2020, 15, e0230957. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Butler, É.M.; Reynolds, A.J.; Derraik, J.G.B.; Wilson, B.C.; Cutfield, W.S.; Grigg, C.P. The views of pregnant women in New Zealand on vaginal seeding: A mixed-methods study. BMC Pregnancy Childbirth 2021, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Zarei, I.; Koistinen, V.M.; Kokla, M.; Klåvus, A.; Babu, A.F.; Lehtonen, M.; Auriola, S.; Hanhineva, K. Tissue-wide metabolomics reveals wide impact of gut microbiota on mice metabolite composition. Sci. Rep. 2022, 12, 15018. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- El Aidy, S.; Hooiveld, G.; Tremaroli, V.; Bäckhed, F.; Kleerebezem, M. The gut microbiota and mucosal homeostasis. Gut Microbes 2013, 4, 118–124. [Google Scholar] [CrossRef]
- Köhler, A.; Delbauve, S.; Smout, J.; Torres, D.; Flamand, V. Very early-life exposure to microbiota-induced TNF drives the maturation of neonatal pre-cDC1. Gut 2021, 70, 511–521. [Google Scholar] [CrossRef]
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front. Immunol. 2021, 12, 683022. [Google Scholar] [CrossRef]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Torow, N.; Hornef, M.W. The Neonatal Window of Opportunity: Setting the Stage for Life-Long Host-Microbial Interaction and Immune Homeostasis. J. Immunol. 2017, 198, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Sakamoto, K.; Zeng, M.Y.; Wang, Y.; Hakim, J.; Matus-Acuña, V.; Inohara, N.; Núñez, G. Maternal Immunization Confers Protection to the Offspring against an Attaching and Effacing Pathogen through Delivery of IgG in Breast Milk. Cell Host Microbe 2019, 25, 313–323. [Google Scholar] [CrossRef]
- Wood, H.; Acharjee, A.; Pearce, H.; Quraishi, M.N.; Powell, R.; Rossiter, A.; Beggs, A.; Ewer, A.; Moss, P.; Toldi, G. Breastfeeding promotes early neonatal regulatory T-cell expansion and immune tolerance of non-inherited maternal antigens. Allergy 2021, 76, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chai, S.; Wen, Q.; Wang, S.; Zhan, S. Risk of type 2 diabetes and long-term antibiotic use in childhood: Evidence from the UK Biobank. Diabetes Res. Clin. Pract. 2024, 209, 111571. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Bauer, H.; Horowitz, R.E.; Levenson, S.M.; Popper, H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am. J. Pathol. 1963, 42, 471–483. [Google Scholar]
- Gordon, H.A.; Bruckner-Kardoss, E.; Staley, T.E.; Wagner, M.; Wostmann, B.S. Characteristics of the germfree rat. Cells Tissues Organs 1966, 64, 367–389. [Google Scholar] [CrossRef]
- Constantinides, M.G.; Link, V.M.; Tamoutounour, S.; Wong, A.C.; Perez-Chaparro, P.J.; Han, S.J.; Chen, Y.E.; Li, K.; Farhat, S.; Weckel, A.; et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366, eaax6624. [Google Scholar] [CrossRef]
