Microbial Community Distribution in Low Permeability Reservoirs and Their Positive Impact on Enhanced Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Reservoir Characteristics of the Experimental Area
2.2. Experimental Materials
- (1)
- Enrichment Pond Water Samples
- (2)
- Wellhead Produced Water Samples
2.3. Experimental Methods
- (1)
- Microbial Concentration Detection:
- (2)
- Microbial Metabolite Monitoring:
- (3)
- Functional Gene Quantification:
- (4)
- High-Throughput Sequencing Analysis:
3. Results
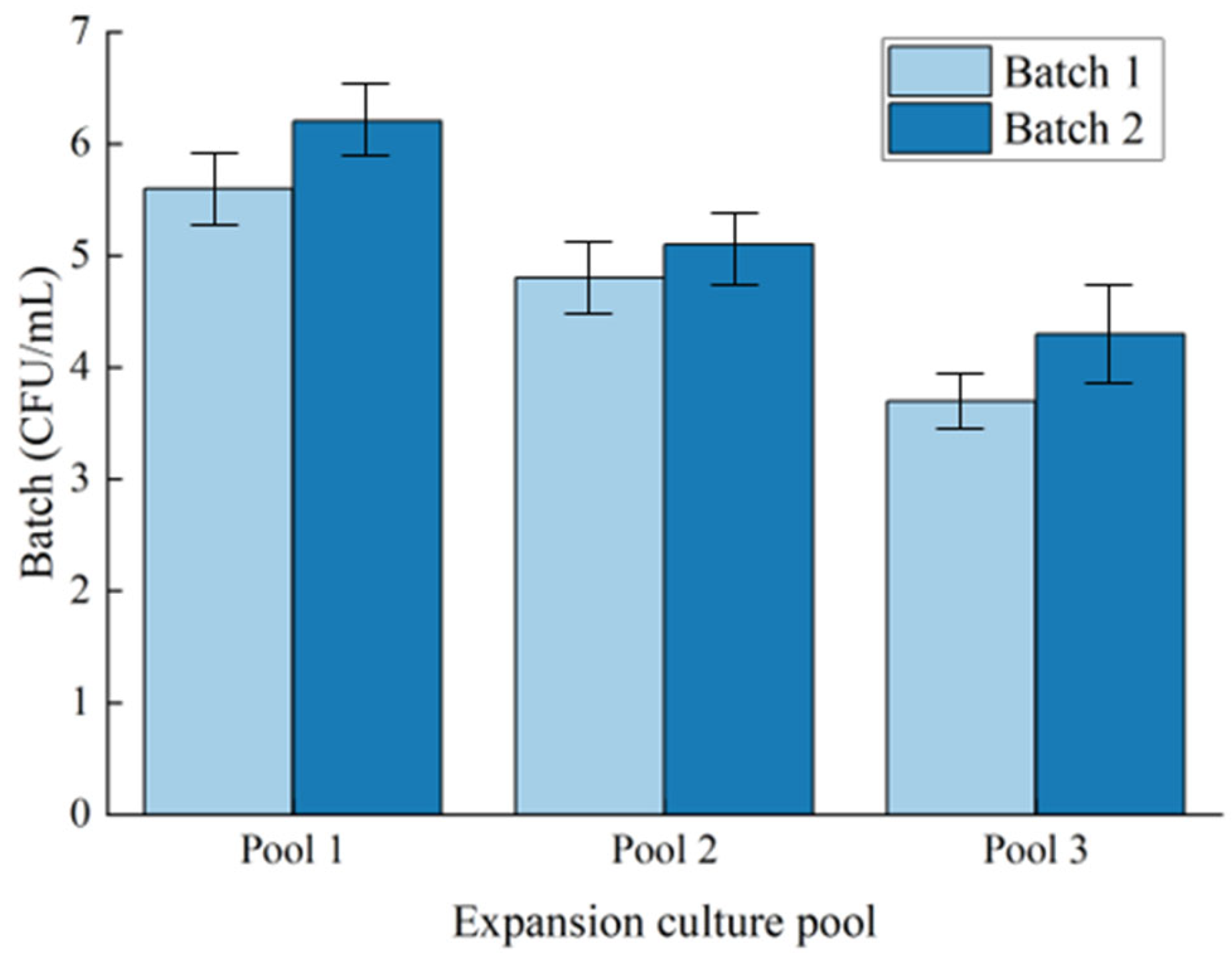
3.1. Microbial Concentration
3.2. Metabolite Concentrations
- (1)
- Surfactants (Lipids)
- (2)
- Short-Chain Organic Acids
- (3)
- Long-Chain Organic Acids
- (4)
- Organic Solvents
- (5)
- Biogas
3.3. Quantitative Analysis of Functional Genes
- (1)
- 16S rRNA Gene Abundance
- (2)
- Arch (Archaeal) Gene Abundance
- (3)
- mcrA (Methanogen) Gene Abundance
3.4. Microbial Community Composition
- (1)
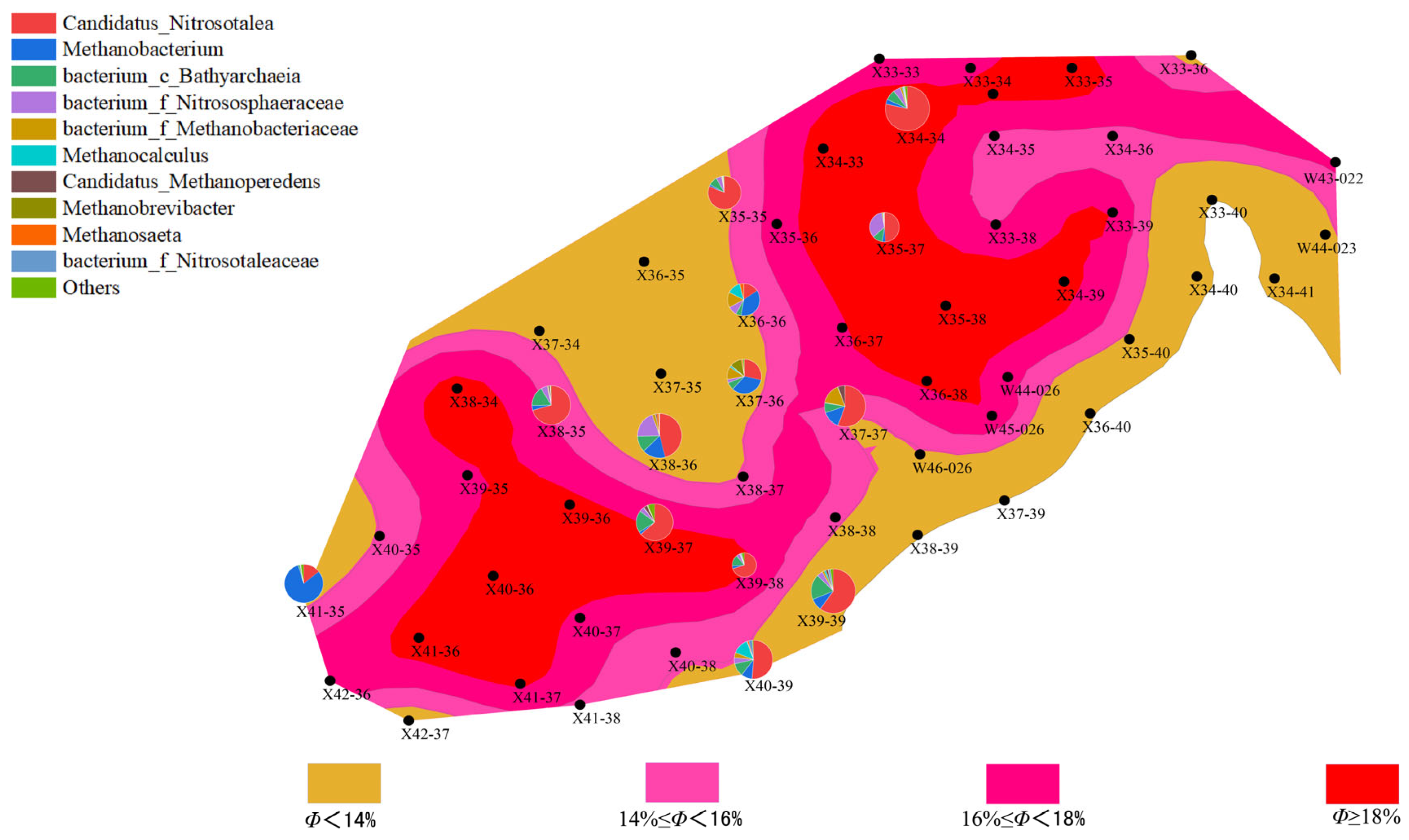
- Distribution Characteristics of Bacterial and Archaeal Communities
- (2)
- Changes in Bacterial and Archaeal Communities
- (3)
- Relationship Between Microbial Communities and Reservoir Characteristics
4. Discussion
4.1. Microbial Growth Status (Concentration) and On-Site Effects
4.2. Microbial Metabolites and Changes
4.3. Distribution Patterns of Microbial Communities
4.4. Important Findings and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, J.; Qian, K.; Dou, X. Experimental study of surfactant flooding system in low permeability reservoir. Sci. Rep. 2025, 15, 12534. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Wu, J.; Wang, Y.J.P. The carrying behavior of water-based fracturing fluid in shale reservoir fractures and molecular dynamics of sand-carrying mechanism. Processes 2024, 12, 2051. [Google Scholar] [CrossRef]
- Wang, X.; Yu, W.; Xie, Y.; He, Y.; Xu, H.; Chu, X.; Li, C. Numerical Simulation of the Dynamic Behavior of Low Permeability Reservoirs Under Fracturing-Flooding Based on a Dual-Porous and Dual-Permeable Media Model. Energies 2024, 17, 6203. [Google Scholar] [CrossRef]
- Adnan, M.T.; Zhang, G.; Chang, B.; Wei, H.; Peng, R.; Chang, W.; Wang, L. Multiscale pore architecture and its influence on porosity, permeability, and fluid flow in tight gas reservoirs of the Shihezi H8 formation, Ordos Basin. Energies 2024, 17, 5952. [Google Scholar] [CrossRef]
- Schmitt, M.; Fernandes, C.P.; Wolf, F.G.; Neto, J.A.B.d.C.; Rahner, C.P.; dos Santos, V.S.S. Characterization of Brazilian tight gas sandstones relating permeability and angstrom-to micron-scale pore structures. J. Nat. Gas Sci. Eng. 2015, 27, 785–807. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhao, H.; Zhou, F.; Tian, W.; Wen, Z.; Fan, Y.; Chen, X. An improved method for predicting the permeability of Chang 7 shale oil reservoirs based on lithology and hydraulic flow units. Sci. Rep. 2025, 15, 9168. [Google Scholar] [CrossRef]
