The Developmental Disorders of Fall Armyworm (Spodoptera frugiperda, Lepidoptera: Noctuidae) Caused by the Infection with Nosema sp. (Microsporidia: Nosematidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Microsporidia
2.2. Latent Period Detect
2.3. Ultra-Histopathology
2.4. Impact of Nosema sp. on Development of S. frugiperda
2.5. RNA-Seq
2.6. Impact of Nosema sp. on Genes of Chitin Synthesis Pathway of S. frugiperda
3. Results
3.1. Ultra-Histopathology Within Latent Period in S. frugiperda Infected by Nosema sp.
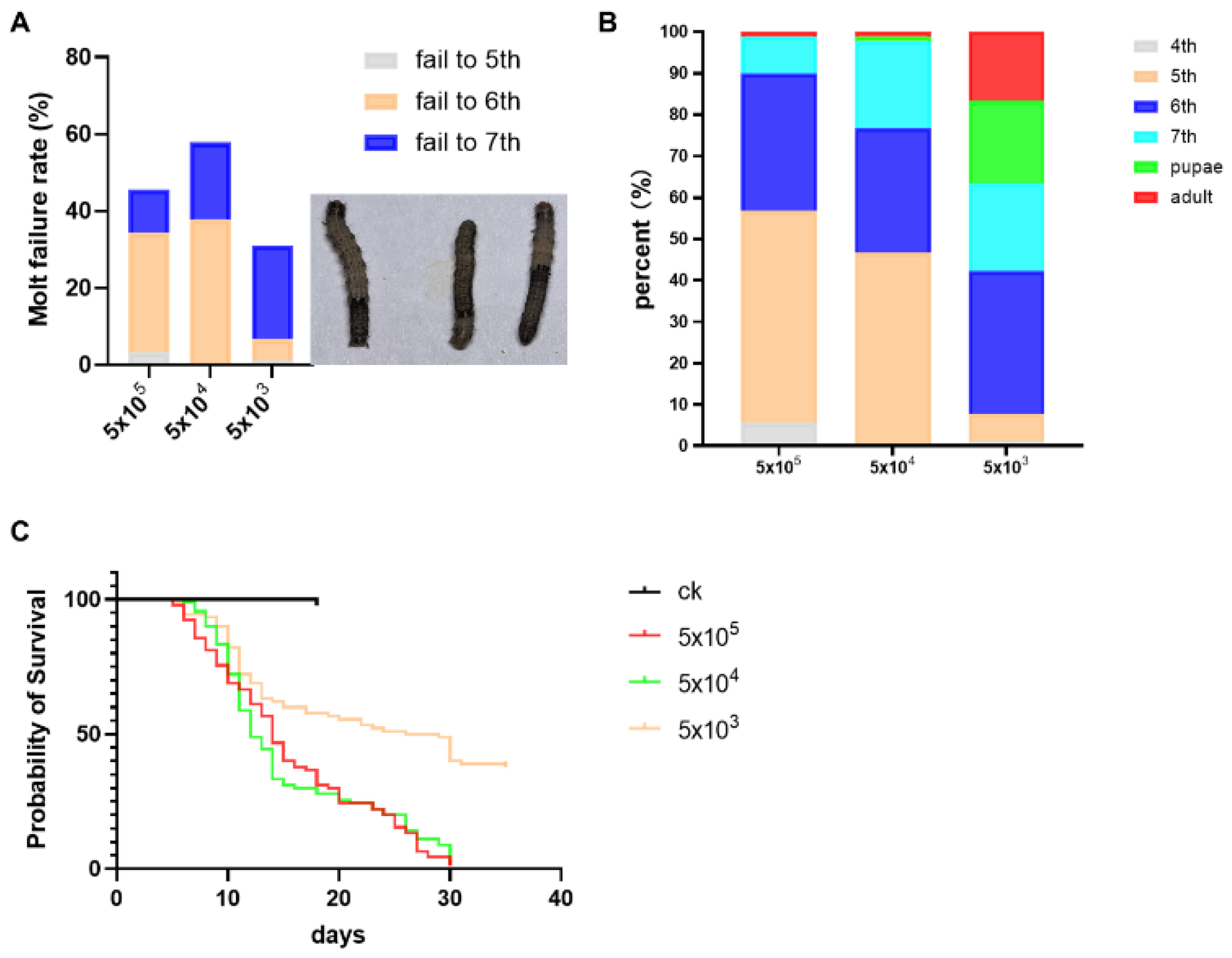
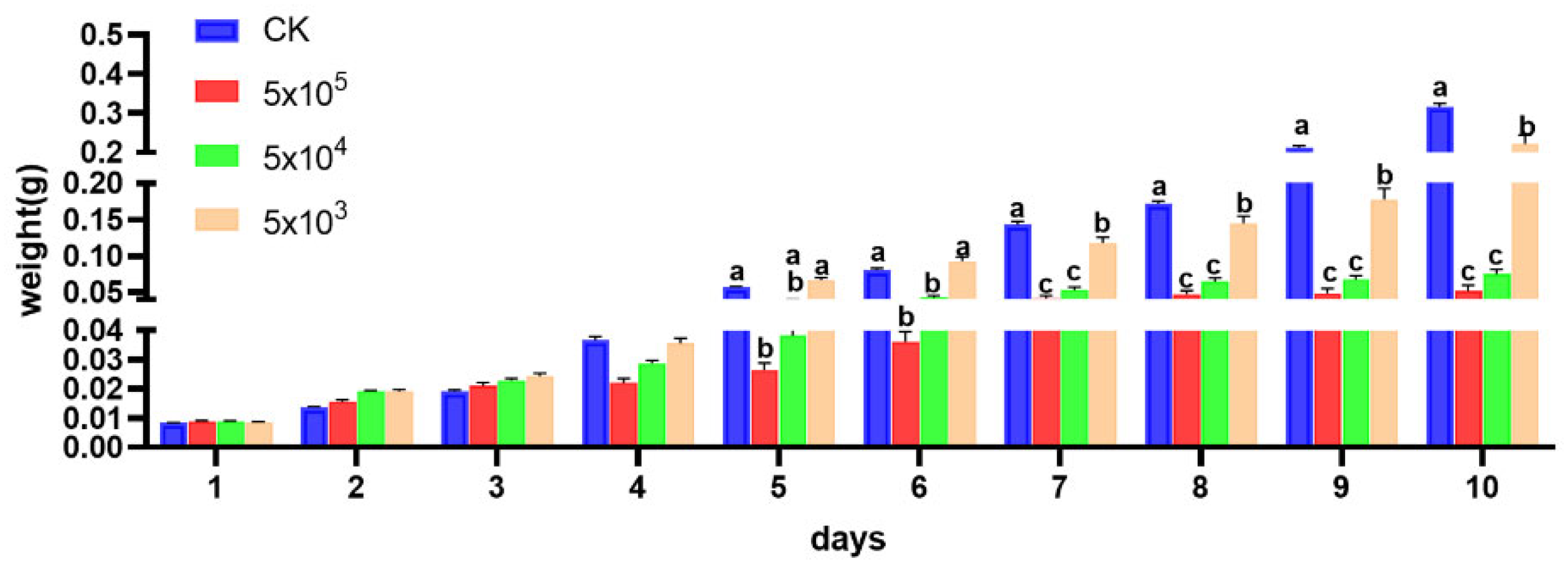
3.2. Impact of Developmental Duration, Weights, and Virulence on S. frugiperda Infected by Nosema sp.
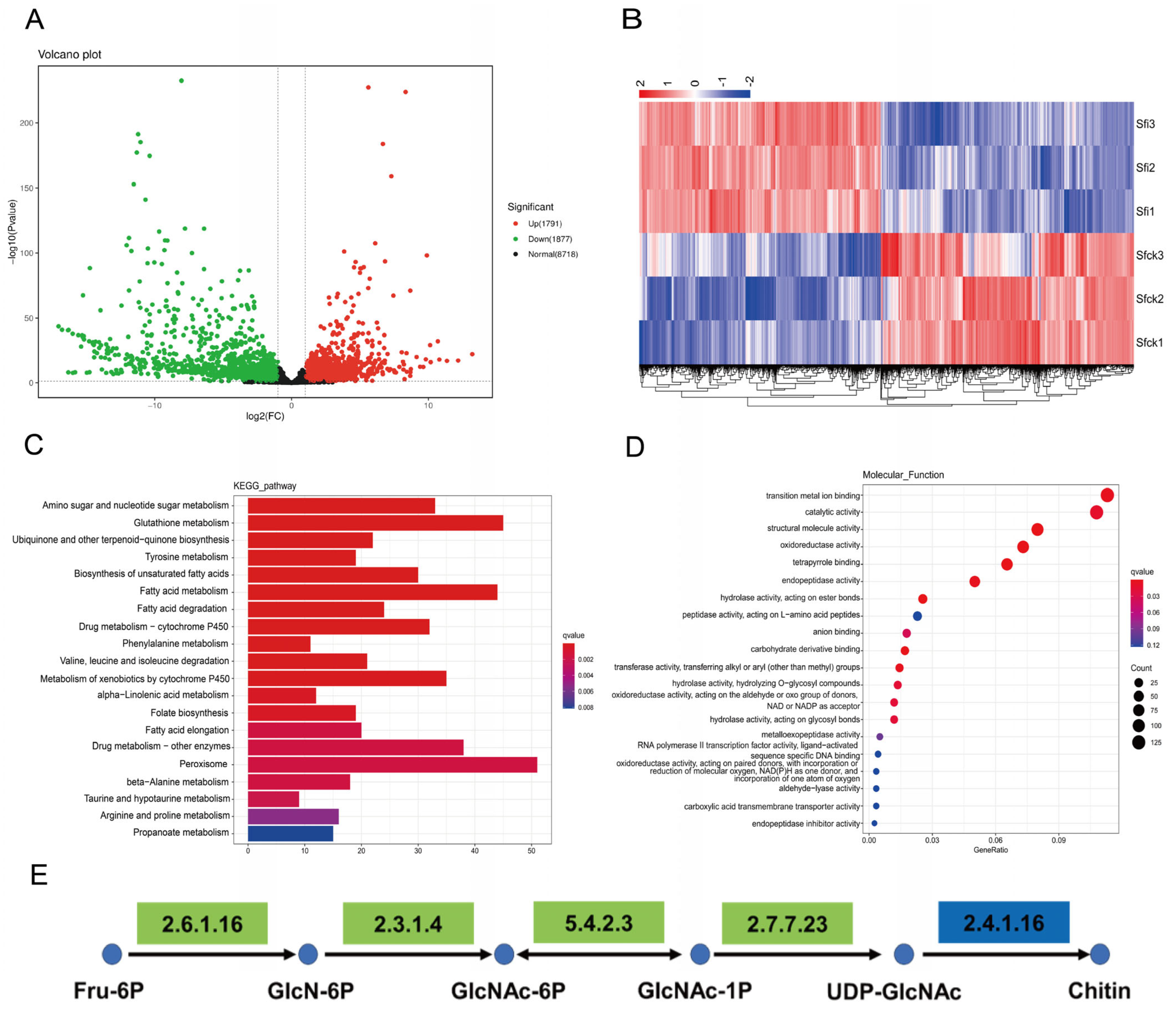
3.3. RNA-Seq Analysis
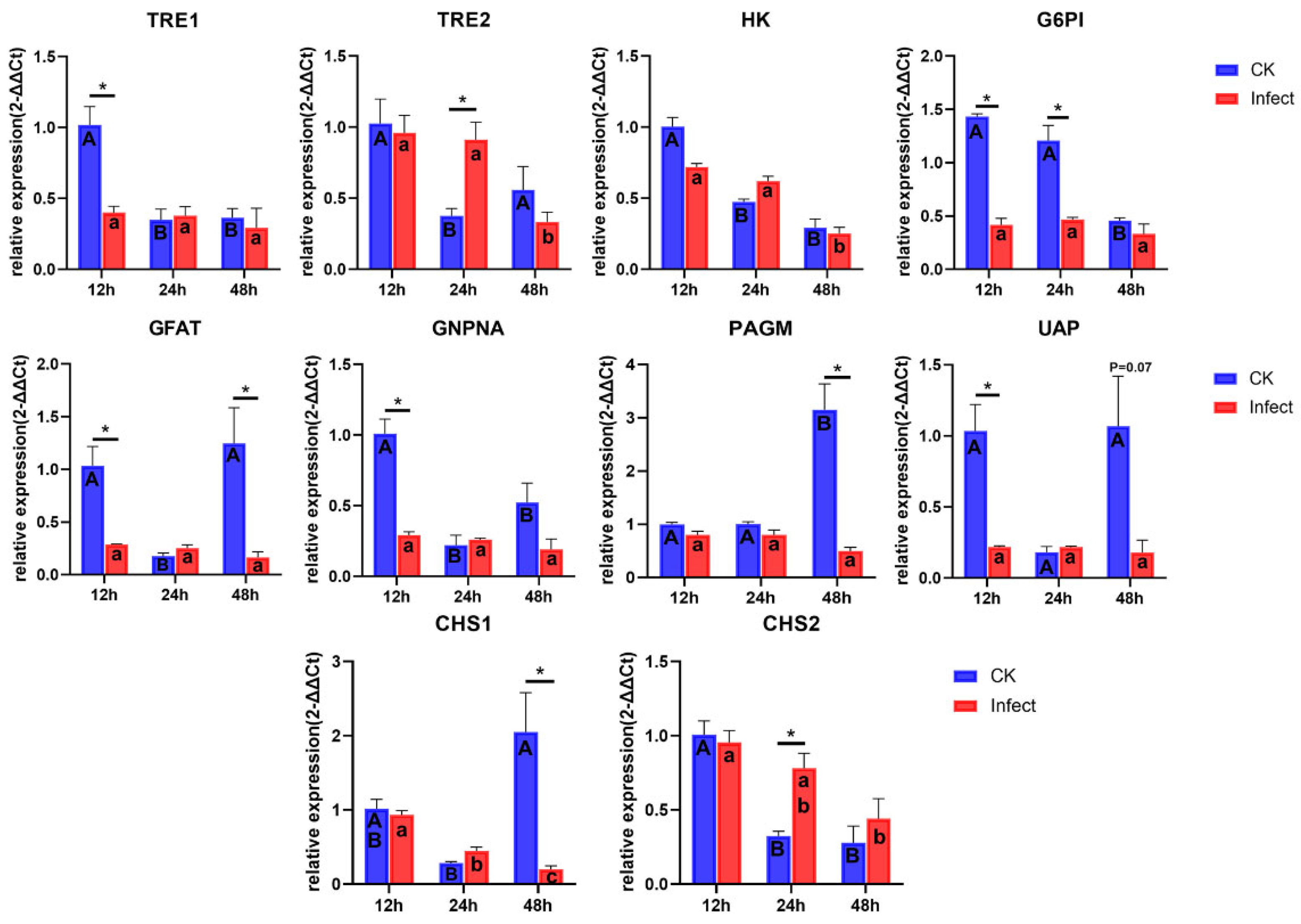
3.4. Gene Expression of Chitin Synthesis Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Makale, F.; Mugambi, I.; Kansiime, M.K.; Yuka, I.; Abang, M.; Lechina, B.S.; Rampeba, M.; Rwomushana, I. Fall armyworm in Botswana: Impacts, farmer management practices and implications for sustainable pest management. Pest Manag. Sci. 2022, 78, 1060–1070. [Google Scholar] [CrossRef]
- Wei, L.; Nei, Y.; Li, Y.J. Research progress on green and sustainable prevention and control technologies for Spodoptera frugiperda. Chin. J. Biol. Control. 2024; in press. [Google Scholar]