- Risnes, K.R.; Belanger, K.; Murk, W.; Bracken, M.B. Antibiotic Exposure by 6 Months and Asthma and Allergy at 6 Years: Findings in a Cohort of 1401 US Children. Am. J. Epidemiol. 2011, 173, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.-M.; Kull, I.; Wickman, M.; Bergström, A. Antibiotic use in early life and development of allergic diseases: Respiratory infection as the explanation. Clin. Exp. Allergy 2010, 40, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Borbet, T.C.; Pawline, M.B.; Zhang, X.; Wipperman, M.F.; Reuter, S.; Maher, T.; Li, J.; Iizumi, T.; Gao, Z.; Daniele, M.; et al. Influence of the early-life gut microbiota on the immune responses to an inhaled allergen. Mucosal Immunol. 2022, 15, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association Between the Use of Antibiotics in the First Year of Life and Pediatric Inflammatory Bowel Disease. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 2687–2692. [Google Scholar] [CrossRef]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic Exposure and IBD Development Among Children: A Population-Based Cohort Study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef]
- Mark-Christensen, A.; Lange, A.; Erichsen, R.; Frøslev, T.; Esen, B.; Sørensen, H.T.; Kappelman, M.D. Early-Life Exposure to Antibiotics and Risk for Crohn’s Disease: A Nationwide Danish Birth Cohort Study. Inflamm. Bowel Dis. 2022, 28, 415–422. [Google Scholar] [CrossRef]
- Ozkul, C.; Ruiz, V.E.; Battaglia, T.; Xu, J.; Roubaud-Baudron, C.; Cadwell, K.; Perez-Perez, G.I.; Blaser, M.J. A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis. Genome Med. 2020, 12, 65. [Google Scholar] [CrossRef]
- Depner, M.; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; Lauener, R.; et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef]
- Gao, Y.; Stokholm, J.; O’Hely, M.; Ponsonby, A.L.; Tang, M.L.K.; Ranganathan, S.; Saffery, R.; Harrison, L.C.; Collier, F.; Gray, L.; et al. Gut microbiota maturity mediates the protective effect of siblings on food allergy. J. Allergy Clin. Immunol. 2023, 152, 667–675. [Google Scholar] [CrossRef]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Rasmussen, S.H.; Shrestha, S.; Bjerregaard, L.G.; Ängquist, L.H.; Baker, J.L.; Jess, T.; Allin, K.H. Antibiotic exposure in early life and childhood overweight and obesity: A systematic review and meta-analysis. Diabetes Obes. Metab. 2018, 20, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Ding, X.; Wang, B.; Li, L.; An, X.; Yao, Q.; Song, R.; Zhang, J.A. Antibiotic Exposure in Early Life Increases Risk of Childhood Obesity: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2017, 8, 170. [Google Scholar] [CrossRef]
- Miller, S.A.; Wu, R.K.S.; Oremus, M. The association between antibiotic use in infancy and childhood overweight or obesity: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Osterman, M.; Hamilton, B.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020; National Vital Statistics Reports; National Center for Health Statistics: Hyattsville, MA, USA, 2021; Volume 70, pp. 1–50.
- Sengupta, A.; Sabastin Sagayam, M.; Reja, T. Increasing trend of C-section deliveries in India: A comparative analysis between southern states and rest of India. Sex. Reprod. Healthc. 2021, 28, 100608. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, S.; Silitonga, P.I.I.; Febriani, E.; Long, Q. Socioeconomic, geographic and health system factors associated with rising C-section rate in Indonesia: A cross-sectional study using the Indonesian demographic and health surveys from 1998 to 2017. BMJ Open 2021, 11, e045592. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Wong, W.S.W.; Sabu, P.; Deopujari, V.; Levy, S.; Shah, A.A.; Clemency, N.; Provenzano, M.; Saadoon, R.; Munagala, A.; Baker, R.; et al. Prenatal and Peripartum Exposure to Antibiotics and Cesarean Section Delivery Are Associated with Differences in Diversity and Composition of the Infant Meconium Microbiome. Microorganisms 2020, 8, 179. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898. [Google Scholar] [CrossRef]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Romero-Soto, H.N.; Stern, D.B.; Maxwell, G.L.; Levy, S.; Hourigan, S.K. Delivery Mode Impacts Gut Bacteriophage Colonization During Infancy. Gut Microbes Rep. 2025, 2, 2464631. [Google Scholar] [CrossRef]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.-A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Salminen, S.; Gibson, G.R.; McCartney, A.L.; Isolauri, E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut 2004, 53, 1388–1389. [Google Scholar] [CrossRef]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat.Commun. 2018, 9, 4169. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Zhu, B.; Liu, F.; Qin, S.; Lv, N.; Feng, Y.; Wang, S.; Yang, H. A health-promoting role of exclusive breastfeeding on infants through restoring delivery mode-induced gut microbiota perturbations. Front. Microbiol. 2023, 14, 1163269. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhou, Q.; Li, M.; Zhou, L.; Xu, L.; Zhang, Y.; Li, D.; Wang, Y.; Dai, W.; Li, S.; et al. Breastfeeding restored the gut microbiota in caesarean section infants and lowered the infection risk in early life. BMC Pediatr. 2020, 20, 532. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Dominguez-Bello, M.G. Microbial seeding in early life. Cell Host Microbe 2023, 31, 331–333. [Google Scholar] [CrossRef]
- Lai, C.; Huang, L.; Wang, Y.; Huang, C.; Luo, Y.; Qin, X.; Zeng, J. Effect of different delivery modes on intestinal microbiota and immune function of neonates. Sci. Rep. 2024, 14, 17452. [Google Scholar] [CrossRef]
- Wampach, L.; Heintz-Buschart, A.; Fritz, J.V.; Ramiro-Garcia, J.; Habier, J.; Herold, M.; Narayanasamy, S.; Kaysen, A.; Hogan, A.H.; Bindl, L.; et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 2018, 9, 5091. [Google Scholar] [CrossRef] [PubMed]
- De Koff, E.M.; van Baarle, D.; van Houten, M.A.; Reyman, M.; Berbers, G.A.M.; van den Ham, F.; Chu, M.L.J.N.; Sanders, E.A.M.; Bogaert, D.; Fuentes, S. Mode of delivery modulates the intestinal microbiota and impacts the response to vaccination. Nat. Commun. 2022, 13, 6638. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Wu, L.; Luo, J.; Liang, X.; Xiao, B.; Zhu, Y. The impacts of delivery mode on infant’s oral microflora. Sci. Rep. 2018, 8, 11938. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.P.; O’Laughlin, B.; Kumar, P.S.; Dabdoub, S.M.; Levy, S.; Myles, I.A.; Hourigan, S.K. Vaginal delivery provides skin colonization resistance from environmental microbes in the NICU. Clin. Transl. Med. 2023, 13, e1506. [Google Scholar] [CrossRef]