- Tohidi, E.; Hesan, M.; Azad, A.; Abbasi, M.; Sadeghnejad, S. Implementing pore size distribution into saturation height function modelling of reservoir rock types: A case study on a carbonate gas reservoir. Gas. Sci. Eng. 2024, 121, 205188. [Google Scholar] [CrossRef]
- Bolton, A.J.; Maltman, A.J.; Fisher, Q. Anisotropic permeability and bimodal pore-size distributions of fine-grained marine sediments. Mar. Pet. Geol. 2000, 17, 657–672. [Google Scholar] [CrossRef]
- Mitchell, T.M.; Faulkner, D.R. Towards quantifying the matrix permeability of fault damage zones in low porosity rocks. Earth Planet. Sci. Lett. 2012, 339, 24–31. [Google Scholar] [CrossRef]
- Williams, K.E.; Lerche, I.; Maubeuge, F. Unconventional gas traps: Low permeability sands and gas accumulations. Energy Explor. Exploit. 1998, 16, 1–87. Available online: https://www.jstor.org/stable/43865279 (accessed on 20 February 2025). [CrossRef]
- Emami-Meybodi, H.; Ma, M.; Zhang, F.; Rui, Z.; Rezaeyan, A.; Ghanizadeh, A.; Hamdi, H.; Clarkson, C.R. Cyclic Gas Injection in Low-Permeability Oil Reservoirs: Progress in Modeling and Experiments. SPE J. 2024, 29, 6217–6250. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J. Research on Optimization of Water Quality Index System for Low Permeability Reservoir Water Injection Development. Chem. Technol. Fuels Oils 2023, 58, 1018–1026. [Google Scholar] [CrossRef]
- Das, A.; Das, N.; Pandey, P.; Pandey, P. Microbial enhanced oil recovery: Process perspectives, challenges, and advanced technologies for its efficient applications and feasibility. Arch. Microbiol. 2025, 207, 106. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Petrisor, I.; Yen, T. Microbial enhanced oil recovery (MEOR). Pet. Sci. Technol. 2007, 25, 1353–1366. [Google Scholar] [CrossRef]
- McInerney, M.J.; Nagle, D.P.; Knapp, R.M. Microbially Enhanced Oil Recovery: Past, Present, and Future. In Petroleum Microbiology; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Li, G.; Gao, P.; Wu, Y.; Tian, H.; Dai, X.; Wang, Y.; Cui, Q.; Zhang, H.; Pan, X.; Dong, H.; et al. Microbial abundance and community composition influence production performance in a low-temperature petroleum reservoir. Environ. Sci. Technol. 2014, 48, 5336–5344. [Google Scholar] [CrossRef]