- Van den Berg, J.; du Plessis, H. Chemical Control and Insecticide Resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Borja, M.; Guzmán-Franco, A.W.; Rodríguez-Leyva, E.; Santillán-Ortega, C.; Pérez-Panduro, A. Interaction of Beauveria bassiana and Metarhizium anisopliae with chlorpyrifos ethyl and spinosad in Spodoptera frugiperda larvae. Pest Manag. Sci. 2018, 74, 2049–2056. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, K.H.; Zhao, Q.; Bai, X.P.; Zhou, J.C.; Zhang, L.S. Morphological defense of the egg mass of Spodoptera frugiperda (Lepidoptera: Noctuidae) affects parasitic capacity and alters behaviors of egg parasitoid wasps. J. Asia-Pac. Entomol. 2021, 24, 671–678. [Google Scholar] [CrossRef]
- Stinguel, P.; Paiva, C.E.C.; Zuim, V.; Azevedo, A.C.T.; Valicente, F.H.; Santos Júnior, H.J.G. Optimization of in vivo production of Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV). Neotrop. Entomol. 2022, 51, 122–132. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, X.L.; Hao, H.Y.; Liu, S.S.; Guo, N.; Shi, J. Identification and pathogenicity of an entomopathogenic microsporidium collected from fall armyworm Spodoptera frugiperda. J. Plant Prot. 2022, 49, 1513–1520. [Google Scholar]
- Han, B.; Weiss, L.M. Microsporidia: Obligate Intracellular Pathogens Within the Fungal Kingdom. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Weiss, L.M.; Becnel, J.J. Microsporidia: Pathogens of Opportunity; Wiley-Blackwell: Oxford, UK, 2014. [Google Scholar]
- Shi, W.; Guo, Y.; Xu, C.; Tan, S.; Miao, J.; Feng, Y.; Zhao, H.; St Leger, R.J.; and Fang, W. Unveiling the mechanism by which microsporidian parasites prevent locust swarm behavior. Proc. Natl. Acad. Sci. USA 2014, 111, 1343–1348. [Google Scholar] [CrossRef]
