Combination of Exhaust Gas Fermentation Effluent and Dairy Wastewater for Microalgae Production: Effect on Growth and FAME Composition of Chlorella sorokiniana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strain
2.2. Pre-Treatment of Wastewaters
2.3. Wastewaters Preliminary Screening for Microalgae Cultivation
2.4. Photobioreactor Working Conditions
2.5. Microalgal Growth Measurement
2.6. FAME Analysis
2.7. VFAs and Alcohol Uptake
2.8. Statistical Analysis
3. Results
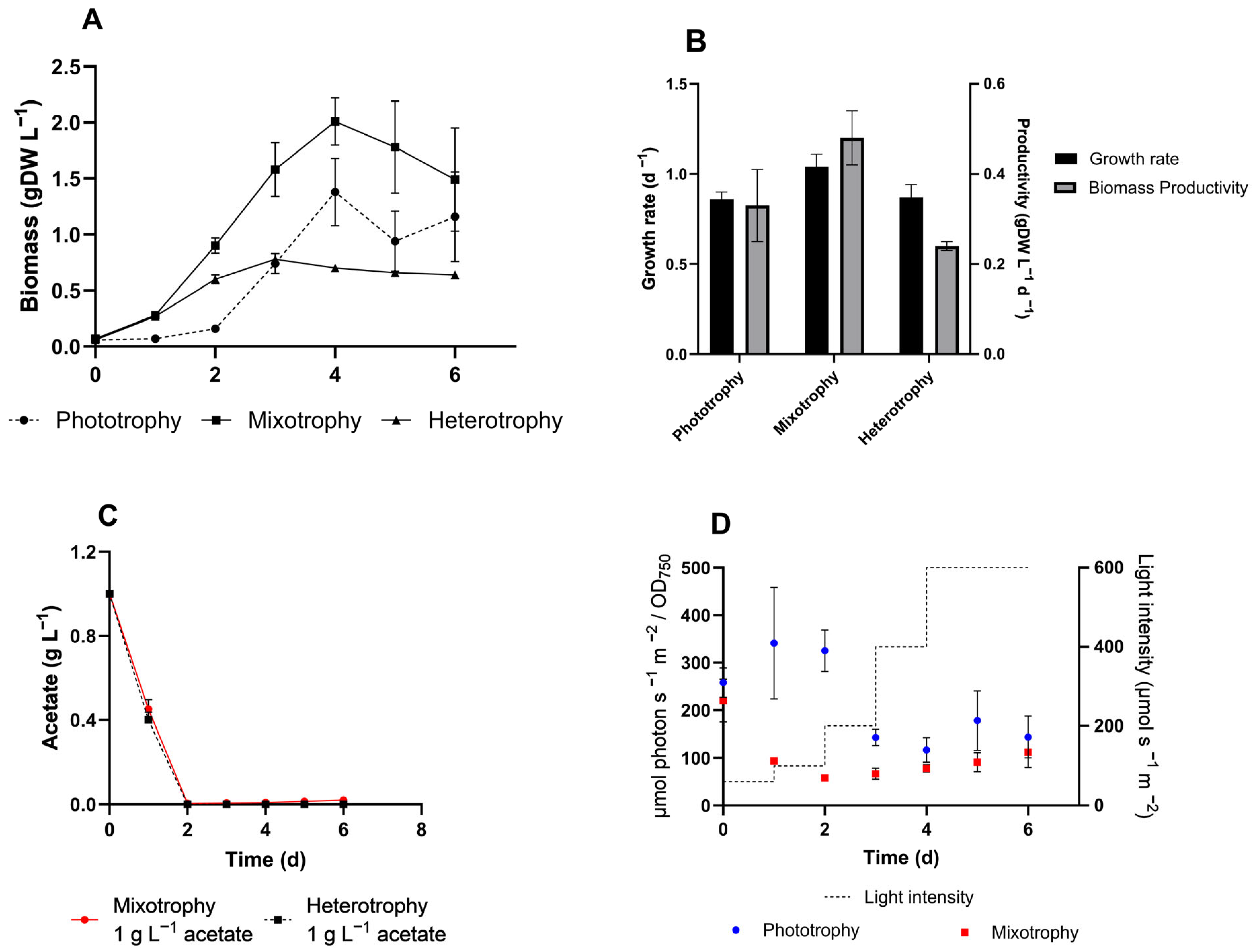
3.1. C. sorokiniana Metabolism Under Standard Conditions
3.2. Chemical Analysis of Exhausted Sludge and Gas Fermentation Effluent
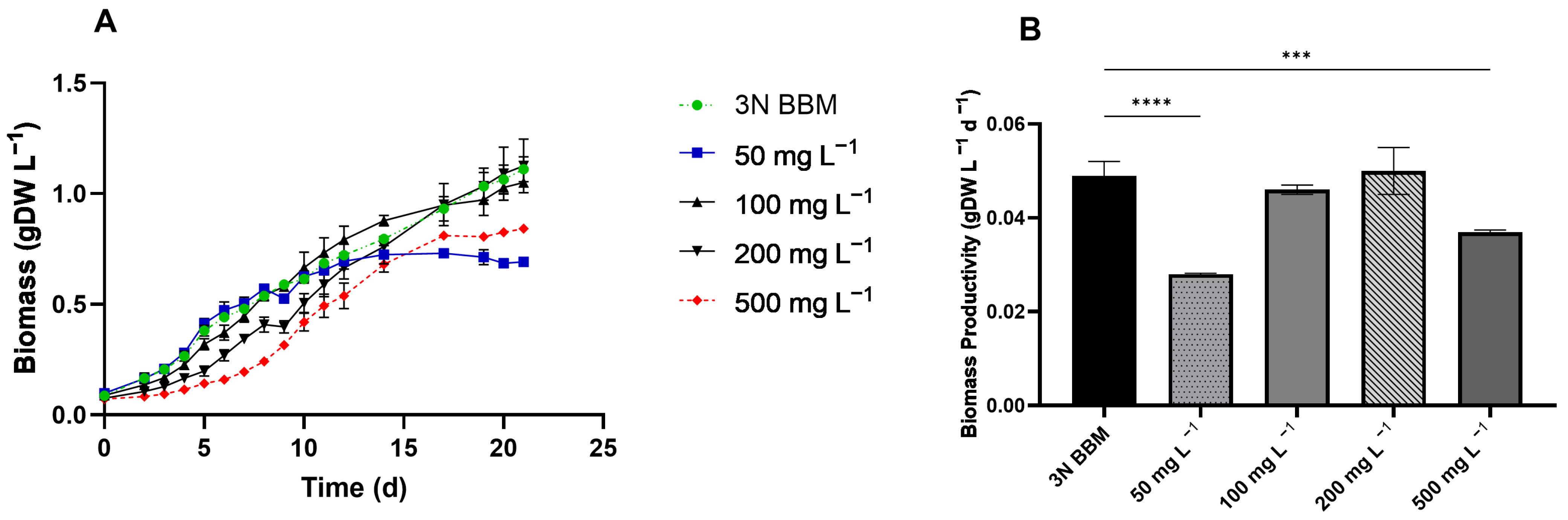
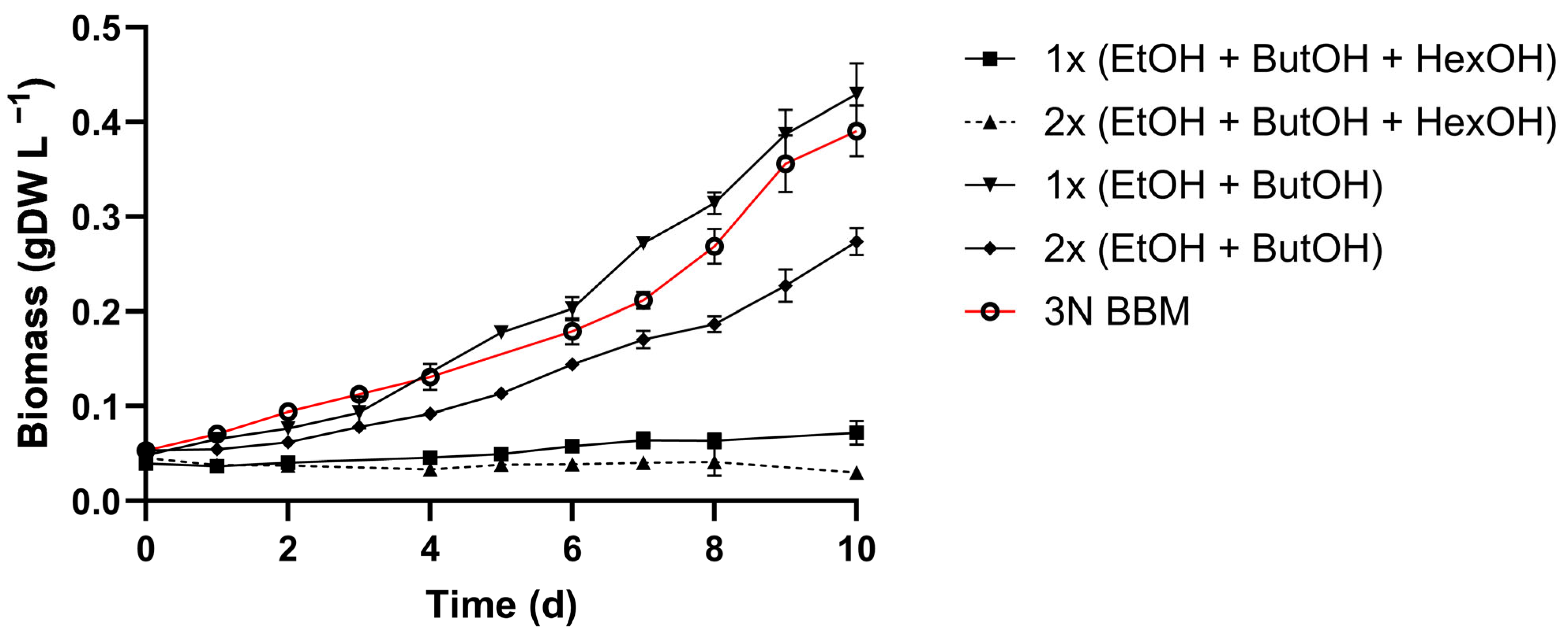
3.3. Ammonium and Alcohol Tolerance of C. sorokiniana
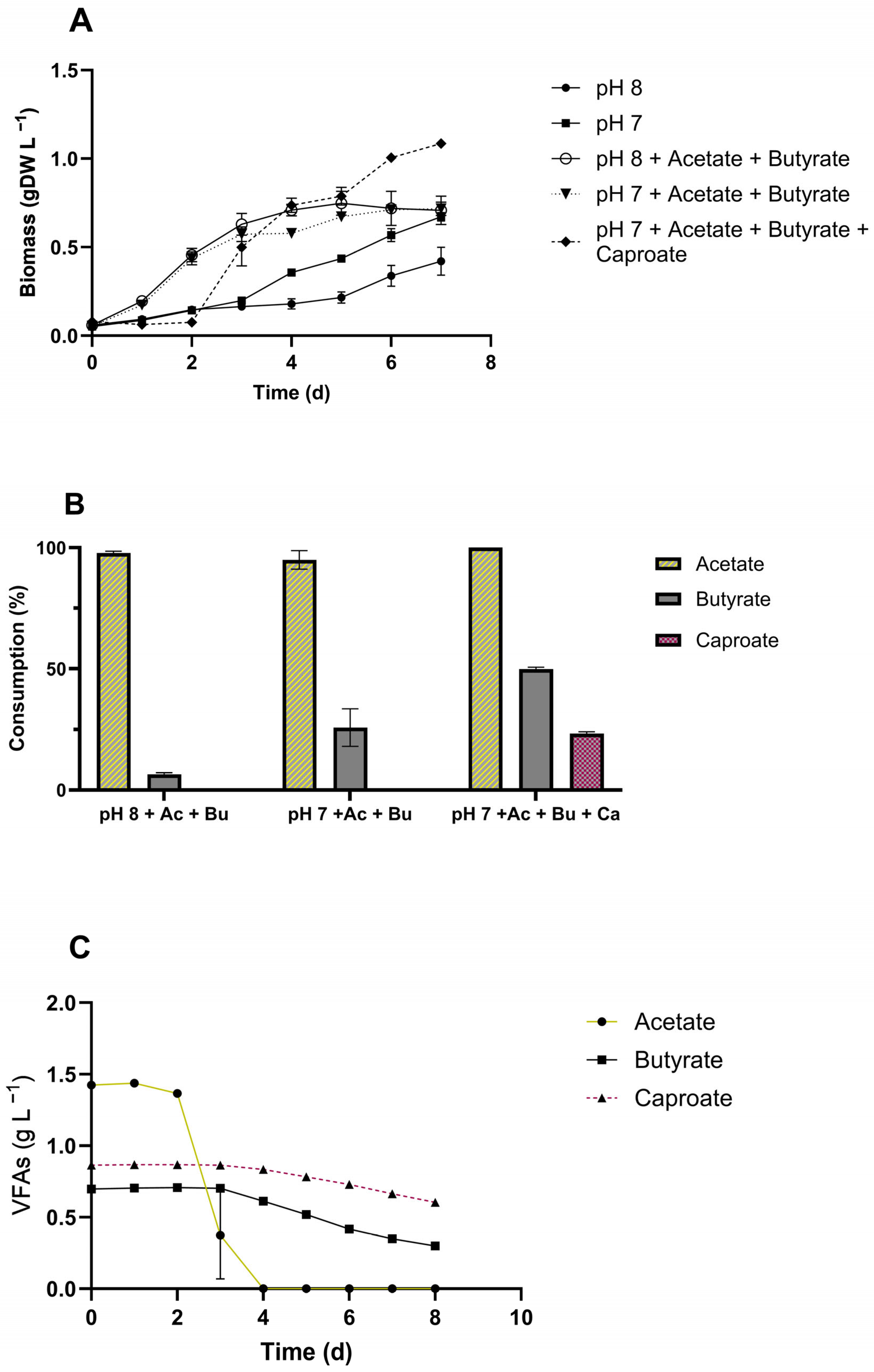
3.4. VFAs Assimilation by C. sorokiniana
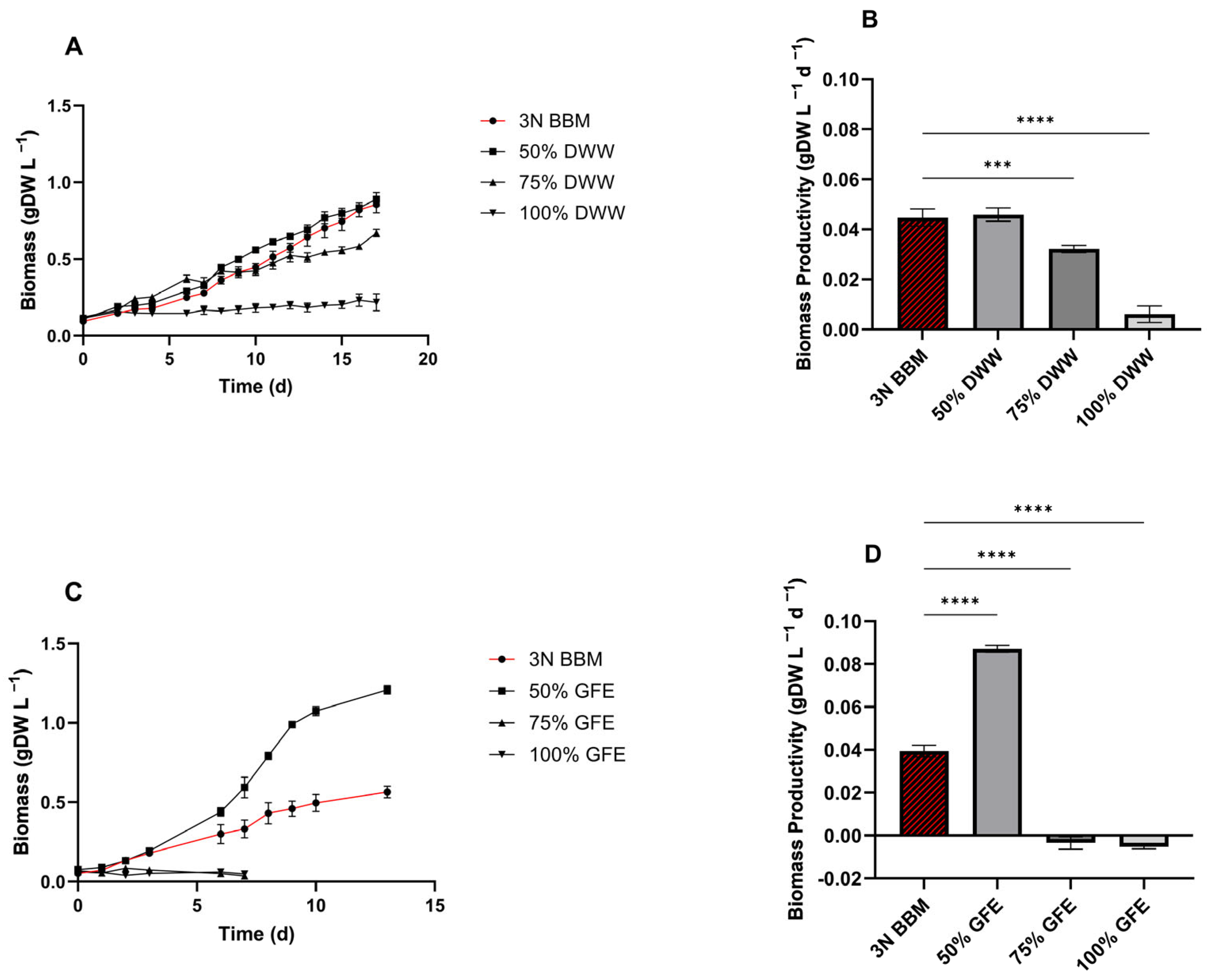
3.5. C. sorokiniana Growth on Wastewater from Dairy and Gas Fermentation Processes
3.6. Combination of Wastewater to Cultivate C. sorokiniana
3.7. FAME Profile of C. sorokininana on Wastewater
4. Discussion
4.1. Chemical Features of Wastewater Selected for C. sorokiniana
4.2. C. sorokiniana Tolerance and Utilization of Organic Carbon Component in Wastewater
4.3. Influence of pH on C. sorokiniana Uptake of Organic Acids
4.4. C. sorokiniana Growth Kinetics on Dairy Wastewater and Gas Fermentation Effluent
4.5. Combining Wastewater: GFE and DWW for Sustainable Cultivation of C. sorokiniana
4.6. Effect of Blending Wastewater on FAME Profile of C. sorokiniana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3NBBM | Basal Bold Medium with 3-fold Nitrogen |
| DW | Dried Weight |
| DWW | Dairy Wastewater |
| FA | Fatty Acids |
| FAME | Fatty Acid Methyl Esters |
| GC | Gas Chromatography |
| GFE | Gas Fermentation Effluent |
| HPLC | High Performance Liquid Chromatography |
| MUFA | Mono-Unsaturated Fatty Acids |