- Vu, K.; Lou, W.; Tun, H.M.; Konya, T.B.; Morales-Lizcano, N.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Mandal, R.; Wishart, D.S.; et al. From Birth to Overweight and Atopic Disease: Multiple and Common Pathways of the Infant Gut Microbiome. Gastroenterology 2021, 160, 128–144. [Google Scholar] [CrossRef]
- Darmasseelane, K.; Hyde, M.J.; Santhakumaran, S.; Gale, C.; Modi, N. Mode of Delivery and Offspring Body Mass Index, Overweight and Obesity in Adult Life: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87896. [Google Scholar] [CrossRef]
- Kuhle, S.; Tong, O.S.; Woolcott, C.G. Association between caesarean section and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 295–303. [Google Scholar] [CrossRef]
- Li, H.T.; Zhou, Y.B.; Liu, J.M. The impact of cesarean section on offspring overweight and obesity: A systematic review and meta-analysis. Int. J. Obes. 2013, 37, 893–899. [Google Scholar] [CrossRef]
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association Between Cesarean Birth and Risk of Obesity in Offspring in Childhood, Adolescence, and Early Adulthood. JAMA Pediatr. 2016, 170, e162385. [Google Scholar] [CrossRef]
- Bridgman, S.L.; Penfold, S.; Field, C.J.; Haqq, A.M.; Mandhane, P.J.; Moraes, T.J.; Turvey, S.E.; Simons, E.; Subbarao, P.; Kozyrskyj, A.L. Pre-labor and post-labor cesarean delivery and early childhood adiposity in the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study. Int. J. Obes. 2024, 48, 717–724. [Google Scholar] [CrossRef]
- Bouhanick, B.; Ehlinger, V.; Delpierre, C.; Chamontin, B.; Lang, T.; Kelly-Irving, M. Mode of delivery at birth and the metabolic syndrome in midlife: The role of the birth environment in a prospective birth cohort study. BMJ Open 2014, 4, e005031. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Martín-Calvo, N.; Yuan, C.; Arvizu, M.; Rich-Edwards, J.W.; Michels, K.B.; Sun, Q. Association of Birth by Cesarean Delivery with Obesity and Type 2 Diabetes Among Adult Women. JAMA Netw. Open 2020, 3, e202605. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Rifas-Shiman, S.L.; Mitchell, C.; Sordillo, J.; Aris, I.M.; Hivert, M.-F.; Oken, E.; Chavarro, J.E. Cesarean delivery and metabolic health and inflammation biomarkers during mid-childhood and early adolescence. Pediatr. Res. 2022, 91, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Park, Y.M.; Lee, S.Y.; Lee, S.H.; Park, M.J.; Ahn, K.; Kim, K.W.; Shin, Y.H.; Suh, D.I.; Hong, S.J. Associations of prenatal antibiotic exposure and delivery mode on childhood asthma inception. Ann. Allergy Asthma Immunol. 2023, 131, 52–58. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, L.; Liu, X.; Chen, J.; Zhou, Y.; Zhang, J.; Su, H.; Yang, Y.; Tian, M.; Li, J.; et al. Cesarean section and the risk of allergic rhinitis in children: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 18361. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef]