- Simões, R.A.; Feliciano, J.R.; Solter, L.F.; Delalibera, I. Impacts of Nosema sp. (Microsporidia: Nosematidae) on the sugarcane borer, Diatraea saccharalis (Lepidoptera: Crambidae). J. Invertebr. Pathol. 2015, 129, 7–12. [Google Scholar] [CrossRef]
- Kermani, N.; Abu-Hassan, Z.A.; Dieng, H.; Ismail, N.F.; Attia, M.; Ghani, I.A. Pathogenicity of Nosema sp. (Microsporidia) in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). PLoS ONE 2013, 8, e62884. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fang, Z.; He, Q.; Wang, C.; Meng, X.; Yu, B.; Zhou, Z. Characterizing the xenoma of Vairimorpha necatrix provides insights into the most efficient mode of microsporidian proliferation. Front. Cell. Infect. Microbiol. 2021, 11, 699239. [Google Scholar]
- Szumowski, S.C.; Troemel, E.R. Microsporidia-host interactions. Curr. Opin. Microbiol. 2015, 26, 10–16. [Google Scholar] [CrossRef]
- Ma, Z.; Li, C.; Pan, G.; Li, Z.; Han, B.; Xu, J.; Lan, X.; Chen, J.; Yang, D.; Chen, Q.; et al. Genome-wide transcriptional response of silkworm (Bombyx mori) to infection by the microsporidian Nosema bombycis. PLoS ONE 2013, 8, e84137. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Higes, M.; Sagastume, S.; Juarranz, Á.; Dias-Almeida, J.; Budge, G.E.; Meana, A.; Boonham, N. Microsporidia infection impacts the host cell’s cycle and reduces host cell apoptosis. PLoS ONE 2017, 12, e0170183. [Google Scholar] [CrossRef]
- Lopez-Ezquerra, A.; Mitschke, A.; Bornberg-Bauer, E.; Joop, G. Tribolium castaneum gene expression changes after Paranosema whitei infection. J. Invertebr. Pathol. 2018, 153, 92–98. [Google Scholar] [CrossRef]