| OD750 | Optical Density at 750 nm |
| PBR | Photo-bioreactor |
| PIPES | 1,4-Piperazinediethanesulfonic acid |
| PUFA | Polyunsaturated Fatty Acids |
| SFA | Saturated Fatty Acids |
| TAG | Triacylglycerols |
| TES | 2-([Tris(hydroxymethyl)methyl]amino)ethane-1-sulfonic acid sodium salt |
| VFA | Volatile Fatty Acids |
| WW | Wastewater |
References
- Sadvakasova, A.K.; Kossalbayev, B.D.; Bauenova, M.O.; Balouch, H.; Leong, Y.K.; Zayadan, B.K.; Huang, Z.; Alharby, H.F.; Tomo, T.; Chang, J.S.; et al. Microalgae as a key tool in achieving carbon neutrality for bioproduct production. Algal Res. 2023, 72, 103096. [Google Scholar] [CrossRef]
- Miazek, K.; Kratky, L.; Sulc, R.; Jirout, T.; Aguedo, M.; Richel, A.; Goffin, D. Effect of Organic Solvents on Microalgae Growth, Metabolism and Industrial Bioproduct Extraction: A Review. Int. J. Mol. Sci. 2017, 18, 1429. [Google Scholar] [CrossRef] [PubMed]
- Velea, S.; Oancea, F.; Fischer, F. 2—Heterotrophic and Mixotrophic Microalgae Cultivation; Gonzalez-Fernandez, C., Muñoz, B., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2017; pp. 45–65. [Google Scholar]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A Review of Microalgae-and Cyanobacteria-Based Biodegradation of Organic Pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Shan, S.; Manyakhin, A.Y.; Wang, C.; Ge, B.; Han, J.; Zhang, X.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. Mixotrophy, a more promising culture mode: Multi-faceted elaboration of carbon and energy metabolism mechanisms to optimize microalgae culture. Bioresour. Technol. 2023, 386, 129512. [Google Scholar] [CrossRef]
- Castillo, T.; Ramos, D.; García-Beltrán, T.; Brito-Bazan, M.; Galindo, E. Mixotrophic cultivation of microalgae: An alternative to produce high-value metabolites. Biochem. Eng. J. 2021, 176, 108183. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-value biomass from microalgae production platforms: Strategies and progress based on carbon metabolism and energy conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar]
- Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies. Sustainability 2022, 14, 15070. [Google Scholar] [CrossRef]
- Musa, M.; Ayoko, G.A.; Ward, A.; Rösch, C.; Brown, R.J.; Rainey, T.J. Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity. Cells 2019, 8, 851. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef]
- Khanum, F.; Giwa, A.; Nour, M.; Al-Zuhair, S.; Taher, H. Improving the economic feasibility of biodiesel production from microalgal biomass via high-value products coproduction. Int. J. Energy Res. 2020, 44, 11453–11472. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Udaypal Goswami, R.K.; Verma, P. Transforming dairy effluent into valuable resources: Harnessing microalgae for sustainable production of nutraceuticals and pharmaceuticals. Process Biochem. 2025, 150, 342–356. [Google Scholar] [CrossRef]
- Choi, H.J. Parametric study of brewery wastewater effluent treatment using Chlorella vulgaris microalgae. Environ. Eng. Res. 2016, 21, 401–408. [Google Scholar] [CrossRef]
- Christodoulou, X.; M’ahmed, C.; Zili, F.; Bessadok, B.; Sadok, S.; Monney, I.; Rothlisberger, R.; Bagnoud, M. Design and development of pilot photobioreactor for simultaneous microalgae cultivation and aquaculture wastewater treatment. Process Biochem. 2025, 153, 182–190. [Google Scholar] [CrossRef]
- Roy, H.P.; Eldiehy, K.S.H.; Deka, D. Phycoremediation of rice bran oil processing wastewater and biodiesel production using green microalgae, Scenedesmus obliquus: A green approach to environmental protection and remediation. Environ. Sci. Pollut. Res. 2024, 31, 65288–65301. [Google Scholar] [CrossRef]
- Lenzuni, M.; Demichelis, F.; Basbus, J.F.; Barbucci, A.; Savorani, F.; Tommasi, T.; Casazza, A.A. Microalgae-based bioremediation of olive mill wastewater: Technical and environmental evaluations using orange peel and orange peel-derived biochar. Sustain. Mater. Technol. 2025, 43, e01338. [Google Scholar] [CrossRef]
