The Strategies Microalgae Adopt to Counteract the Toxic Effect of Heavy Metals
Abstract
1. Introduction
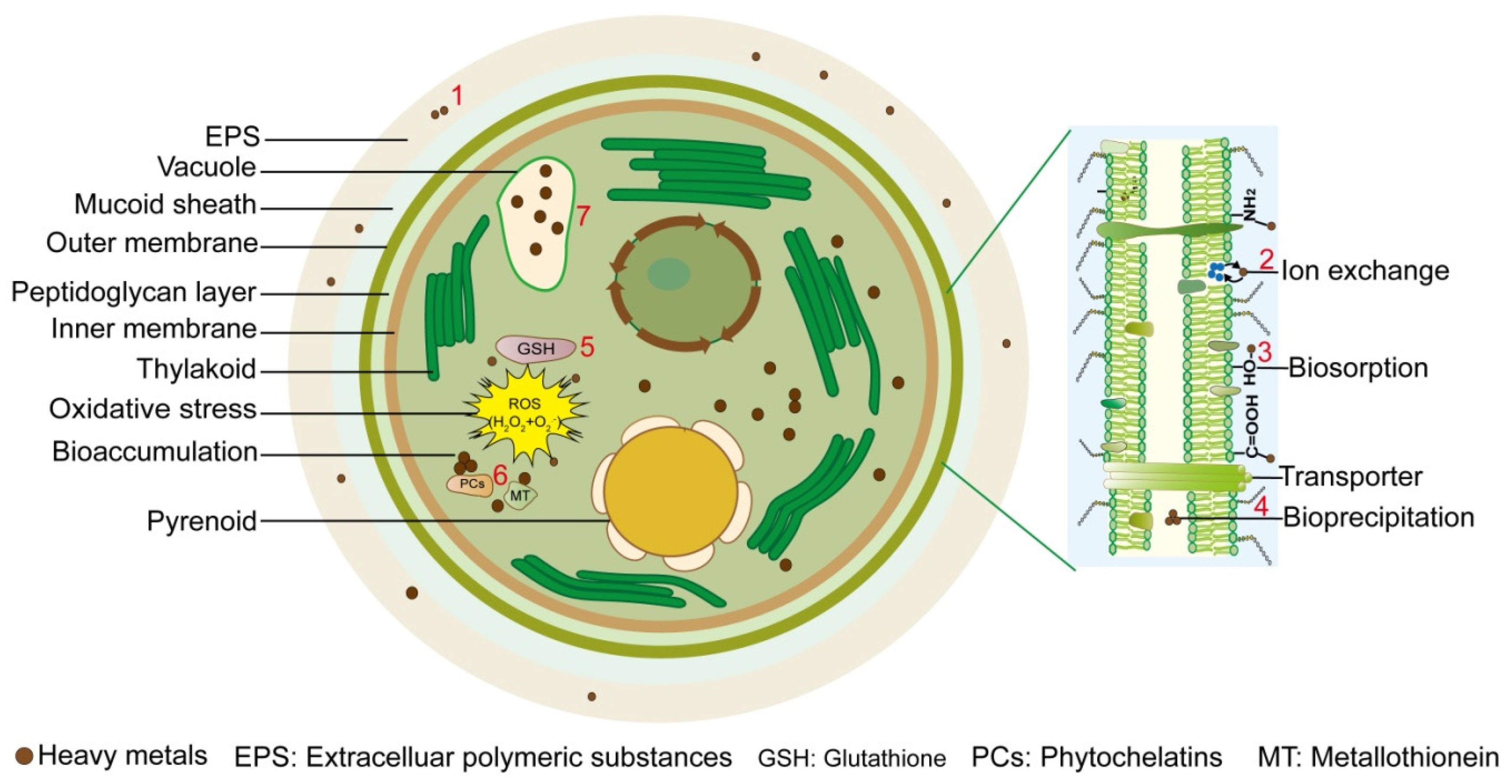
2. Mechanisms for Removal of Heavy Metals by Microalgae
3. Microalgae Show High Removal Efficiencies to Low Levels of Heavy Metals
4. Exogenous Chemical Additives
| Microalga Species | Additives | Effects on Algal Growth or HM Biosorption Capacity | Reference |
|---|---|---|---|
| Scenedesmus subspicatus | EDTA Fulvic acid | Significantly reduce the concentration of Cu adsorbed by the cell wall | [67] |
| Chlorella pyrenoidosa | Citric acid | Removal rate increased from 81% to 87% for 0.0016–0.025 mM Cu | [68] |
| Fulvic acid | Removal rate increased from 81% to 87% for 0.0016–0.025 mM Cu | ||
| Humic acid | Removal rate increased from 81% to 88% for 0.0016–0.025 mM Cu | ||
| Chlorella vulgaris | Fulvic acid | Specific growth rate increased by 10% under 0.5 mg/L Cr; removal rate increased from 54% to 62% | [69] |
| Chlamydomonas moewusii | Sulfate ions | 1 mM sulphate increased EC50 from 0.5 mg Cd/L to 4.46 mg Cd/L | [73] |
| Multiple microalgae | Phosphate | 100% higher Chl content at 5 mg/L ZnSO4·7H2O in a microalgal-bacterial symbiosis system | [80] |
| Chlorella pyrenoidosa | Salicylic acid | 60% higher cell density at 3.0 mg/L Cd and 96 h | [82] |
| Chlorella vulgaris | Homoserine lactones | 10% higher Chl content at 100 μg/L Cd in a algae-bacteria consortia | [84] |
| Parachlorella kessleri R-3 | Sodium nitro-prusside (SNP) | Lipid content increased from 51% to 60% at 5 μg/L Tl+ (control content was 38%) | [85] |
5. Genetic Manipulation
6. Microalgal Strain Selection
| Microalga Species | Types of HMs | Effects on Algal Growth or HM Biosorption Capacity | Reference |
|---|---|---|---|
| Scenedesmus acutus | Cd | Inhibition rate of growth decreased from 82% to 58% at 4.5 μM Cd | [42] |
| Dyctiosphaerium chlorelloides | Cr | IC50 of K2Cr2O7 increased by 18 times; IC50 of K2CrO4 increased by 208 times | [93] |
| Chlamydomonas CPCC 121 | Cd | 10–25% higher relative cell division rate than the control strain at 100–600 μM Cd | [94] |
| Desmodesmus sp. MAS1 | Cd | strain MAS1 was tolerant 20 mg L−1 Cd; Control strain MAS3 was tolerant 5 mg L−1 Cd | [97] |
| Chlamydomonas reinhardtii | U | Ancestral strain of 4.30 mg U g−1 DW; Selected strain of 6.34 mg U g−1 DW | [99] |
| Coelastrella sp. PCV | U | 25–55% removal of 70–1100 ng in 20 mL culture medium | [100] |
7. Immobilization Methods
8. Coupling HM Bioremediation and Biofuel Production
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.V.; Singh, R.P. Algal consortia based metal detoxification of municipal wastewater: Implication on photosynthetic performance, lipid production, and defense responses. Sci. Total Environ. 2022, 814, 151928. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.L.; Yu, Y.D.; Huang, H. Making waves: Microbe-photocatalyst hybrids may provide new opportunities for treating heavy metal polluted wastewater. Water Res. 2021, 195, 116984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Wang, R.; Tang, C.C.; Varrone, C.; He, Z.W.; Li, Z.H.; Wang, X.C. Advances, challenges, and prospects in microalgal-bacterial symbiosis system treating heavy metal wastewater. Chemosphere 2023, 345, 140448. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Cervera, B.; Garcia-Balboa, C.; Diaz-Alejo, H.M.; Costas, E.; Lopez-Rodas, V. Rapid colonization of uranium mining-impacted waters, the biodiversity of successful lineages of phytoplankton extremophiles. Microb. Ecol. 2020, 79, 576–587. [Google Scholar] [CrossRef]
- Watson, J.; Swoboda, M.; Aierzhati, A.; Wang, T.; Si, B.; Zhang, Y. Biocrude oil from algal bloom microalgae: A novel integration of biological and thermochemical techniques. Environ. Sci. Technol. 2021, 55, 1973–1983. [Google Scholar] [CrossRef]