- Youssef, N.H.; Elshahed, M.S.; McInerney, M.J. Microbial processes in oil fields: Culprits, problems, and opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Pereira, J.F.; Rodrigues, L.R.; Coutinho, J.A.; Teixeira, J.A. Isolation and study of microorganisms from oil samples for application in Microbial Enhanced Oil Recovery. Int. Biodeterior. Biodegrad. 2012, 68, 56–64. [Google Scholar] [CrossRef]
- Xiao, M.; Sun, S.S.; Zhang, Z.Z.; Wang, J.M.; Qiu, L.W.; Sun, H.Y.; Song, Z.Z.; Zhang, B.Y.; Gao, D.L.; Zhang, G.Q.; et al. Analysis of bacterial diversity in two oil blocks from two low-permeability reservoirs with high salinities. Sci. Rep. 2016, 6, 19600. [Google Scholar] [CrossRef]
- Gray, M.R.; Yeung, A.; Foght, J. Potential microbial enhanced oil recovery processes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar] [CrossRef]
- Nazina, T.; Sokolova, D.; Grouzdev, D.; Semenova, E.; Babich, T.; Bidzhieva, S.; Serdukov, D.; Volkov, D.; Bugaev, K.; Ershov, A.; et al. The Potential Application of Microorganisms for Sustainable Petroleum Recovery from Heavy Oil Reservoirs. Sustainability 2020, 12, 15. [Google Scholar] [CrossRef]
- Liang, K.; Liu, M.; Liang, Q.; Yang, H.P.; Li, J.; Yao, Z.; Li, S.; Yan, W. Shifts in Bacterial and Archaeal Community Composition in Low-Permeability Oil Reservoirs by a Nutrient Stimulation for Enhancing Oil Recovery. Appl. Sci. 2022, 12, 8075. [Google Scholar] [CrossRef]
- Al-Sulaimani, H.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bahry, S.N.; Elshafie, A.; Al-Bemani, A.S. Microbial biotechnology for enhancing oil recovery: Current developments and future prospects. Integr. Manuf. Syst. 2000, 11, 218–238. [Google Scholar] [CrossRef]
- Wood, D.A. Microbial improved and enhanced oil recovery (MIEOR): Review of a set of technologies diversifying their applications. Adv. Geo-Energy Res. 2018, 3, 122–140. [Google Scholar] [CrossRef]
- Yin, J.; Wei, X.; Hu, F.; Cheng, C.; Zhuang, X.; Song, M.; Zhuang, G.; Wang, F.; Ma, A. Halotolerant Bacillus velezensis sustainably enhanced oil recovery of low permeability oil reservoirs by producing biosurfactant and modulating the oil microbiome. Chem. Eng. J. 2022, 453, 139912. [Google Scholar] [CrossRef]
- You, J.; Wu, G.; Ren, F.; Chang, Q.; Yu, B.; Xue, Y.; Mu, B. Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Appl. Microbiol. Biotechnol. 2016, 100, 1469–1478. [Google Scholar] [CrossRef]
- Alkan, H.; Mukherjee, S.; Kögler, F. Reservoir engineering of in-situ MEOR; impact of microbial community. J. Pet. Sci. Eng. 2020, 195, 107928. [Google Scholar] [CrossRef]
- Agarry, S.E.; Salam, K.; Arinkoola, A.; Aremu, M.O. Biosurfactant production by indigeneous Pseudomonas and Bacillus species isolated from auto-mechanic soil environment towards microbial enhanced oil recovery. Eur. J. Eng. Technol. 2015, 3, 27–39. [Google Scholar]