- Li, Y.; Miros, S.; Kiani, H.; Eckhardt, H.G.; Blanco, A.; Mulcahy, S.; McDonnell, H.; Tiwari, B.K.; Halim, R. Mechanism of lactose assimilation in microalgae for the bioremediation of dairy processing side-streams and co-production of valuable food products. J. Appl. Phycol. 2023, 35, 1649–1661. [Google Scholar] [CrossRef]
- Rana Qul ain Latif, S.; Perveen, S.; Haq, A.; Ali, S.; Irfan, M.; Gauttam, R.; Shah, T.A.; Dawoud, T.M.; Wondmie, G.F.; Bourhia, M.; et al. Utilization of microalgae for agricultural runoff remediation and sustainable biofuel production through an integrated biorefinery approach. Bioresour. Bioprocess 2024, 11, 8. [Google Scholar]
- Thi Cam Van, D.; Thi Mai, D.; Thi Thu Uyen, B.; Thi Phuong Dung, N.; Thi Thu Ha, L.; Thi Lieu, N.; Minh, D.N.; Thuan, T.D.; Giang, L.T. Sustainable remediation of piggery wastewater using a novel mixotrophic Chlorella sorokiniana Cbeo for high value biomass production. Biochem. Eng. J. 2025, 213, 109555. [Google Scholar] [CrossRef]
- Pham, T.L.; Bui, M.H. Removal of Nutrients from Fertilizer Plant Wastewater Using Scenedesmus sp.: Formation of Bioflocculation and Enhancement of Removal Efficiency. J. Chem. 2020, 2020, 8094272. [Google Scholar] [CrossRef]
- Walters, C.; Steyn, M.; Ndlela, L.; Nocanda, X.; Moloi, M.; Oberholster, P. Phycoremediation of industrial wastewater: Review of algae consortia. Int. J. Environ. Sci. Technol. 2025, 22, 6209–6224. [Google Scholar] [CrossRef]
- Allzrag, A.M.; Lalung, J.; Hamid, N.K.A.; Rashed, J.A.; Kausar, R.; Kassim, M.A. A Comparative phycoremediation study of aquaculture wastewater treatment by Chlorella sp. immobilized in different hybrid-alginate matrices. Env. Technol. Innov. 2025, 38, 104158. [Google Scholar] [CrossRef]