- Yu, A.; Beck, M.; Merzendorfer, H.; Yang, Q. Advances in understanding insect chitin biosynthesis. Insect Biochem. Mol. Biol. 2024, 164, 104058. [Google Scholar] [CrossRef]
- Yao, Q.; Quan, L.F.; Xu, S.; Dong, Y.Z.; Li, W.J.; Chen, B.X. Effect of diflubenzuron on the chitin biosynthesis pathway in Conopomorpha sinensis eggs. Insect Sci. 2021, 28, 1061–1075. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Chen, J.; Tang, B.; Chen, H.; Yao, Q.; Huang, X.; Chen, J.; Zhang, D.; Zhang, W. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef]
- Pearson, J.C.; Juarez, M.T.; Kim, M.; Drivenes, Ø.; McGinnis, W. Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 2224–2229. [Google Scholar] [CrossRef]
- Johny, S.; Kanginakudru, S.; Muralirangan, M.C.; Nagaraju, J. Morphological and molecular characterization of a new microsporidian (Protozoa: Microsporidia) isolated from Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Parasitology 2006, 132, 803–814. [Google Scholar] [CrossRef]
- Hopper, J.V.; Mills, N.J. Pathogenicity, prevalence and intensity of a microsporidian infection by Nosema fumiferanae postvittana in the light brown apple moth, Epiphyas postvittana, in California. J. Invertebr. Pathol. 2016, 134, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Pu, Z.L. Insect Pathology; Guangdong Science and Technology Press: Guangdong, China, 1994. [Google Scholar]
- Wiwat, C.; Thaithanun, S.; Pantuwatana, S.; Bhumiratana, A. Toxicity of chitinase-producing Bacillus thuringiensis ssp. kurstaki HD-1 (G) toward Plutella xylostella. J. Invertebr. Pathol. 2000, 76, 270–277. [Google Scholar] [CrossRef]