- Chiu, S.; Kao, C.; Chen, T.; Chang, Y.; Kuo, C.; Lin, C. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Kundu, D.; Dutta, D.; Samanta, P.; Dey, S.; Sherpa, K.C.; Kumar, S.; Dubey, B.K. Valorization of wastewater: A paradigm shift towards circular bioeconomy and sustainability. Sci. Total Environ. 2022, 848, 157709. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Lindberg, R.H.; Tysklind, M.; Funk, C. Northern green algae have the capacity to remove active pharmaceutical ingredients. Ecotoxicol. Environ. Saf. 2019, 170, 644–656. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Giráldez, I.; Vega, J.M.; Vigara, J. Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquat. Toxicol. 2021, 239, 105941. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.; Jaiswal, K.K.; Kumar, V.; Verma, M.; Vlaskin, M.S.; Gururani, P.; Kim, H.; Alajmi, M.F.; Hussain, A. Bio-remediation capacity for Cd(II) and Pb(II) from the aqueous medium by two novel strains of microalgae and their effect on lipidomics and metabolomics. J. Water Proc. Eng. 2021, 44, 102404. [Google Scholar] [CrossRef]
- Das, S.; Kumar, S.; Kumar Mehta, A.; Ghangrekar, M.M. Heavy metals removal by algae and usage of activated metal-enriched biomass as cathode catalyst for improving performance of photosynthetic microbial fuel cell. Bioresour. Technol. 2024, 406, 131038. [Google Scholar] [CrossRef]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of two different strains of green microalgae for Cu and Ni removal from aqueous media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal extracellular polymeric substances and their interactions with metal(loid)s: A review. Crit. Rev. Environ. Sci. Tec. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Tripathi, S.; Poluri, K.M. Heavy metal detoxification mechanisms by microalgae: Insights from transcriptomics analysis. Environ. Pollut. 2021, 285, 117443. [Google Scholar] [CrossRef]
- Pagliaccia, B.; Carretti, E.; Severi, M.; Berti, D.; Lubello, C.; Lotti, T. Heavy metal biosorption by Extracellular Polymeric Substances (EPS) recovered from anammox granular sludge. J. Hazard. Mater. 2022, 424, 126661. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Schiewer, S.; Wong, M.H. Ionic strength effects in biosorption of metals by marine algae. Chemosphere 2000, 41, 271–282. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.H.; Chang, J.S. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef] [PubMed]
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef]
- Yang, T.; Chen, M.L.; Wang, J.H. Genetic and chemical modification of cells for selective separation and analysis of heavy metals of biological or environmental significance. Trends Anal. Chem. 2015, 66, 90–102. [Google Scholar] [CrossRef]
- Priya, A.K.; Jalil, A.A.; Vadivel, S.; Dutta, K.; Rajendran, S.; Fujii, M.; Soto-Moscoso, M. Heavy metal remediation from wastewater using microalgae: Recent advances and future trends. Chemosphere 2022, 305, 135375. [Google Scholar] [CrossRef]
- Caner, C.; Erdaği, D.; Şeker, B.; Altundağ, H.; Çeti, N.G.; Tunca, H. Effects of molybdenum to growth parameters and lipid content of two algae in Scenedesmaceae taxa. Heliyon 2024, 11, e40847. [Google Scholar] [CrossRef]
- Li, H.G.; Watson, J.; Zhang, Y.H.; Lu, H.F.; Liu, Z.D. Environment-enhancing process for algal wastewater treatment, heavy metal control and hydrothermal biofuel production: A critical review. Bioresour. Technol. 2020, 298, 122421. [Google Scholar] [CrossRef]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Parthiba Karthikeyan, O.; Kumar, G.; Kumar Tyagi, V.; Rajesh Banu, J. Algal-based system for removal of emerging pollutants from wastewater: A review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef]
- Cao, M.; Yang, D.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Sun, K. Extracellular polymeric substances altered the physicochemical properties of molybdenum disulfide nanomaterials to mitigate its toxicity to Chlorella vulgaris. NanoImpact 2023, 32, 100485. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Yuan, S.; Guo, Y.C.; Tan, Y.Y.; Mao, H.T.; Cao, Y.; Chen, Y.E. Highly efficient and sustainable removal of Cr (VI) in aqueous solutions by photosynthetic bacteria supplemented with phosphor salts. Chemosphere 2021, 283, 131031. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Min, S.N.; Jian, X.Y.; Guo, Y.C.; He, S.H.; Huang, C.Y.; Zhang, Z.; Yuan, S.; Chen, Y.E. Bioreduction mechanisms of high-concentration hexavalent chromium using sulfur salts by photosynthetic bacteria. Chemosphere 2023, 311 Pt 1, 136861. [Google Scholar] [CrossRef]