- Song, W.R.L.; Keong, Y.S.; Yusoff FMd Ping, T.J.; Rahman, N.A. Potential of culturing microalgae Chlorella vulgaris and Nannochloropsis oculata with aquaculture wastewater for simultaneous aquafeed production and wastewater remediation. Blue Biotechnol. 2024, 1, 19. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Unnikannan, P.; Sruthi, P. Phycoremediation of Tannery Wastewater Using Microalgae Scenedesmus Species. Int. J. Phytoremediat. 2015, 17, 907–916. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Asmare, A.M. Phycoremediation of textile wastewater using indigenous microalgae. Water Pract. Technol. 2018, 13, 274–284. [Google Scholar] [CrossRef]
- Calatrava, V.; Gonzalez-Ballester, D.; Dubini, A. Microalgae for bioremediation: Advances, challenges, and public perception on genetic engineering. BMC Plant Biol. 2024, 24, 1261. [Google Scholar] [CrossRef]
- Ahmad, A.; Ashraf, S.S. Harnessing microalgae: Innovations for achieving UN Sustainable Development Goals and climate resilience. J. Water Process Eng. 2024, 68, 106506. [Google Scholar] [CrossRef]
- Yun, H.S.; Kim, Y.S.; Yoon, H.S. Characterization of Chlorella sorokiniana and Chlorella vulgaris fatty acid components under a wide range of light intensity and growth temperature for their use as biological resources. Heliyon 2020, 6, e04447. [Google Scholar] [CrossRef]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular basis of autotrophic vs mixotrophic growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef]
- Khalaji, M.; Hosseini, S.A.; Ghorbani, R.; Agh, N.; Rezaei, H.; Kornaros, M.; Koutra, E. Treatment of dairy wastewater by microalgae Chlorella vulgaris for biofuels production. Biomass Convers. Biorefin. 2023, 13, 3259–3265. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Pareek, N. Comparative Appraisal of Biomass Production, Remediation, and Bioenergy Generation Potential of Microalgae in Dairy Wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S.; Charalambous, P.; Vyrides, I. Dairy wastewater management in EU: Produced amounts, existing legislation, applied treatment processes and future challenges. J. Environ. Manag. 