- Bybee, K. MEOR in northwest Peru. J. Pet. Technol. 2006, 58, 48–49. [Google Scholar] [CrossRef]
- Bryant, R.; Stepp, A.; Bertus, K.; Burchfield, T.; Dennis, M. Microbial enhanced waterflooding field tests. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, 17–20 April 1994; SPE: Richardson, TX, USA, 1994; p. SPE-27751-MS. [Google Scholar]
- Ghaffari, A.; Sarafzadeh, P.; Hassanpour, S.; Setoodeh, P.; Hezave, A.Z.; Rahimpour, M.R. Examining the effect of reservoir conditions on efficiency of microbial enhanced oil recovery processes using Rhodococcus erythropolis strain; experimental approach. Braz. J. Chem. Eng. 2023, 40, 573–583. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Use of Microorganisms in the Recovery of Oil From Recalcitrant Oil Reservoirs: Current State of Knowledge, Technological Advances and Future Perspectives. Front. Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Liao, Z.; Liu, T.; Cheng, W.; Gao, G.; Yang, M.; Ma, T.; Li, G. Investigation of the transport and metabolic patterns of oil-displacing bacterium FY-07-G in the microcosm model using X-CT technology. J. Appl. Microbiol. 2023, 134, lxad281. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhang, Z.; Zhang, Z.; Sun, S.; Li, H.; Fu, P. Stimulation of indigenous microbes by optimizing the water cut in low permeability reservoirs for green and enhanced oil recovery. Sci. Rep. 2019, 9, 15772. [Google Scholar] [CrossRef]
- Basak, S.; Shetty, P.H. Conventional Microbial Counting and Identification Techniques. In Techniques to Measure Food Safety and Quality; Khan, M.S., Shafiur Rahman, M., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Jo, S.J.; Lee, Y.M.; Cho, K.; Park, S.Y.; Kwon, H.; Giri, S.S.; Lee, S.B.; Jung, W.J.; Park, J.H.; Hwang, M.H.; et al. Standardization of the Agar Plate Method for Bacteriophage Production. Antibiotics 2025, 14, 2. [Google Scholar] [CrossRef]
- Hockett, K.L.; Baltrus, D.A. Use of the Soft-agar Overlay Technique to Screen for Bacterially Produced Inhibitory Compounds. J. Vis. Exp. 2017, 119, 55064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, R.H.; Allen, M.J.; Geldreich, E.E. Standard plate count: A comparison of pour plate and spread plate methods. Am. Water Work. Assoc. 1983, 75, 35–37. [Google Scholar] [CrossRef]