- Qian, Z.; Yanqiu, S.; Lin, G.; Hongmei, D.; Lihan, Z.; Shuangnan, M.; Shu, Y.; Yanger, C.; Qi, L. Sulfur source promotes the biosorption and bioprecipitation of Cd in purple non-sulfur bacteria. Int. Biodeter. Biodegr. 2024, 188, 105742. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Lee, D.J.; Tay, J.H.; Zhang, Y.; Wan, C.L.; Chen, X.F. Recent advances on biosorption by aerobic granular sludge. J. Hazard. Mater. 2018, 357, 253–270. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, R.A.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Microalgae in aquatic environs: A sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innov. 2021, 21, 101340. [Google Scholar] [CrossRef]
- Ghomi, A.G.; Asasian-Kolur, N.; Sharifian, S.; Golnaraghi, A. Biosorpion for sustainable recovery of precious metals from wastewater. J. Environ. Chem. Eng. 2020, 8, 103996. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Ahuja, V.; Chandel, N.; Mehariya, S.; Kumar, P.; Vinayak, V.; Saratale, G.D.; Raj, T.; Kim, S.H.; Yang, Y.H. An overview on microalgal-bacterial granular consortia for resource recovery and wastewater treatment. Bioresour. Technol. 2022, 351, 127028. [Google Scholar] [CrossRef]
- Bhuvaneshwari, M.; Thiagarajan, V.; Nemade, P.; Chandrasekaran, N.; Mukherjee, A. Toxicity and trophic transfer of P25 TiO2 NPs from Dunaliella salina to Artemia salina: Effect of dietary and waterborne exposure. Environ. Res. 2018, 160, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, E.; Gorbi, G.; Pawlik-Skowronska, B.; Di Toppi, L.S.; Corradi, M.G. Cadmium tolerance, cysteine and thiol peptide levels in wild type and chromium-tolerant strains of Scenedesmus acutus (Chlorophyceae). Aquat. Toxicol. 2004, 68, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zheng, Y.; Ge, Y. Phytochelatin synthesis in Dunaliella salina induced by arsenite and arsenate under various phosphate regimes. Ecotoxicol. Environ. Saf. 2017, 136, 150–160. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Biol. 2000, 3, 211–216. [Google Scholar] [CrossRef]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. Arab. Book 2011, 9, e0142. [Google Scholar] [CrossRef]
- Li, M.; Barbaro, E.; Bellini, E.; Saba, A.; Sanità di Toppi, L.; Varotto, C. Ancestral function of the phytochelatin synthase C-terminal domain in inhibition of heavy metal-mediated enzyme overactivation. J. Exp. Bot. 2020, 71, 6655–6669. [Google Scholar] [CrossRef]
- Tsuji, N.; Hirayanagi, N.; Okada, M.; Miyasaka, H.; Hirata, K.; Zenk, M.H.; Miyamoto, K. Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochem. Biophys. Res. Commun. 2002, 293, 653–659. [Google Scholar] [CrossRef]
- Clemens, S. Evolution and function of phytochelatin synthases. J. Plant Physiol. 2006, 163, 319–332. [Google Scholar] [CrossRef]
- Cai, X.H.; Brown, C.; Adhiya, J.; Traina, S.J.; Sayre, R.T. Growth and heavy metal binding properties of transgenic Chlamydomonas expressing a foreign metallothionein gene. Int. J. Phytoremediat. 1999, 1, 53–65. [Google Scholar] [CrossRef]
- Han, S.; Hu, Z.; Lei, A. Expression and function analysis of the metallothionein-like (MT-like) gene from Festuca rubra in Chlamydomonas reinhardtii chloroplast. Sci. China C Life Sci. 2008, 51, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Wan, C.; Zhao, X.Q.; Chen, L.J.; Chang, J.S.; Bai, F.W. Enhanced removal of Zn2+ or Cd2+ by the flocculating Chlorella vulgaris JSC-7. J. Hazard. Mater. 2015, 289, 38–45. [Google Scholar] [CrossRef]
- Birungi, Z.S.; Chirwa, E.M.N. The adsorption potential and recovery of thallium using green microalgae from eutrophic water sources. J. Hazard. Mater. 2015, 299, 67–77. [Google Scholar] [CrossRef]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative uptake study of arsenic, boron, copper, manganese and zinc from water by different green microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef]