2022, 303, 114152. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas Fermentation Process Development for Production of Biofuels and Chemicals: A Review; Bioresource Technology Reports; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 7. [Google Scholar]
- Antonicelli, G.; Ricci, L.; Tarraran, L.; Fraterrigo Garofalo, S.; Re, A.; Vasile, N.S.; Verga, F.; Pirri, C.F.; Menin, B.; Agostino, V. Expanding the product portfolio of carbon dioxide and hydrogen-based gas fermentation with an evolved strain of Clostridium carboxidivorans. Bioresour. Technol. 2023, 387, 129689. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, G.; Vasile, N.; Piro, E.; Fraterrigo Garofalo, S.; Menin, B.; Verga, F.; Pirri, F.; Agostino, V. Harnessing an adapted strain of Clostridium carboxidivorans to unlock hexanol production from carbon dioxide and hydrogen in elevated-pressure stirred tank reactors. Bioresour. Technol. 2025, 418, 131966. [Google Scholar] [CrossRef]
- Usai, G.; Cordara, A.; Mazzocchi, E.; Re, A.; Fino, D.; Pirri, C.F.; Menin, B. Coupling dairy wastewaters for nutritional balancing and water recycling: Sustainable heterologous 2-phenylethanol production by engineered cyanobacteria. Front. Bioeng. Biotechnol. 2024, 12, 1359032. [Google Scholar] [CrossRef]
- Usai, G.; Cordara, A.; Re, A.; Polli, M.F.; Mannino, G.; Bertea, C.M.; Fino, D.; Pirri, C.F.; Menin, B. Combining metabolite doping and metabolic engineering to improve 2-phenylethanol production by engineered cyanobacteria. Front. Bioeng. Biotechnol. 2022, 10, 1005960. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Fraterrigo Garofalo, S.; Cavallini, N.; Demichelis, F.; Savorani, F.; Mancini, G.; Fino, D.; Tommasi, T. From tuna viscera to added-value products: A circular approach for fish-waste recovery by green enzymatic hydrolysis. Food Bioprod. Process. 