- Selvam, K.; Manigundan, K.; Rajesh Kannan, V.; Annamalai, K.K.; Manikkam, R.; Venugopal, G.; Krishnan, S. Analysis and Identification of Short-Chain Fatty Acid Postbiotics by Gas Chromatography. In Postbiotics; Methods and Protocols in Food Science; Dharumadurai, D., Ed.; Humana: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Naveed, M.; Ishfaq, H.; Rehman, S.U.; Javed, A.; Waseem, M.; Makhdoom, S.I.; Aziz, T.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. GC-MS profiling of Bacillus spp. metabolites with an in vitro biological activity assessment and computational analysis of their impact on epithelial glioblastoma cancer genes. Front. Chem. 2023, 11, 1287599. [Google Scholar] [CrossRef]
- Mandodan, S.; Gangmei, K.; Vijayakumar, A.; Kunnikuruvan, A.; Lukose, J.; Padmanaban, H.; Bora, B.; Ashokkumar, M.; Irudayaraj, G.; Subbiah, P. Molecular identification and GC-MS analysis of a newly isolated novel bacterium (Lysinibacillus sp. VCRC B655) for mosquito control. Mol. Biol. Rep. 2024, 51, 800. [Google Scholar] [CrossRef]
- Kosa, G.; Shapaval, V.; Kohler, A.; Zimmermann, B. FTIR spectroscopy as a unified method for simultaneous analysis of intra- and extracellular metabolites in high-throughput screening of microbial bioprocesses. Microb. Cell Fact. 2017, 16, 195. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, X.; Shi, H.; Li, J.; Zhang, M.; Zhao, J.; Su, X. Comprehensive Assessment of 16S rRNA Gene Amplicon Sequencing for Microbiome Profiling across Multiple Habitats. Microbiol. Spectr. 2023, 11, e0056323. [Google Scholar] [CrossRef]
- Bender, J.M.; Li, F.; Adisetiyo, H.; Lee, D.; Zabih, S.; Hung, L.; Wilkinson, T.A.; Pannaraj, P.S.; She, R.C.; Bard, J.D.; et al. Quantification of variation and the impact of biomass in targeted 16S rRNA gene sequencing studies. Microbiome 2018, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Chen, X.; Zhang, W.; Li, S.; Huang, Q.; Zhang, Y.; Yan, C.; Li, S. High Throughput Sequencing Reveals Distinct Bacterial Communities and Functional Diversity in Two Typical Coastal Bays. J. Mar. Sci. Eng. 2022, 10, 1878. [Google Scholar] [CrossRef]
- Gong, B.; Cao, H.; Peng, C.; Perčulija, V.; Tong, G.; Fang, H.; Wei, X.; Ouyang, S. High-throughput sequencing and analysis of microbial communities in the mangrove swamps along the coast of Beibu Gulf in Guangxi, China. Sci. Rep. 2019, 9, 9377. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Bian, Z.; Chen, Y.; Zhang, X.; Wu, Y.; Wu, H. Horizontal and Vertical Comparison of Microbial Community Structures in a Low Permeability Reservoir at the Local Scale. Microorganisms 2023, 11, 2862. [Google Scholar] [CrossRef]
- Yin, J.; Wei, X.; Hu, F.; Cheng, C.; Song, M.; Zhuang, G.; Ma, A. Alternative stable microbiome state triggered by the introduction of functional microbes in oil reservoirs drives sustainable microbial enhanced oil recovery. Chem. Eng. J. 2023, 475, 146073. [Google Scholar] [CrossRef]