- Andrade, L.M.; Tito, C.A.; Mascarenhas, C.; Lima, F.A.; Dias, M.; Andrade, C.J.; Mendes, M.A.; Nascimento, C.A.O. Chlorella vulgaris phycoremediation at low Cu2+ contents: Proteomic profiling of microalgal metabolism related to fatty acids and CO2 fixation. Chemosphere 2021, 284, 131272. [Google Scholar] [CrossRef]
- Chandrashekharaiah, P.S.; Sanyal, D.; Dasgupta, S.; Banik, A. Cadmium biosorption and biomassproduction by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa: An integrated approach. Chemosphere 2021, 269, 128755. [Google Scholar]
- Rahmani, A.; Zerrouki, D.; Tabchouche, A.; Djafer, L. Oilfield-produced water as a medium for the growth of Chlorella pyrenoidosa outdoor in an arid region. Environ. Sci. Pollut. Res. Int. 2022, 29, 87509–87518. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.F.; Kong, F.; Song, Q.; Ren, N.Q.; Ren, H.Y. New insights into rare earth element-induced microalgae lipid accumulation: Implication for biodiesel production and adsorption mechanism. Water Res. 2024, 251, 121134. [Google Scholar] [CrossRef]
- Shen, L.; Saky, S.A.; Yang, Z.; Ho, S.H.; Chen, C.; Qin, L.; Zhang, G.; Wang, Y.; Lu, Y. The critical utilization of active heterotrophic microalgae for bioremoval of Cr(VI) in organics co-contaminated wastewater. Chemosphere 2019, 228, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Tattibayeva, Z.; Tazhibayeva, S.; Kujawski, W.; Zayadan, B.; Musabekov, K. Peculiarities of adsorption of Cr (VI) ions on the surface of Chlorella vulgaris ZBS1 algae cells. Heliyon 2022, 8, e10468. [Google Scholar] [CrossRef]
- Aththanayake, A.M.K.C.B.; Rathnayake, I.V.N.; Deeyamulla, M.P.; Megharaj, M. Potential use of Chlorella vulgaris KCBAL01 from a freshwater stream receiving treated textile effluent in hexavalent chromium [Cr(VI)] removal in extremely acidic conditions. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2022, 57, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, C.; Yañez-Mansilla, E.; Jeison, D. Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res. 2019, 43, 101659. [Google Scholar] [CrossRef]
- Tambat, V.S.; Tseng, Y.S.; Kumar, P.; Chen, C.W.; Singhania, R.R.; Chang, J.S.; Dong, C.D.; Patel, A.K. Effective and sustainable bioremediation of molybdenum pollutants from wastewaters by potential microalgae. Environ. Technol. Innov. 2023, 30, 103091. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Leon-Miranda, E.; Llamas, A. Chlamydomonas reinhardtii—A reference microorganism for eukaryotic molybdenum metabolism. Microorganisms 2023, 11, 1671. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, W.; Wang, Z.; Witkamp, G.J. Accumulation, assimilation and growth inhibition of copper on freshwater alga (Scenedesmus subspicatus 86.81 SAG) in the presence of EDTA and fulvic acid. Aquat. Toxicol. 2003, 63, 221–228. [Google Scholar] [CrossRef]
- Shi, W.; Jin, Z.; Hu, S.; Fang, X.; Li, F. Dissolved organic matter affects the bioaccumulation of copper and lead in Chlorella pyrenoidosa: A case of long-term exposure. Chemosphere 2017, 174, 447–455. [Google Scholar] [CrossRef]
- Luo, L.; Yang, C.; Jiang, X.; Guo, W.; Ngo, H.H.; Wang, X.C. Impacts of fulvic acid and Cr (VI) on metabolism and chromium removal pathways of green microalgae. J. Hazard. Mater. 2023, 459, 132171. [Google Scholar] [CrossRef]
- Wu, Y.J.; Chen, B.L. Effect of fulvic acid coating on biochar surface structure and sorption properties towards 4-chlorophenol. Sci. Total Environ. 2019, 691, 595–604. [Google Scholar] [CrossRef]
- Kong, D.; Ma, H.; Zhu, C.; Hao, Y.; Li, C. Unraveling the toxicity response and metabolic compensation mechanism of tannic acid-Cr(III) complex on alga Raphidocelis subcapitata. Sci. Total Environ. 2024, 930, 172034. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, T.; Dzakpasu, M.; Jiang, X.; Guo, W.; Ngo, H.H.; Wang, X.C. Interplay of humic acid and Cr(VI) on green microalgae: Metabolic responses and chromium enrichment. J. Hazard. Mater. 2024, 480, 135885. [Google Scholar] [CrossRef] [PubMed]