2023, 137, 155–167. [Google Scholar] [CrossRef]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Effect of lignocellulose related compounds on microalgae growth and product biosynthesis: A review. Energies 2014, 7, 4446–4481. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Xing, D.; Wang, H.; Li, S.; Jin, C.; Zhao, Y.; Gao, M.; Guo, L. Insight into the effect of carbon composition on microalgae cultivation using acidogenic fermentation liquid: Growth kinetics, biochemical composition synthesis, and physiological characteristics. Chem. Eng. J. 2024, 488, 150794. [Google Scholar] [CrossRef]
- Tan, X.B.; Zhao, X.C.; Zhang, Y.L.; Zhou, Y.Y.; Yang, L.B.; Zhang, W.W. Enhanced lipid and biomass production using alcohol wastewater as carbon source for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour. Technol. 2018, 247, 784–793. [Google Scholar] [CrossRef]
- Wise, D.L. Carbon Nutrition and Metabolism of Polytomella caeca*. J. Protozool. 1959, 6, 19–23. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.; Dixon, R.; Wang, Y.P. Deciphering the Principles of Bacterial Nitrogen Dietary Preferences: A Strategy or Nutrient Containment. mBio 2016, 7, e00792-16. [Google Scholar] [CrossRef]
- De Lourdes, F.M.M.; Dolores Josefina, R.R.M.; Ulises, M.M.C.; De Jesús, M.R.A. Tolerance and nutrients consumption of Chlorella vulgaris growing in mineral medium and real wastewater under laboratory conditions. Open Agric. 2017, 2, 394–400. [Google Scholar] [CrossRef]
- Ngangkham, M.; Kumar Ratha, S.; Prasanna, R.; Saxena, A.K.; Dhar, D.W.; Sarika, C.; Badari, R.; Prasad, N. Biochemical Modulation of Growth, Lipid Quality and Productivity in Mixotrophic Cultures of Chlorella sorokiniana. 2012. Available online: http://www.springerplus.com/content/1/1/33 (accessed on 9 August 2012).