- Scanlon, M.; Leitch, G.J.; Visvesvara, G.S.; Shaw, A.P. Relationship between the host cell mitochondria and the parasitophorous vacuole in cells infected with Encephalitozoon microsporidia. J. Eukaryot. Microbiol. 2004, 51, 81–87. [Google Scholar] [CrossRef]
- Han, B.; Ma, Y.; Tu, V.; Tomita, T.; Mayoral, J.; Williams, T.; Horta, A.; Huang, H.; Weiss, L.M. Microsporidia interact with host cell mitochondria via voltage-dependent anion channels using sporoplasm surface protein 1. mBio 2019, 10, e01944-19. [Google Scholar] [CrossRef]
- Solter, L.E.; Becnel, J.J.; Oi, D.H. Microsporidian entomopathogens. In Insect Pathology; Vega, F.E., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 221–263. [Google Scholar]
- Castro, B.M.C.E.; Martínez, L.C.; Plata-Rueda, A.; Soares, M.A.; Tavares, W.S.; Serrão, J.E.; Zanuncio, J.C. Chlorantraniliprole degenerates microvilli goblet cells of the Anticarsia gemmatalis (Lepidoptera: Noctuidae) midgut. Chemosphere 2019, 229, 525–528. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Khan, M.A.M.; Osborne, T.; Collins, D.; Srivastava, M.; Reynolds, O.L. A walk on the wild side: Gut bacteria fed to mass-reared larvae of Queensland fruit fly [Bactrocera tryoni (Froggatt)] influence development. BMC Biotechnol. 2019, 19 (Suppl. S2), 95. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, S.; Wang, J.; Ni, Y.; Huang, W.; Liu, Q.; Ling, E. Peptide hormones in the insect midgut. Front. Physiol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Myung-Sae, H.; Hitoshi, W. Transovarial transmission of two microsporidia in the silkworm, Bombyx mori, and disease occurrence in the progeny population. J. Invertebr. Pathol. 1988, 51, 41. [Google Scholar]