- Mera, R.; Torres, E.; Abalde, J. Sulphate, more than a nutrient, protects the microalga Chlamydomonas moewusii from cadmium toxicity. Aquat. Toxicol. 2014, 148, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.K.; Mulchandani, P.; Hunter, T.C. Role of CdS quantum crystallites in cadmium resistance in Candida Glabrata. Biochem. Biophys. Res. Commun. 1994, 200, 1193–1200. [Google Scholar] [CrossRef]
- Dameron, C.T.; Winge, D.R. Characterization of peptide-coated cadmium-sulfide crystallites. Inorg. Chem. 1990, 29, 1343–1348. [Google Scholar] [CrossRef]
- Li, X.M.; Peng, W.H.; Jia, Y.Y.; Lu, L.; Fan, W.H. Bioremediation of lead contaminated soil with Rhodobacter sphaeroides. Chemosphere 2016, 156, 228–235. [Google Scholar] [CrossRef]
- Su, Y.; Shi, Q.; Li, Z.; Deng, H.; Zhou, Q.; Li, L.; Zhao, L.; Yuan, S.; Liu, Q.; Chen, Y. Rhodopseudomonas palustris shapes bacterial community, reduces Cd bioavailability in Cd contaminated flooding paddy soil, and improves rice performance. Sci. Total Environ. 2024, 926, 171824. [Google Scholar] [CrossRef]
- Shan, S.; Guo, Z.; Lei, P.; Wang, Y.; Li, Y.; Cheng, W.; Zhang, M.; Wu, S.; Yi, H. Simultaneous mitigation of tissue cadmium and lead accumulation in rice via sulfate- reducing bacterium. Ecotox. Environ. Saf. 2019, 169, 292–300. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Lin, M.; Gui, H.; Xiang, H.; Zhao, Q.; Fan, W. Biosafety of cadmium contaminated sediments after treated by indigenous sulfate reducing bacteria: Based on biotic experiments and DGT technique. J. Hazard. Mater. 2020, 384, 121439. [Google Scholar] [CrossRef]
- Tang, C.C.; Hu, Y.R.; Zhang, M.; Chen, S.L.; He, Z.W.; Li, Z.H.; Tian, Y.; Wang, X.C. Role of phosphate in microalgal-bacterial symbiosis system treating wastewater containing heavy metals. Environ. Pollut. 2024, 349, 123951. [Google Scholar] [CrossRef]
- Mao, H.T.; Chen, L.X.; Zhang, M.Y.; Shi, Q.Y.; Xu, H.; Zhang, D.Y.; Zhang, Z.W.; Yuan, M.; Yuan, S.; Su, Y.Q.; et al. Melatonin improves the removal and the reduction of Cr(VI) and alleviates the chromium toxicity by antioxidative machinery in Rhodobacter sphaeroides. Environ. Pollut. 2023, 319, 120973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, M.; Yan, H.; Li, C. Effects of salicylic acid on heavy metal resistance in eukaryotic algae and its mechanisms. Int. J. Environ. Res. Public Health 2022, 19, 13415. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, R.; Cui, X.; He, M.; Zheng, S.; Du, W.; Gao, M.; Wang, C. Co-culture of bacteria and microalgae for treatment of high concentration biogas slurry. J. Water Process Eng. 2021, 41, 102014. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, J.; Ye, M.; Wei, Y.; Zhang, C.; Ge, Y. N-acyl homoserine lactones (AHLs) enhanced removal of cadmium and other pollutants by algae-bacteria consortia. J. Environ. Manag. 2024, 366, 121792. [Google Scholar] [CrossRef]
- Song, X.; Kong, F.; Liu, B.F.; Song, Q.; Ren, N.Q.; Ren, H.Y. Thallium-mediated NO signaling induced lipid accumulation in microalgae and its role in heavy metal bioremediation. Water Res. 2023, 239, 120027. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.; Lau, F.; Chang, J.; Ling, T.C. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Henríquez, V.; Mayfield, S.P. In metabolic engineering of eukaryotic microalgae: Potential and challenges come with great diversity. Front. Microbiol. 2015, 6, 1376. [Google Scholar] [CrossRef]
- Hassanien, A.; Saadaoui, I.; Schipper, K.; Al-Marri, S.; Dalgamouni, T.; Aouida, M.; Saeed, S.; Al-Jabri, H.M. Genetic engineering to enhance microalgal-based produced water treatment with emphasis on CRISPR/Cas9: A review. Front. Bioeng. Biotechnol. 2023, 10, 1104914. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef]