- Fu, Z.-Y.; Chen, W.-T.; Qi, G.-N.; Hou, Z.-W.; Liu, Y.-F.; Shou, L.-B.; Zhou, L.; Yang, S.-Z.; Wu, X.-L.; Gu, J.-D.; et al. Microbiome changes and characteristics under nutrient injection for enhanced oil production at Daqing oilfield. Int. Biodeterior. Biodegrad. 2025, 196, 105934. [Google Scholar] [CrossRef]
- Niu, J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Pet. Sci. Eng. 2020, 192, 107350. [Google Scholar] [CrossRef]
- Karim, G.A.M.; Salim, M.A.H.; Zain, M.Z.; Talib, N.N. Microbial enhanced oil recovery (MEOR) technology in Bokor Field, Sarawak. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 8–9 October 2001; SPE: Richardson, TX, USA, 2001; p. SPE-72125-MS. [Google Scholar]
- Town, K.; Sheehy, A.J.; Govreau, B.R. MEOR success in southern Saskatchewan. SPE Reserv. Eval. Eng. 2010, 13, 773–781. [Google Scholar] [CrossRef]
- Strappa, L.; De Lucia, J.; Maure, M.; Llopiz, M.L. A novel and successful MEOR pilot project in a strong water-drive reservoir Vizcacheras Field, Argentina. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, 17–21 April 2004; SPE: Richardson, TX, USA, 2004; p. SPE-89456-MS. [Google Scholar]
- Chowdhury, S.; Shrivastava, S.; Kakati, A.; Sangwai, J.S. Comprehensive Review on the Role of Surfactants in the Chemical Enhanced Oil Recovery Process. Ind. Eng. Chem. Res. 2022, 61, 21–64. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Kapse, N.; Dagar, S.S.; Dhakephalkar, P.K. Appropriate characterization of reservoir properties and investigation of their effect on microbial enhanced oil recovery through simulated laboratory studies. Sci. Rep. 2024, 14, 15401. [Google Scholar] [CrossRef] [PubMed]
- Vilcáez, J.; Chowdhury, E. Biogenic hydrogen production from oil hydrocarbons at geological carbon storage conditions. Energy Convers. Manag. 2025, 325, 119438. [Google Scholar] [CrossRef]
- Liu, K.; Wei, X. Oil Recovery: Experiences and Economics of Microbially Enhanced Oil Recovery (MEOR). In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Handbook of Hydrocarbon and Lipid Microbiology; Lee, S., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Nazina, T.N.; Sokolova, D.S.; Grigor’Yan, A.A.; Xue, Y.-F.; Belyaev, S.S.; Ivanov, M.V. Production of Oil-Releasing Compounds by Microorganisms from the Daqing Oil Field, China. Microbiology 2003, 72, 173–178. [Google Scholar] [CrossRef]
- Liang, Y.; Van Nostrand, J.D.; Deng, Y.; He, Z.; Wu, L.; Zhang, X.; Li, G.; Zhou, J. Functional gene diversity of soil microbial communities from five oil-contaminated fields in China. ISME J. 2011, 5, 403–413. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wu, X.; Hou, Z.; Wang, M.; Yang, E. Research on Microbial Community Structure in Different Blocks of Alkaline–Surfactant–Polymer Flooding to Confirm Optimal Stage of Indigenous Microbial Flooding. Appl. Sci. 2024, 14, 5243. [Google Scholar] [CrossRef]