- Mubanga, M.; Lundholm, C.; Rohlin, E.S.; Rejnö, G.; Brew, B.K.; Almqvist, C. Mode of delivery and offspring atopic dermatitis in a Swedish nationwide study. Pediatr. Allergy Immunol. 2023, 34, e13904. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhou, L.; Chen, Y.; Wang, J.; Zhao, L.; Li, M.; Chen, I.; Krewski, D.; Wen, S.W.; Xie, R.H. Prevalence of eczema between cesarean-born and vaginal-born infants within 1 year of age: A systematic review and meta-analysis. Eur. J. Pediatr. 2022, 181, 2237–2247. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, C.; Guo, C.; Wang, J.; Chen, I.; Wen, S.W.; Krewski, D.; Yue, L.; Xie, R.H. The prevalence of food allergy in cesarean-born children aged 0–3 years: A systematic review and meta-analysis of cohort studies. Front. Pediatr. 2022, 10, 1044954. [Google Scholar] [CrossRef]
- Beigelman, A.; Bacharier, L.B. Early-life respiratory infections and asthma development: Role in disease pathogenesis and potential targets for disease prevention. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 172–178. [Google Scholar] [CrossRef]
- Magnus, M.C.; Håberg, S.E.; Stigum, H.; Nafstad, P.; London, S.J.; Vangen, S.; Nystad, W. Delivery by Cesarean section and early childhood respiratory symptoms and disorders: The Norwegian mother and child cohort study. Am. J. Epidemiol. 2011, 174, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.L.; Thornton, C.; de Jonge, A.; Khashan, A.; Tracy, M.; Downe, S.; Feijen-de Jong, E.I.; Dahlen, H.G. The effect of medical and operative birth interventions on child health outcomes in the first 28 days and up to 5 years of age: A linked data population-based cohort study. Birth 2018, 45, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.K.; Leung, T.F.; Tam, W.H.; Leung, A.S.Y.; Chan, O.M.; Ng, R.W.Y.; Yau, J.W.K.; Yuen, L.-Y.; Tong, S.L.Y.; Ho, W.C.S.; et al. Development of the early-life gut microbiome and associations with eczema in a prospective Chinese cohort. mSystems 2023, 8, e00521–e00523. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Simonsen, J.; Nielsen, N.M.; Frisch, M. Cesarean section and offspring’s risk of inflammatory bowel disease: A national cohort study. Inflamm. Bowel Dis. 2012, 18, 857–862. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Stene, L.C.; Joner, G.; Cinek, O.; Svensson, J.; Goldacre, M.J.; Parslow, R.C.; Pozzilli, P.; Brigis, G.; Stoyanov, D.; et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia 2008, 51, 726–735. [Google Scholar] [CrossRef]
- Decker, E.; Engelmann, G.; Findeisen, A.; Gerner, P.; Laass, M.; Ney, D.; Posovszky, C.; Hoy, L.; Hornef, M.W. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 2010, 125, e1433–e1440. [Google Scholar] [CrossRef]
- Kristensen, K.; Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol. 2016, 137, 587–590. [Google Scholar] [CrossRef]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef]
- Cao, Y.; Nguyen, L.H.; Tica, S.; Otegbeye, E.; Zong, X.; Roelstraete, B.; Chan, A.T.; Warner, B.B.; Stephansson, O.; Ludvigsson, J.F. Evaluation of Birth by Cesarean Delivery and Development of Early-Onset Colorectal Cancer. JAMA Netw. Open 2023, 6, e2310316. [Google Scholar] [CrossRef]
- Marcoux, S.; Soullane, S.; Lee, G.E.; Auger, N. Association between caesarean birth and childhood cancer: An age-lagged approach. Acta Paediatr. 2023, 112, 313–320. [Google Scholar] [CrossRef]