- Ibuot, A.; Dean, A.P.; McIntosh, O.A.; Pittman, J.K. Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res. 2017, 24, 89–96. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, M.W.; Hsieh, J.L.; Lin, W.H.; Chen, P.C.; Chien, L.F. Expression of mercuric reductase from Bacillus megaterium MB1 in eukaryotic microalga Chlorella sp. DT: An approach for mercury phytoremediation. Appl. Microbiol. Biotechnol. 2006, 72, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, K.; Kavanagh, K.; Khan, I.; Slaveykova, V.I.; Sieber, S. Surface displayed MerR increases mercury accumulation by green microalga Chlamydomonas reinhardtii. Environ. Int. 2024, 189, 108813. [Google Scholar] [CrossRef] [PubMed]
- D’ors, A.; Pereira, M.; Bartolomé, M.C.; López-Rodas, V.; Costas, E.; Sánchez-Fortún, S. Toxic effects and specific chromium acquired resistance in selected strains of Dyctiosphaerium chlorelloides. Chemosphere 2010, 81, 282–287. [Google Scholar] [CrossRef]
- Samadani, M.; Perreault, F.; Oukarroum, A.; Dewez, D. Effect of cadmium accumulation on green algae Chlamydomonas reinhardtii and acid-tolerant Chlamydomonas CPCC 121. Chemosphere 2018, 191, 174–182. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Cole, N.; Dharmarajan, R.; Venkateswarlu, K.; Megharaj, M. Sustainable production of biomass and biodiesel by acclimation of non-acidophilic microalgae to acidic conditions. Bioresour. Technol. 2019, 271, 316–324. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Panneerselvan, L.; Venkateswarlu, K.; Megharaj, M. Potential of acid-tolerant microalgae, Desmodesmus sp. MAS1 and Heterochlorella sp. MAS3, in heavy metal removal and biodiesel production at acidic pH. Bioresour. Technol. 2019, 278, 9–16. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Perera, I.A.; Megharaj, M. Acid-tolerant microalgae can withstand higher concentrations of invasive cadmium and produce sustainable biomass and biodiesel at pH 3.5. Bioresour. Technol. 2019, 281, 469–473. [Google Scholar] [CrossRef]
- García-Balboa, C.; Baselga-Cervera, B.; García-Sanchez, A.; Igual, J.M.; Lopez-Rodas, V.; Costas, E. Rapid adaptation of microalgae to bodies of water with extreme pollution from uranium mining: An explanation of how mesophilic organisms can rapidly colonise extremely toxic environments. Aquat. Toxicol. 2013, 44–45, 116–123. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; Romero-Lopez, J.; Garcia-Balboa, C.; Costas, E.; Lopez-Rodas, V. Improvement of the uranium sequestration ability of a Chlamydomonas sp. (ChlSP Strain) isolated from extreme uranium mine tailings through selection for potential bioremediation application. Front. Microbiol. 2018, 9, 523. [Google Scholar] [CrossRef]
- Beaulier, C.; Dannay, M.; Devime, F.; Galeone, A.; Baggio, C.; El Sakkout, N.; Raillon, C.; Courson, O.; Bourguignon, J.; Alban, C.; et al. Characterization of a uranium-tolerant green microalga of the genus Coelastrella with high potential for the remediation of metal-polluted waters. Sci. Total Environ. 2024, 908, 168195. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Jin, S.; Zhu, L.; Liu, C.; Zheng, H.; Zhou, T.; Liu, Y.; Ruan, R. Lignocellulosic residue as bio-carrier for algal biofilm growth: Effects of carrier physicochemical proprieties and toxicity on algal biomass production and composition. Bioresour. Technol. 2019, 293, 122091. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; Cubero-Cardoso, J.; Trujillo-Reyes, Á.; Fermoso, F.G.; León, R.; Funk, C.; Vigara, J.; Urbano, J. Enhanced wastewater bioremediation by a sulfur-based copolymer asscaffold for microalgae immobilization (AlgaPol). Chemosphere 2023, 315, 137761. [Google Scholar] [CrossRef]
- Tao, S.; Li, C.; Fan, X.; Zeng, G.; Lu, P.; Zhang, X.; Wen, Q.; Zhao, W.; Luo, D.; Fan, C. Activated coke impregnated with cerium chloride used for elemental mercury removal from simulated flue gas. Chem. Eng. J. 2012, 210, 547–556. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Tao, Q.; Li, B.; Chen, Y.; Zhao, J.; Li, Q.; Chen, Y.; Peng, Q.; Yuan, S.; Li, H.; Huang, R.; et al. An integrated method to produce fermented liquid feed and biologically modified biochar as cadmium adsorbents using corn stalks. Waste Manag. 2021, 127, 112–120. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, S.; Yin, X.; Tian, Y.; Liu, Y.; Deng, Z.; Wang, L. Contrasting effects of a novel biochar-microalgae complex on arsenic and mercury removal. Ecotox. Environ. Saf. 2023, 262, 115144. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, X.; Gao, T.; Li, X.; Wang, G.; Pan, X.; Wang, J. Dielectrophoresis-assisted removal of Cd and Cu heavy metal ions by using Chlorella microalgae. Environ. Pollut. 2023, 334, 122110. [Google Scholar] [CrossRef]