- Gong, C.; Hasnain, A.; Wang, Q.; Liu, D.; Xu, Z.; Zhan, X.; Liu, X.; Pu, J.; Sun, M.; Wang, X. Eco-friendly deacetylated chitosan base siRNA biological-nanopesticide loading cyromazine for efficiently controlling Spodoptera frugiperda. Int. J. Biol. Macromol. 2023, 241, 124575. [Google Scholar] [CrossRef]
- Chen, J.P.; Xie, J.; Zhao, K.; Hu, Z.; Zhang, X.; Nan, X.H.; Sun, R.F. Effects of a novel 2,6-difluorobenzoylurea compound on the bioactivity and chitin synthesis of Spodoptera frugiperda. Chin. J. Pestic. Sci. 2023, 25, 798–807. [Google Scholar]
- Zou, H.; Zhang, B.; Zou, C.; Ma, W.; Zhang, S.; Wang, Z.; Bi, B.; Li, S.; Gao, J.; Zhang, C.; et al. Knockdown of GFAT disrupts chitin synthesis in Hyphantria cunea larvae. Pestic. Biochem. Physiol. 2022, 188, 105245. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Du, J.; Li, S.W.; Shakeel, M.; Li, J.J.; Meng, X.G. Cloning, characterization, and RNA interference effect of the UDP-N-acetylglucosamine pyrophosphorylase gene in Cnaphalocrocis medinalis. Genes 2021, 12, 464. [Google Scholar] [CrossRef]
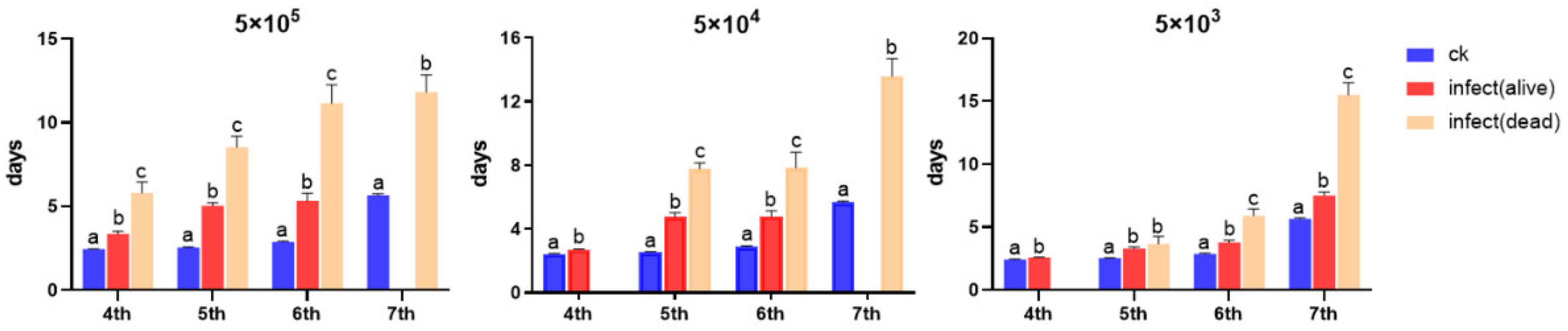
| ck | 5 × 105 | 5 × 104 | 5 × 103 | ||||
|---|---|---|---|---|---|---|---|
| Instar | Alive | Alive | Dead | Alive | Dead | Alive | Dead |
| 4th | 2.44 ± 0.32 n = 90 | 3.36 ± 1.53 n = 85 | 5.80 ± 1.48 n = 5 | 2.70 ± 0.35 n = 90 | / | 2.58 ± 0.23 n = 89 | / |
| 5th | 2.54 ± 0.40 n = 90 | 5.03 ± 1.20 n = 38 | 8.53 ± 4.39 n = 47 | 4.75 ± 1.85 n = 48 | 7.77 ± 2.50 n = 42 | 3.31 ± 1.03 n = 83 | 3.67 ± 1.50 n = 6 |