- Fritz, J.; Lamadrid-Figueroa, H.; Muñoz-Rocha, T.V.; Huerta-García, Y.; Martínez-Silva, G.; Trejo-Valdivia, B.; Martínez-Medina, S.; Hernandez-Chavez, C.; Osorio-Valencia, E.; Burris, H.H.; et al. Cesarean birth is associated with lower motor and language development scores during early childhood: A longitudinal analysis of two cohorts. Sci. Rep. 2024, 14, 23438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sidorchuk, A.; Sevilla-Cermeño, L.; Vilaplana-Pérez, A.; Chang, Z.; Larsson, H.; Mataix-Cols, D.; Fernández de la Cruz, L. Association of Cesarean Delivery With Risk of Neurodevelopmental and Psychiatric Disorders in the Offspring: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1910236. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Adams, S.H.; Li, X.; Badger, T.M.; Pivik, R.T.; Glasier, C.M.; Ramakrishnaiah, R.H.; Rowell, A.C.; Ou, X. Cesarean Delivery Impacts Infant Brain Development. AJNR Am. J. Neuroradiol. 2019, 40, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Hyötyläinen, T.; Petrone, J.R.; Igelström, K.; George, C.D.; Garrett, T.J.; Orešič, M.; Triplett, E.W.; Ludvigsson, J. Infant microbes and metabolites point to childhood neurodevelopmental disorders. Cell 2024, 187, 1853–1873. [Google Scholar] [CrossRef]
- Naspolini, N.F.; Natividade, A.P.; Asmus, C.I.F.; Moreira, J.C.; Dominguez-Bello, M.G.; Meyer, A. Early-life gut microbiome is associated with behavioral disorders in the Rio birth cohort. Sci. Rep. 2025, 15, 8674. [Google Scholar] [CrossRef]
- Dewey, K.G.; Nommsen-Rivers, L.A.; Heinig, M.J.; Cohen, R.J. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 2003, 112, 607–619. [Google Scholar] [CrossRef]
- Declercq, E.; MacDorman, M.; Osterman, M.; Belanoff, C.; Iverson, R. Prepregnancy Obesity and Primary Cesareans among Otherwise Low-Risk Mothers in 38 U.S. States in 2012. Birth 2015, 42, 309–318. [Google Scholar] [CrossRef]
- QuickStats: Rate of Cesarean Delivery, by Maternal Prepregnancy Body Mass Index Category—United States. 2020. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7048a7.htm#suggestedcitation (accessed on 21 January 2025).
- Mueller, N.T.; Differding, M.K.; Sun, H.; Wang, J.; Levy, S.; Deopujari, V.; Appel, L.J.; Blaser, M.J.; Kundu, T.; Shah, A.A.; et al. Maternal Bacterial Engraftment in Multiple Body Sites of Cesarean Section Born Neonates after Vaginal Seeding-a Randomized Controlled Trial. mBio 2023, 14, e0049123. [Google Scholar] [CrossRef]
- Song, S.J.; Wang, J.; Martino, C.; Jiang, L.; Thompson, W.K.; Shenhav, L.; McDonald, D.; Marotz, C.; Harris, P.R.; Hernandez, C.D.; et al. Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Med 2021, 2, 951–964. [Google Scholar] [CrossRef]
- Mueller, N.T.; Dominguez-Bello, M.G.; Appel, L.J.; Hourigan, S.K. ‘Vaginal seeding’ after a caesarean section provides benefits to newborn children: FOR: Does exposing caesarean-delivered newborns to the vaginal microbiome affect their chronic disease risk? The critical need for trials of ‘vaginal seeding’ during caesarean section. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 301. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qiu, W.; Wang, J.; Zhao, A.; Zhou, C.; Sun, T.; Xiong, Z.; Cao, P.; Shen, W.; Chen, J.; et al. Effects of vaginal microbiota transfer on the neurodevelopment and microbiome of cesarean-born infants: A blinded randomized controlled trial. Cell Host Microbe 2023, 31, 1232–1247. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.-T.; Zhou, S.-J.; Zhou, H.-H.; Xiong, Y.; Yang, J.; Zhou, Y.-B.; Chen, D.-J.; Liu, J.-M. Effects of vaginal seeding on gut microbiota, body mass index, and allergy risks in infants born through cesarean delivery: A randomized clinical trial. Am. J. Obstet. Gynecol. MFM 2023, 5, 100793. [Google Scholar] [CrossRef]