- Tang, C.C.; Tian, Y.; Liang, H.; Zuo, W.; Wang, Z.W.; Zhang, J.; He, Z.W. Enhanced nitrogen and phosphorus removal from domestic wastewater via algae-assisted sequencing batch biofilm reactor. Bioresour. Technol. 2018, 250, 185–190. [Google Scholar] [CrossRef]
- Tang, C.C.; Tian, Y.; He, Z.W.; Zuo, W.; Zhang, J. Performance and mechanism of a novel algal-bacterial symbiosis system based on sequencing batch suspended biofilm reactor treating domestic wastewater. Bioresour. Technol. 2018, 265, 422–431. [Google Scholar] [CrossRef]
- Tang, C.C.; Wang, T.Y.; Zhang, X.Y.; Wang, R.; He, Z.W.; Li, Z.; Wang, X.C. Role of types and dosages of cations with low valance states on microalgal-bacterial symbiosis system treating wastewater. Bioresour. Technol. 2022, 361, 127755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Q.; Cui, L.; Cheng, J.; Zhou, H.; Peng, A.; Qiu, G.; Shen, L. Effect of extracellular proteins on Cd(II) adsorption in fungus and algae symbiotic system. J. Environ. Manag. 2022, 323, 116173. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef]
- Craggs, R.; Heubeck, S.; Lundquist, T.; Benemann, J. Algal biofuels from wastewater treatment high rate algal ponds. Water Sci. Technol. 2011, 63, 660–665. [Google Scholar] [CrossRef]
- Kesaano, M.; Sims, R.C. Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, X.; Su, G.; Danquah, M.K.; Zhang, S.; Lu, Y.; Sun, Y.; Lin, L. Bioprocess considerations for microalgal-based wastewater treatment and biomass production. Renew. Sustain. Energy Rev. 2015, 42, 1385–1392. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Raeesossadati, M.J.; Ahmadzadeh, H.; McHenry, M.P.; Moheimani, N.R. CO2 bioremediation by microalgae in photobioreactors: Impacts of biomass and CO2 concentrations, light, and temperature. Algal Res. 2014, 6 Pt A, 78–85. [Google Scholar] [CrossRef]
- Craggs, R.; Sutherland, D.; Campbell, H. Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol. 2012, 24, 329–337. [Google Scholar] [CrossRef]
- Cao, S.; Teng, F.; Lv, J.; Zhang, Q.; Wang, T.; Zhu, C.; Li, X.; Cai, Z.; Xie, L.; Tao, Y. Performance of an immobilized microalgae-based process for wastewater treatment and biomass production: Nutrients removal, lipid induction, microalgae harvesting and dewatering. Bioresour. Technol. 2022, 356, 127298. [Google Scholar] [CrossRef] [PubMed]
- Changko, S.; Rajakumar, P.D.; Young, R.E.B.; Purton, S. The phosphite oxidoreductase gene, ptxD as a bio-contained chloroplast marker and crop-protection tool for algal biotechnology using Chlamydomonas. Appl. Microbiol. Biotechnol. 2020, 104, 675–686. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, L.; Lin, W.; Xie, Z.; Luo, J. Potential of using sodium bicarbonate as external carbon source to cultivate microalga in non-sterile condition. Bioresour. Technol. 2018, 266, 109–115. [Google Scholar] [CrossRef]
- de la Broise, D.; Ventura, M.; Chauchat, L.; Guerreiro, M.; Michez, T.; Vinet, T.; Gautron, N.; Le Grand, F.; Bideau, A.; Goïc, N.L.; et al. Scale-up to pilot of a non-axenic culture of Thraustochytrids using digestate from methanization as nitrogen source. Mar. Drugs 2022, 20, 499. [Google Scholar] [CrossRef]
- Phyu, K.; Zhi, S.; Graham, D.W.; Cao, Y.; Xu, X.; Liu, J.; Wang, H.; Zhang, K. Impact of indigenous vs. cultivated microalgae strains on biomass accumulation, microbial community composition, and nutrient removal in algae-based dairy wastewater treatment. Bioresour. Technol. 2025, 426, 132349. [Google Scholar] [CrossRef]
- Raslavičius, L.; Semenov, V.G.; Chernova, N.I.; Keršys, A.; Kopeyka, A.K. Producing transportation fuels from algae: In search of synergy. Renew. Sustain. Energy Rev. 2014, 40, 133–142. [Google Scholar] [CrossRef]