| 6th | 2.88 ± 0.50 n = 90 | 5.33 ± 1.35 n = 9 | 11.16 ± 5.93 n = 29 | 4.80 ± 1.50 n = 20 | 7.85 ± 5.00 n = 27 | 3.77 ± 1.38 n = 52 | 5.90 ± 3.05 n = 31 |
| 7th | 5.66 ± 0.80 n = 88 | / | 11.81 ± 2.95 n = 9 | / | 13.58 ± 4.83 n = 25 | 7.53 ± 1.51 n = 33 | 15.50 ± 4.29 n = 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liu, H.; Huang, X.; Tan, S.; Shi, W. The Developmental Disorders of Fall Armyworm (Spodoptera frugiperda, Lepidoptera: Noctuidae) Caused by the Infection with Nosema sp. (Microsporidia: Nosematidae). Microorganisms 2025, 13, 994. https://doi.org/10.3390/microorganisms13050994
Xu Y, Liu H, Huang X, Tan S, Shi W. The Developmental Disorders of Fall Armyworm (Spodoptera frugiperda, Lepidoptera: Noctuidae) Caused by the Infection with Nosema sp. (Microsporidia: Nosematidae). Microorganisms. 2025; 13(5):994. https://doi.org/10.3390/microorganisms13050994
Chicago/Turabian StyleXu, Yudi, Haoyu Liu, Xinzheng Huang, Shuqian Tan, and Wangpeng Shi. 2025. "The Developmental Disorders of Fall Armyworm (Spodoptera frugiperda, Lepidoptera: Noctuidae) Caused by the Infection with Nosema sp. (Microsporidia: Nosematidae)" Microorganisms 13, no. 5: 994. https://doi.org/10.3390/microorganisms13050994
APA StyleXu, Y., Liu, H., Huang, X., Tan, S., & Shi, W. (2025). The Developmental Disorders of Fall Armyworm (Spodoptera frugiperda, Lepidoptera: Noctuidae) Caused by the Infection with Nosema sp. (Microsporidia: Nosematidae). Microorganisms, 13(5), 994. https://doi.org/10.3390/microorganisms13050994