- Wilson, B.C.; Butler, É.M.; Grigg, C.P.; Derraik, J.G.B.; Chiavaroli, V.; Walker, N.; Thampi, S.; Creagh, C.; Reynolds, A.J.; Vatanen, T.; et al. Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial. EBioMedicine 2021, 69, 103443. [Google Scholar] [CrossRef]
- Duar, R.M.; Kyle, D.; Tribe, R.M. Reintroducing B. infantis to the cesarean-born neonate: An ecologically sound alternative to “vaginal seeding”. FEMS Microbiol. Lett. 2020, 367, fnaa032. [Google Scholar] [CrossRef]
- Korpela, K.; Helve, O.; Kolho, K.-L.; Saisto, T.; Skogberg, K.; Dikareva, E.; Stefanovic, V.; Salonen, A.; Andersson, S.; de Vos, W.M. Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 2020, 183, 324–334. [Google Scholar] [CrossRef]
- Liu, T.; Kress, A.M.; Debelius, J.; Zhao, N.; Smirnova, E.; Bandyopadhyay, S.; Bonham, K.; Comstock, S.S.; Gill, S.; Gern, J.E.; et al. Maternal Vaginal and Fecal Microbiota in Later Pregnancy Contribute to Child Fecal Microbiota Development in the ECHO Cohort. iScience 2025, 28, 112211. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Dominguez-Bello, M.G.; Mueller, N.T. Can maternal-child microbial seeding interventions improve the health of infants delivered by Cesarean section? Cell Host Microbe 2022, 30, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, S.K.; Mueller, N.T.; Dominguez-Bello, M.G. Can Vaginal Seeding Improve Health Outcomes of Infants Born by Cesarean Delivery? JAMA Pediatr. 2025, 179, 361–362. [Google Scholar] [CrossRef]
- Namasivayam, S.; Tilves, C.; Ding, H.; Wu, S.; Domingue, J.C.; Ruiz-Bedoya, C.; Shah, A.; Bohrnsen, E.; Schwarz, B.; Bacorn, M.; et al. Fecal transplant from vaginally seeded infants decreases intraabdominal adiposity in mice. Gut Microbes 2024, 16, 2353394. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Committee Opinion No. 725: Vaginal Seeding. Obs. Gynecol 2017, 130, e274–e278. [Google Scholar] [CrossRef]
- Limaye, M.A.; Ratner, A.J. ‘Vaginal seeding’ after a caesarean section provides benefits to newborn children. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 302. [Google Scholar] [CrossRef]
- Huynh, J.; Palasanthiran, P.; McMullan, B. Potential Transmission of Herpes Simplex Virus Via Vaginal Seeding. Pediatr. Infect. Dis. J. 2018, 37, e278. [Google Scholar] [CrossRef] [PubMed]
- Amstey, M.S.; Lewin, E.; Colaice, J. Vaginal colonization with invasive Escherichia coli during pregnancy. Am. J. Obs. Gynecol. 1980, 137, 534–535. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Hill, E.M.; Kane, P.J.; Rutt, L.; Gyles, T.; Folts, L.; Rock, K.D.; Howard, C.D.; Morrison, K.E.; Ravel, J.; et al. The composition of human vaginal microbiota transferred at birth affects offspring health in a mouse model. Nat. Commun. 2021, 12, 6289. [Google Scholar] [CrossRef]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef]
