Assess the Variability and Robustness of an Aluminum-Based Adsorption–Precipitation Method for Virus Detection in Wastewater Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
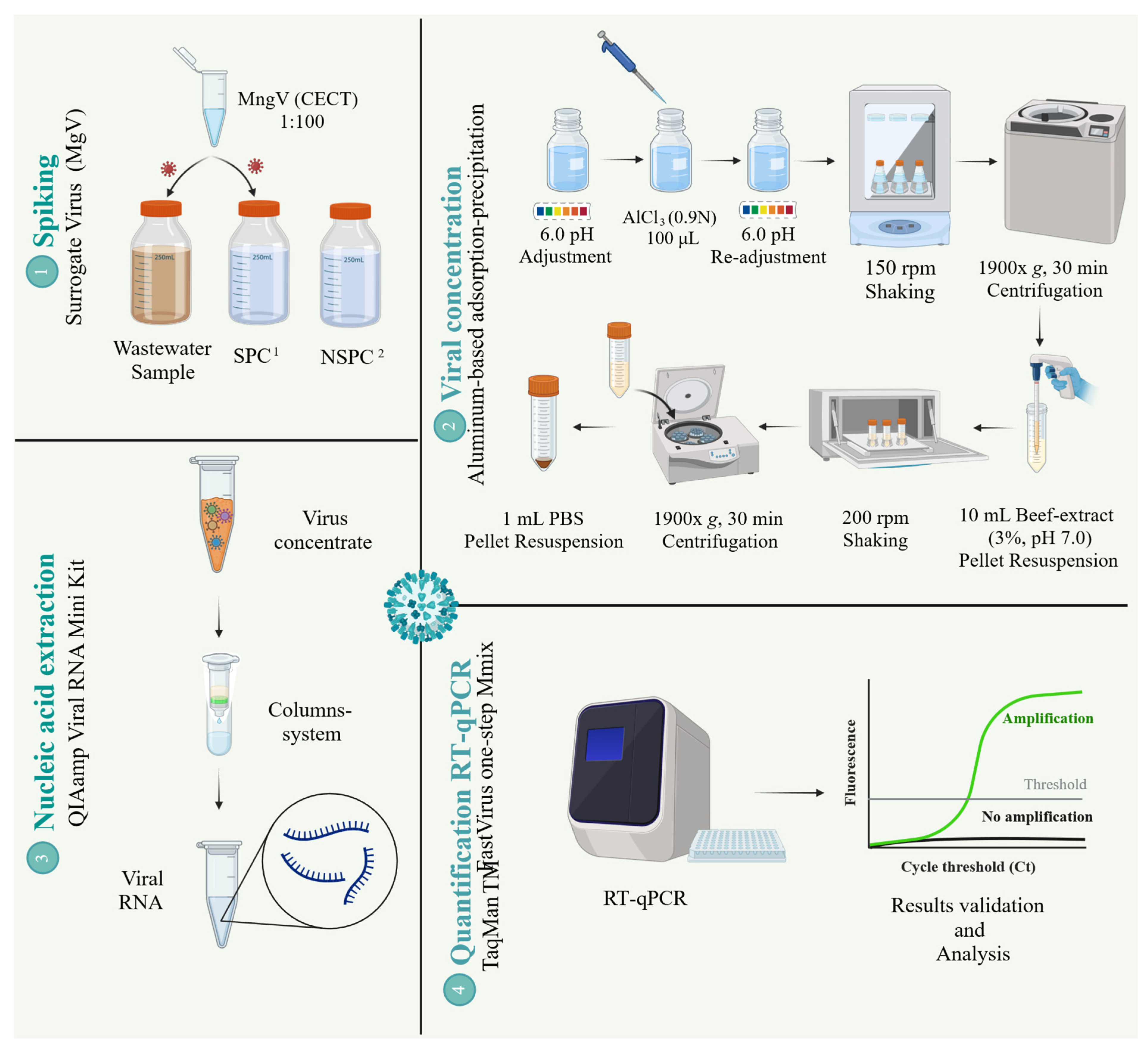
2.2. Virus Detection Strategy
2.2.1. Process Controls Used in This Study
2.2.2. Virus Concentration Step
2.2.3. Nucleic Acid Extraction Step
2.2.4. Virus Quantification by RT-qPCR
2.3. Methodology Robustness Study
2.4. Recovery Rates
2.5. Physicochemical Data
2.6. Statistical Analysis and Plots
3. Results
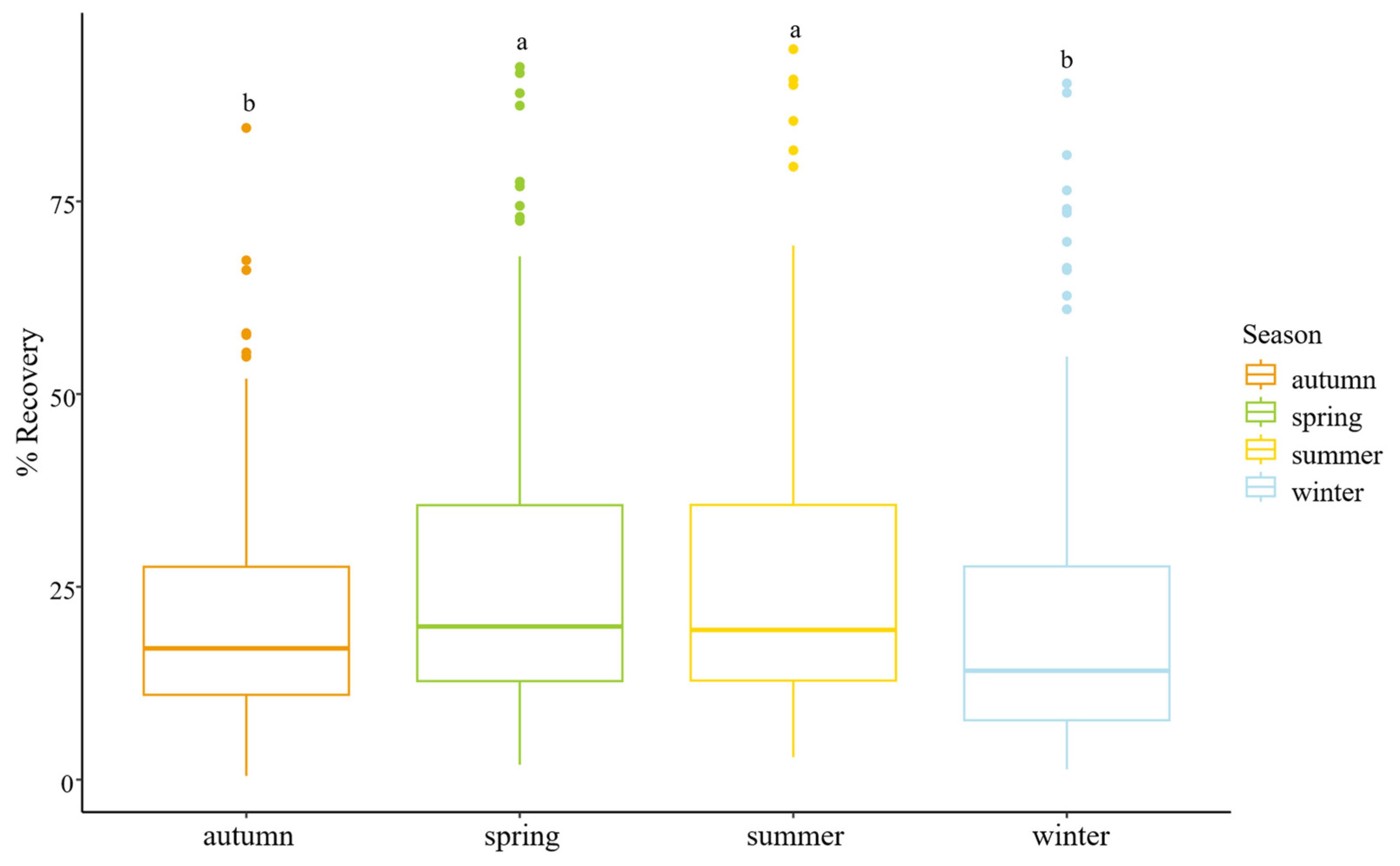
3.1. Robustness of the Methodology
3.2. Effect of the Physicochemical Parameters on the Recovery Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, W.J. Isolation of B Typhosus from sewage and shellfish. BMJ 1928, 1, 1061–1062. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.R.; Trask, J.D.; Culotta, C.S. Poliomyelitic Virus in Sewage. Science 1939, 90, 258–259. [Google Scholar] [CrossRef] [PubMed]
- OMS. Guidelines for Environmental Surveillance of Poliovirus Circulation. Available online: https://iris.who.int/bitstream/handle/10665/67854/WHO_V-B_03.03_eng.pdf?sequence=1&isAllowed=y (accessed on 25 April 2025).