- Raslavičius, L.; Striūgas, N.; Felneris, M. New insights into algae factories of the future. Renew. Sustain. Energy Rev. 2018, 81, 643–654. [Google Scholar] [CrossRef]
| Microalga Species | Types of HMs | Removal Efficiency | Reference |
|---|---|---|---|
| Chlorella vulgaris | Cu | 39% removal of 11.9 mg/L | [15] |
| Desmodesmus sp. | Cu | 43% removal of 11.9 mg/L | |
| Chlorella vulgaris | Ni | 32% removal of 5.7 mg/L | |
| Desmodesmus sp. | Ni | 39% removal of 5.7 mg/L | |
| Flocculating Chlorella vulgaris JSC-7 | Zn | 89% for 20 mg/L | [54] |
| Cd | 62% for 4 mg/L | ||
| Non-flocculating Chlorella vulgaris CNW11 | Zn | 40% for 20 mg/L | |
| Cd | 25% for 4 mg/L | ||
| Scenedesmus acuminutus | Tl | 100% for 150 mg/L; 91% for 250 mg/L; 87% for 500 mg/L | [55] |
| Chlorella vulgaris | Tl | 100% for 150 mg/L; 89% for 250 mg/L; 96% for 500 mg/L | |
| Chlamydomonas reinhardtii | Tl | 100% for 150 mg/L; 94% for 250 mg/L; 95% for 500 mg/L | |
| Chlorella vulgaris | Mn | 99.4% for 3 mg/L | [56] |
| Cu | 87.9% for 3 mg/L | ||
| Zn | 88.8% for 3 mg/L | ||
| Scenedesmus almeriensis | As | 40.7% for 12 mg/L | |
| B | 38.6% for 60 mg/L | ||
| Chlorella vulgaris | Cu | 100, 74, 38 and 26% for 0.1, 0.3, 0.6 and 0.9 mg/L | [57] |
| Chlorella pyrenoidosa | Cd | 45.45% removal of 1.5 ppm | [58] |
| Scenedesmus acutus | Cd | 57.14% removal of 1.5 ppm | |
| Chlorella pyrenoidosa | Pb | 72.86% for 3.64 mg/L | [59] |
| Cu | 73.39% for 3.27 mg/L | ||
| Cd | 48.42% for about 3 mg/L | ||
| Parachlorella kessleri R-3 | Ce | 66.2% of 100 μg/L Ce3+ | [60] |
| Gd | 48.4% of 250 μg/L Gd3+ | ||
| La | 59.9% of 1 mg/L La3+ | ||
| Botryocossuss sp. NJD-1 | Cr(VI) | 94.2% of 5 mg/L Cr(VI) | [61] |
| Chlorella vulgaris ZBS1 | Cr(VI) | 75.46% of 2.1 mg/L Cr(VI) | [62] |
| Chlorella vulgaris | Cr(VI) | 60.38% of 5 mg/L Cr(VI) at pH 2 | [63] |
| Chlorella vulgaris | Mo(VI) | 80.3% of 0.5 mg/L Mo(VI) | [64] |
| Chlorella sorokiniana TU5 | Mo(VI) | 57.8% with 115.65 mg/L Mo(VI) | [65] |
| Microalga Species | Genetic Modification Methods | Types of HMs | Effects on Algal Growth or HM Biosorption | Reference |
|---|---|---|---|---|
| Chlamydomonas reinhardtii | Expression of a class-II metallothionein | Cd | One-time higher cell density at 40 μM Cd | [50] |
| Chlamydomonas reinhardtii | Expression of metallothionein (MT)-like gene from Festuca rubra | Cd | IC50 of Cd increased by 55.43% | [51] |
| Chlamydomonas reinhardtii | Expression of a mothbean Δ1-pyrroline-5-carboxylate synthetase (P5CS) | Cd | Up to 75% higher cell density at 100 μM Cd | [89] |
| Chlamydomonas reinhardtii | Over-expression of metal tolerance protein CrMTP4 | Cd | Cell density increased by 50% at 0.4 mM Cd | [90] |
| Chlorella sp. DT | Expression of a Bacillus megaterium strain MB1 mercuric reductase (MerA) | Hg | Removal rate increased from <1% to 68% for 40 μM Hg | [91] |
| Chlamydomonas reinhardtii | Expression of a surface displayed metalloregulatory protein MerR | Hg | Five folds higher Hg2+ accumulation at 10−9 to 10−7 M Hg2+ | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-Y.; Wei, Y.-X.; Su, Y.-Q.; Zhang, Z.-W.; Tang, X.-Y.; Chen, Y.-E.; Yuan, M.; Yuan, S. The Strategies Microalgae Adopt to Counteract the Toxic Effect of Heavy Metals. Microorganisms 2025, 13, 989. https://doi.org/10.3390/microorganisms13050989
Yang X-Y, Wei Y-X, Su Y-Q, Zhang Z-W, Tang X-Y, Chen Y-E, Yuan M, Yuan S. The Strategies Microalgae Adopt to Counteract the Toxic Effect of Heavy Metals. Microorganisms. 2025; 13(5):989. https://doi.org/10.3390/microorganisms13050989
Chicago/Turabian StyleYang, Xin-Yue, Yu-Xin Wei, Yan-Qiu Su, Zhong-Wei Zhang, Xiao-Yan Tang, Yang-Er Chen, Ming Yuan, and Shu Yuan. 2025. "The Strategies Microalgae Adopt to Counteract the Toxic Effect of Heavy Metals" Microorganisms 13, no. 5: 989. https://doi.org/10.3390/microorganisms13050989
APA StyleYang, X.-Y., Wei, Y.-X., Su, Y.-Q., Zhang, Z.-W., Tang, X.-Y., Chen, Y.-E., Yuan, M., & Yuan, S. (2025). The Strategies Microalgae Adopt to Counteract the Toxic Effect of Heavy Metals. Microorganisms, 13(5), 989. https://doi.org/10.3390/microorganisms13050989