- Mueller, N.T.; Hourigan, S.K.; Hoffmann, D.E.; Levy, L.; von Rosenvinge, E.C.; Chou, B.; Dominguez-Bello, M.G. Bacterial Baptism: Scientific, Medical, and Regulatory Issues Raised by Vaginal Seeding of C-Section-Born Babies. J. Law Med. Ethics 2019, 47, 568–578. [Google Scholar] [CrossRef]
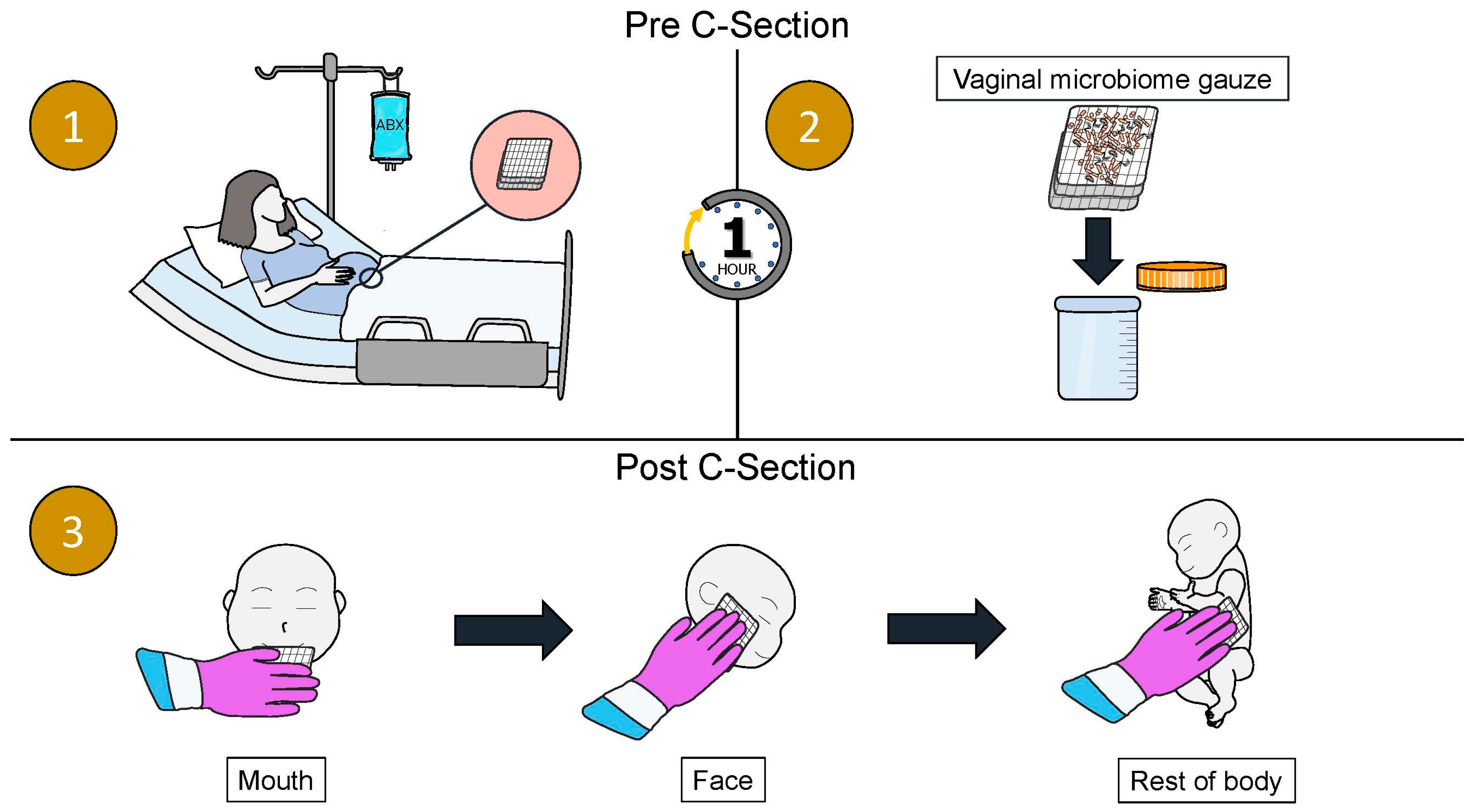
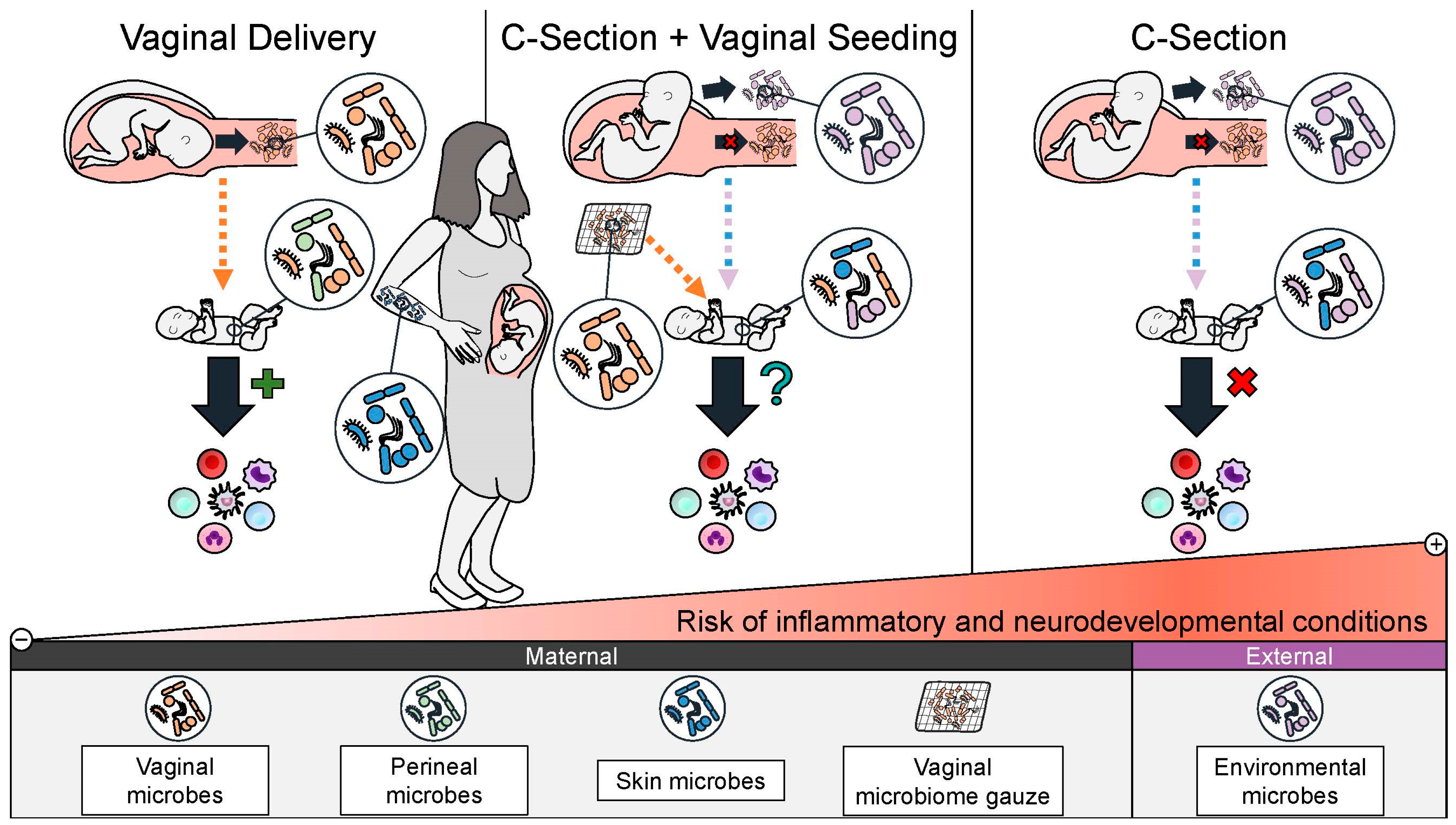
| Authors | Title | Journal Published | Year Published | Location and Patient Population Studied | Key Findings |
|---|---|---|---|---|---|
| Zhou L, Qiu W, Wang J, et al. | Effects of vaginal microbiota transfer on the neurodevelopment and microbiome of Cesarean-born infants: A blinded randomized controlled trial [104] | Cell Host & Microbe | 2023 | US, 32 US women of child-bearing age exposed to vaginal seeding, 36 US women of child-bearing age undergoing normal vaginal delivery | Vaginal seeding procedure is associated with improved neurodevelopment in Cesarean-born infants. |
| Liu Y, Li HT, Zhou SJ, et al. | Effects of Vaginal Seeding on Gut Microbiota, Body Mass Index, and Allergy Risks in Cesarean-Delivered Infants: A Randomized Clinical Trial [105] | American Journal of Obstetrics and Gynecology MFM | 2022 | China, 60 Chinese infants receiving vaginal seeding, 60 controls | For infants born via C-section, vaginal seeding has no significant impacts on the gut microbiota, growth, or allergy risks during the first 2 years of life. |
| Namasivayam S, Tilves C, Ding H, et al. | Fecal transplant from vaginally seeded infants decreases intraabdominal adiposity in mice [112] | Gut Microbes | 2024 | US, 8 US healthy infants, 4 receiving vaginal seeding and 4 controls | There was a reduction in IAAT volume in male mice that received stool from vaginally seeded infants compared to control infants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaPoint, P.; Banks, K.; Bacorn, M.; Prasad, R.; Romero-Soto, H.N.; Namasivayam, S.; Chen, Q.; Patel, A.; Levy, S.; Hourigan, S.K. Can Vaginal Seeding at Birth Improve Health Outcomes of Cesarean Section-Delivered Infants? A Scoping Review. Microorganisms 2025, 13, 1236. https://doi.org/10.3390/microorganisms13061236
LaPoint P, Banks K, Bacorn M, Prasad R, Romero-Soto HN, Namasivayam S, Chen Q, Patel A, Levy S, Hourigan SK. Can Vaginal Seeding at Birth Improve Health Outcomes of Cesarean Section-Delivered Infants? A Scoping Review. Microorganisms. 2025; 13(6):1236. https://doi.org/10.3390/microorganisms13061236
Chicago/Turabian StyleLaPoint, Phoebe, Keona Banks, Mickayla Bacorn, Ruhika Prasad, Hector N. Romero-Soto, Sivaranjani Namasivayam, Qing Chen, Anal Patel, Shira Levy, and Suchitra K. Hourigan. 2025. "Can Vaginal Seeding at Birth Improve Health Outcomes of Cesarean Section-Delivered Infants? A Scoping Review" Microorganisms 13, no. 6: 1236. https://doi.org/10.3390/microorganisms13061236
APA StyleLaPoint, P., Banks, K., Bacorn, M., Prasad, R., Romero-Soto, H. N., Namasivayam, S., Chen, Q., Patel, A., Levy, S., & Hourigan, S. K. (2025). Can Vaginal Seeding at Birth Improve Health Outcomes of Cesarean Section-Delivered Infants? A Scoping Review. Microorganisms, 13(6), 1236. https://doi.org/10.3390/microorganisms13061236