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of therapeutic drugs in the environment. Lancet 2000, 355, 1789–1790. [Google Scholar] [CrossRef]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Calamari, D.; Bagnati, R.; Schiarea, S.; Fanelli, R. Cocaine in surface waters: A new evidence-based tool to monitor community drug abuse. Environ. Health 2005, 4, 14. [Google Scholar] [CrossRef]
- Lorenzo, M.; Picó, Y. Wastewater-based epidemiology: Current status and future prospects. Curr. Opin. Environ. Sci. Health 2019, 9, 77–84. [Google Scholar] [CrossRef]
- Xiao, K.; Zhang, L. Wastewater pathogen surveillance based on One Health approach. Lancet Microbe 2023, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Diemert, S.; Yan, T. Clinically Unreported Salmonellosis Outbreak Detected via Comparative Genomic Analysis of Municipal Wastewater Salmonella Isolates. Appl. Environ. Microbiol. 2019, 85, e00139-19. [Google Scholar] [CrossRef]
- Hellmér, M.; Paxéus, N.; Magnius, L.; Enache, L.; Arnholm, B.; Johansson, A.; Bergström, T.; Norder, H. Detection of Pathogenic Viruses in Sewage Provided Early Warnings of Hepatitis A Virus and Norovirus Outbreaks. Appl. Environ. Microbiol. 2014, 80, 6771–6781. [Google Scholar] [CrossRef]
- Adhikari, S.; Halden, R.U. Opportunities and limits of wastewater-based epidemiology for tracking global health and attainment of UN sustainable development goals. Environ. Int. 2022, 163, 107217. [Google Scholar] [CrossRef]
- Singer, A.C.; Thompson, J.R.; Filho, C.R.M.; Street, R.; Li, X.; Castiglioni, S.; Thomas, K.V. A world of wastewater-based epidemiology. Nat. Water 2023, 1, 408–415. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.B.; O’Dell, H.D.; Norton, G.; Shin, G.; Hsu, F.-C.; Meschke, J.S. Evaluation of a novel electropositive filter for the concentration of viruses from diverse water matrices. Water Sci. Technol. 2010, 61, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Katayama, H.; Asami, M.; Akiba, M. Development of a novel method for simultaneous concentration of viruses and protozoa from a single water sample. J. Virol. Methods 2012, 182, 62–69. [Google Scholar] [CrossRef]
- Hill, V.R.; Polaczyk, A.L.; Hahn, D.; Narayanan, J.; Cromeans, T.L.; Roberts, J.M.; Amburgey, J.E. Development of a Rapid Method for Simultaneous Recovery of Diverse Microbes in Drinking Water by Ultrafiltration with Sodium Polyphosphate and Surfactants. Appl. Environ. Microbiol. 2005, 71, 6878–6884. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Metcalf, T.G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988, 54, 1983–1988. [Google Scholar] [CrossRef]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef]
- Fumian, T.M.; Leite, J.P.G.; Castello, A.A.; Gaggero, A.; Caillou, M.S.L.D.; Miagostovich, M.P. Detection of rotavirus A in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. J. Virol. Methods 2010, 170, 42–46. [Google Scholar] [CrossRef]
- Calgua, B.; Rodriguez-Manzano, J.; Hundesa, A.; Suñen, E.; Calvo, M.; Bofill-Mas, S.; Girones, R. New methods for the concentration of viruses from urban sewage using quantitative PCR. J. Virol. Methods 2013, 187, 215–221. [Google Scholar] [CrossRef]
- Goyal, S.M.; Gerba, C.P. Viradel method for detection of rota virus from seawater. J. Virol. Methods 1983, 7, 279–285. [Google Scholar] [CrossRef]
- Wylie, T.N.; Wylie, K.M.; Herter, B.N.; Storch, G.A. Enhanced virome sequencing using targeted sequence capture. Genome Res. 2015, 25, 1910–1920. [Google Scholar] [CrossRef]