| Gene Name | Primer Name | Primer Sequence | Length of PCR Product |
|---|---|---|---|
| 16S rRNA | 338F | ACTCCTACGGGAGGCAGCAG | 480 bp |
| 806R | GGACTACHVGGGTWTCTAAT | ||
| ARCH | 524F_10_ext | TGYCAGCCGCCGCGGTAA | 350 bp |
| Arch958R_mod | YCCGGCGTTGAVTCCAATT | ||
| mcrA | MLf | GGTGGTGTMGGATTCACACARTAYGCWACAGC | 476 bp |
| MLr | TTCATTGCRTAGTTWGGRTAGTT |
| Metabolite Types | Classification | Batch 1 | Batch 2 | ||||
|---|---|---|---|---|---|---|---|
| Pool 1 | Pool 2 | Pool 3 | Pool 1 | Pool 2 | Pool 3 | ||
| Short-Chain Organic Acids (mg/L) | Formic Acid | - | 16 | 8 | - | 4 | 2 |
| Acetic Acid | 97 | 111 | 117 | 24 | 63 | 32 | |
| Propionic Acid | - | - | - | 3 | 7 | 2 | |
| Butyric Acid | 4 | 6 | 5 | 3 | 8 | 3 | |
| Valeric Acid | 6 | - | - | 6 | - | - | |
| Total | 108 | 133 | 130 | 36 | 82 | 39 | |
| Long-Chain Organic Acids (mg/L) | - | 0.34 | 0.61 | 1.1 | 0.3 | 0.86 | 0.43 |
| Surfactants (mg/L) | Lipids | 4.98 | 1.86 | 1.57 | 1.52 | 1.33 | 6.06 |
| Organic Solvents (mg/L) | Alcohols | 2.66 | 2.81 | 1.74 | 0.06 | 3.12 | 0 |
| Ketones | 2.48 | 4.12 | 2.55 | 0.81 | 1.94 | 3.85 | |
| Aldehydes | 0 | 1.22 | 1.24 | 0.12 | 0.30 | 0.60 | |
| Ethers | 1.73 | 0 | 1.77 | 0.11 | 0.51 | 0 | |
| Total | 6.87 | 8.15 | 7.3 | 1.1 | 5.77 | 4.55 | |
| Biogas (mL/L) | Carbon Dioxide | 16 | 33 | 31 | 2 | 4 | 2 |
| Ethane | 2 | 3 | 3 | 2 | 5 | 1 | |
| Propane | - | 2 | 4 | - | 2 | 1 | |
| Total | 18 | 38 | 38 | 4 | 11 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.; Wu, T.; Yu, X.; Zhou, C.; Gao, J.; Chen, H. Microbial Community Distribution in Low Permeability Reservoirs and Their Positive Impact on Enhanced Oil Recovery. Microorganisms 2025, 13, 1230. https://doi.org/10.3390/microorganisms13061230
Pang J, Wu T, Yu X, Zhou C, Gao J, Chen H. Microbial Community Distribution in Low Permeability Reservoirs and Their Positive Impact on Enhanced Oil Recovery. Microorganisms. 2025; 13(6):1230. https://doi.org/10.3390/microorganisms13061230
Chicago/Turabian StylePang, Jin, Tongtong Wu, Xinan Yu, Chunxi Zhou, Jiaao Gao, and Haotian Chen. 2025. "Microbial Community Distribution in Low Permeability Reservoirs and Their Positive Impact on Enhanced Oil Recovery" Microorganisms 13, no. 6: 1230. https://doi.org/10.3390/microorganisms13061230
APA StylePang, J., Wu, T., Yu, X., Zhou, C., Gao, J., & Chen, H. (2025). Microbial Community Distribution in Low Permeability Reservoirs and Their Positive Impact on Enhanced Oil Recovery. Microorganisms, 13(6), 1230. https://doi.org/10.3390/microorganisms13061230