- Kramberger, P.; Petrovič, N.; Štrancar, A.; Ravnikar, M. Concentration of plant viruses using monolithic chromatographic supports. J. Virol. Methods 2004, 120, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, M.; Ashoori, R.; Paital, B.; Kabdaşlı, I.; Frontistis, Z.; Hashemi, M.; Sandoval, M.A.; Sherchan, S.; Das, K.; Emamjomeh, M.M. Wastewater Based Epidemiology Perspective as a Faster Protocol for Detecting Coronavirus RNA in Human Populations: A Review with Specific Reference to SARS-CoV-2 Virus. Pathogens 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Farmer-Diaz, K.; Matthew-Bernard, M.; Cheetham, S.; Mitchell, K.; Macpherson, C.N.L.; Ramos-Nino, M.E. Optimized Aluminum Hydroxide Adsorption–Precipitation for Improved Viral Detection in Wastewater. IJERPH 2025, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.; Helmi, A.; Slama, I.; Hamdi, R.; Mastouri, M.; Aouni, M. Evaluation of three viral concentration methods for detection and quantification of SARS-CoV-2 in wastewater. J. Water Health 2023, 21, 354–360. [Google Scholar] [CrossRef]
- Ahmed, W.; Bivins, A.; Bertsch, P.M.; Bibby, K.; Choi, P.M.; Farkas, K.; Gyawali, P.; Hamilton, K.A.; Haramoto, E.; Kitajima, M.; et al. Surveillance of SARS-CoV-2 RNA in wastewater: Methods optimization and quality control are crucial for generating reliable public health information. Curr. Opin. Environ. Sci. Health 2020, 17, 82–93. [Google Scholar] [CrossRef]
- D’Agostino, M.; Cook, N.; Rodriguez-Lazaro, D.; Rutjes, S. Nucleic Acid Amplification-Based Methods for Detection of Enteric Viruses: Definition of Controls and Interpretation of Results. Food Environ. Virol. 2011, 3, 55–60. [Google Scholar] [CrossRef]
- Diez-Valcarce, M.; Cook, N.; Hernández, M.; Rodríguez-Lázaro, D. Analytical Application of a Sample Process Control in Detection of Foodborne Viruses. Food Anal. Methods 2011, 4, 614–618. [Google Scholar] [CrossRef]
- Hennechart-Collette, C.; Martin-Latil, S.; Guillier, L.; Perelle, S. Determination of which virus to use as a process control when testing for the presence of hepatitis A virus and norovirus in food and water. Int. J. Food Microbiol. 2015, 202, 57–65. [Google Scholar] [CrossRef]
- ISO 15216-1:2017; Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis a Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification. Spanish Association for Standardization (UNE): Madrid, Spain, 2017.
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Maresca, M.; Longobardi, C.; Mancon, A.; Romeri, F.; et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Hays, A.; Islam, R.; Matys, K.; Williams, D. Best Practices in qPCR and dPCR Validation in Regulated Bioanalytical Laboratories. AAPS J. 2022, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Barril, P.A.; Pianciola, L.A.; Mazzeo, M.; Ousset, M.J.; Jaureguiberry, M.V.; Alessandrello, M.; Sánchez, G.; Oteiza, J.M. Evaluation of viral concentration methods for SARS-CoV-2 recovery from wastewaters. Sci. Total Environ. 2021, 756, 144105. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cataluña, A.; Cuevas-Ferrando, E.; Randazzo, W.; Falcó, I.; Allende, A.; Sánchez, G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021, 758, 143870. [Google Scholar] [CrossRef]
- Philo, S.E.; Keim, E.K.; Swanstrom, R.; Ong, A.Q.W.; Burnor, E.A.; Kossik, A.L.; Harrison, J.C.; Demeke, B.A.; Zhou, N.A.; Beck, N.K.; et al. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci. Total Environ. 2021, 760, 144215. [Google Scholar] [CrossRef]
- Cuevas-Ferrando, E.; Randazzo, W.; Pérez-Cataluña, A.; Sánchez, G. HEV Occurrence in Waste and Drinking Water Treatment Plants. Front. Microbiol. 2020, 10, 2937. [Google Scholar] [CrossRef]
- Ahmed, W.; Bertsch, P.M.; Bivins, A.; Bibby, K.; Farkas, K.; Gathercole, A.; Haramoto, E.; Gyawali, P.; Korajkic, A.; McMinn, B.R.; et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020, 739, 139960. [Google Scholar] [CrossRef]
- North, D.; Bibby, K. Comparison of viral concentration techniques for native fecal indicators and pathogens from wastewater. Sci. Total Environ. 2023, 905, 167190. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Cuevas-Ferrando, E.; Sanjuán, R.; Domingo-Calap, P.; Sánchez, G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health 2020, 230, 113621. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, B.; Yue, Q.; Sun, S.; Wang, Y.; Li, Q. Determination of active ingredients of a new coagulant aid-Enteromorpha by floc characteristics on-line monitoring in Yellow River water treatment. Chem. Eng. J. 2013, 232, 310–318. [Google Scholar] [CrossRef]
- Fumagalli, M.J.; Capato, C.F.; De Castro-Jorge, L.A.; De Souza, W.M.; Arruda, E.; Figueiredo, L.T.M. Stability of SARS-CoV-2 and other airborne viruses under different stress conditions. Arch. Virol. 2022, 167, 183–187. [Google Scholar] [CrossRef]
- Rock, C.; Alum, A.; Abbaszadegan, M. PCR Inhibitor Levels in Concentrates of Biosolid Samples Predicted by a New Method Based on Excitation-Emission Matrix Spectroscopy. Appl. Environ. Microbiol. 2010, 76, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Girón-Guzmán, I.; Cuevas-Ferrando, E.; Barranquero, R.; Díaz-Reolid, A.; Puchades-Colera, P.; Falcó, I.; Pérez-Cataluña, A.; Sánchez, G. Urban wastewater-based epidemiology for multi-viral pathogen surveillance in the Valencian region, Spain. Water Res. 2024, 255, 121463. [Google Scholar] [CrossRef]
- Honap, T.P.; Sankaranarayanan, K.; Schnorr, S.L.; Ozga, A.T.; Warinner, C.; Lewis, C.M. Biogeographic study of human gut-associated crAssphage suggests impacts from industrialization and recent expansion. PLoS ONE 2020, 15, e0226930. [Google Scholar] [CrossRef] [PubMed]
- Bivins, A.; Crank, K.; Greaves, J.; North, D.; Wu, Z.; Bibby, K. Cross-assembly phage and pepper mild mottle virus as viral water quality monitoring tools—Potential, research gaps, and way forward. Curr. Opin. Environ. Sci. Health 2020, 16, 54–61. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, Y.; Xu, X.; Li, S.; Zhang, Y.; Ding, J.; On, H.Y.; Lai, J.C.C.; In Yau, C.; Chin, A.W.H.; et al. Comparison of virus concentration methods and RNA extraction methods for SARS-CoV-2 wastewater surveillance. Sci. Total Environ. 2022, 824, 153687. [Google Scholar] [CrossRef]





| Control | Lot | Mean | Median | Sd | Min | Max | Range | CV (%) |
|---|---|---|---|---|---|---|---|---|
| EC | Lot 1 | 24.21 | 24.17 | 0.75 | 22.46 | 25.62 | 3.17 | 3.09 |
| Lot 2 | 21.51 | 21.40 | 0.70 | 20.20 | 23.52 | 3.32 | 3.28 | |
| SPC | Lot 1 | 26.03 | 25.67 | 1.49 | 23.71 | 29.72 | 6.01 | 5.74 |
| Lot 2 | 24.11 | 24.23 | 1.30 | 22.19 | 27.66 | 5.48 | 5.39 | |
| SPC-EC | 2.17 | 1.95 | 1.17 | 0.40 | 5.12 | 4.73 | 53.75 |
| Comparisons | p-Adjusted | |
|---|---|---|
| Sample_1 | Sample_4 | 0.023 |
| Sample_10 | 0.020 | |
| Sample_11 | 0.019 | |
| Sample_3 | Sample_4 | 0.021 |
| Sample_4 | Sample_6 | 0.017 |
| Sample_10 | Sample_3 | 0.012 |
| Sample_6 | 0.008 | |
| Sample_9 | 0.033 | |
| Sample_12 | 0.015 | |
| Sample_11 | Sample_3 | 0.016 |
| Sample_6 | 0.015 | |
| Sample_12 | 0.028 | |
| Sample_12 | Sample_4 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Martín, L.; Hernández, M.; Eiros, J.M.; Valero, A.; Rodríguez-Lázaro, D. Assess the Variability and Robustness of an Aluminum-Based Adsorption–Precipitation Method for Virus Detection in Wastewater Samples. Microorganisms 2025, 13, 1186. https://doi.org/10.3390/microorganisms13061186
Casado-Martín L, Hernández M, Eiros JM, Valero A, Rodríguez-Lázaro D. Assess the Variability and Robustness of an Aluminum-Based Adsorption–Precipitation Method for Virus Detection in Wastewater Samples. Microorganisms. 2025; 13(6):1186. https://doi.org/10.3390/microorganisms13061186
Chicago/Turabian StyleCasado-Martín, Lorena, Marta Hernández, José M. Eiros, Antonio Valero, and David Rodríguez-Lázaro. 2025. "Assess the Variability and Robustness of an Aluminum-Based Adsorption–Precipitation Method for Virus Detection in Wastewater Samples" Microorganisms 13, no. 6: 1186. https://doi.org/10.3390/microorganisms13061186
APA StyleCasado-Martín, L., Hernández, M., Eiros, J. M., Valero, A., & Rodríguez-Lázaro, D. (2025). Assess the Variability and Robustness of an Aluminum-Based Adsorption–Precipitation Method for Virus Detection in Wastewater Samples. Microorganisms, 13(6), 1186. https://doi.org/10.3390/microorganisms13061186