- Abiusi, F.; Wijffels, R.H.; Janssen, M. Doubling of Microalgae Productivity by Oxygen Balanced Mixotrophy. ACS Sustain. Chem. Eng. 2020, 8, 6065–6074. [Google Scholar] [CrossRef]
- Proietti Tocca, G.; Agostino, V.; Menin, B.; Tommasi, T.; Fino, D.; Di Caprio, F. Mixotrophic and heterotrophic growth of microalgae using acetate from different production processes. Rev. Environ. Sci. Biotechnol. 2024, 23, 93–132. [Google Scholar] [CrossRef]
- Wen, X.; Tao, H.; Peng, X.; Wang, Z.; Ding, Y.; Xu, Y.; Liang, L.; Du, K.; Zhang, A.; Liu, C.; et al. Sequential phototrophic–mixotrophic cultivation of oleaginous microalga Graesiella sp. WBG-1 in a 1000 m2 open raceway pond. Biotechnol. Biofuels 2019, 12, 27. [Google Scholar] [CrossRef]
- Ruiz, J.; Wijffels, R.H.; Dominguez, M.; Barbosa, M.J. Heterotrophic vs autotrophic production of microalgae: Bringing some light into the everlasting cost controversy. Algal Res. 2022, 64, 102698. [Google Scholar] [CrossRef]
- Silva, T.L.; Moniz, P.; Silva, C.; Reis, A. The Role of Heterotrophic Microalgae in Waste Conversion to Biofuels and Bioproducts. Processes 2021, 9, 1090. [Google Scholar] [CrossRef]
- Baldasso, C.; Barros, T.C.; Tessaro, I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Kaur, N. Different treatment techniques of dairy wastewater. Groundw. Sustain. Dev. 2021, 14, 100640. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. [Google Scholar] [CrossRef]
- Sawayama, S.; Rao, K.K.; Hall, D.O. Nitrate and phosphate ion removal from water by Phormidium laminosum immobilized on hollow fibres in a photobioreactor. Appl. Microbiol. Biotechnol. 1998, 49, 463–468. [Google Scholar] [CrossRef]
- Vassalle, L.; Díez-Montero, R.; Machado, A.T.R.; Moreira, C.; Ferrer, I.; Mota, C.R.; Passos, F. Upflow anaerobic sludge blanket in microalgae-based sewage treatment: Co-digestion for improving biogas production. Bioresour. Technol. 2020, 300, 122677. [Google Scholar] [CrossRef]
- Hussain, J.; Dang, K.; Chatterjee, S.; Haque, E. Microalgae Mediated Sludge Treatment BT—Waste Management, Processing and Valorisation; Yaser, A.Z., Tajarudin, H.A., Embrandiri, A., Eds.; Springer: Singapore, 2022; pp. 159–174. [Google Scholar]
- Gonzalez, L.E.; Canizares, R.O.; Baena, S. EFFICIENCY OF AMMONIA AND PHOSPHORUS REMOVAL FROM A COLOMBIAN AGROINDUSTRIAL WASTEWATER BY THE MICROALGAE CHLORELLA VULGARIS AND SCENEDESMUS DIMORPHUS. Bioresour. Technol. 1997, 60, 259–262. [Google Scholar] [CrossRef]
- Chai, W.S.; Chew, C.H.; Munawaroh, H.S.H.; Ashokkumar, V.; Cheng, C.K.; Park, Y.K.; Show, P.L. Microalgae and ammonia: A review on inter-relationship. Fuel 2021, 303, 121303. [Google Scholar] [CrossRef]
- Kim, S.; Park Jeun Cho, Y.B.; Hwang, S.J. Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour. Technol. 2013, 144, 8–13. [Google Scholar] [CrossRef]
- Zhao, X.C.; Tan, X.B.; Yang LBin Liao, J.Y.; Li, X.Y. Cultivation of Chlorella pyrenoidosa in anaerobic wastewater: The coupled effects of ammonium, temperature and pH conditions on lipids compositions. Bioresour. Technol. 2019, 284, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Pugazhendhi, A.; Mathimani, T. Lipid enhancement through nutrient starvation in Chlorella sp. and its fatty acid profiling for appropriate bioenergy feedstock. Biocatal. Agric. Biotechnol. 2019, 20, 101179. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, Q.; Gu, M.; Cong, W. Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J. Appl. Phycol. 2013, 25, 311–318. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Bessa, L.P.; Vieira, L.M.; Moreira, F.S.; de Souza Ferreira, J.; Batista, F.R.X.; Cardoso, V.L. Dark fermentation effluent as substrate for hydrogen production from Rhodobacter capsulatus highlighting the performance of different fermentation systems. 3 Biotech 2019, 9, 153. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fayet, A.; Fouilland, E.; Steyer, J.P. Raw dark fermentation effluent to support heterotrophic microalgae growth: Microalgae successfully outcompete bacteria for acetate. Algal Res. 2015, 12, 119–125. [Google Scholar] [CrossRef]
- Lacroux, J.; Seira, J.; Trably, E.; Bernet, N.; Steyer, J.P.; van Lis, R. Mixotrophic Growth of Chlorella sorokiniana on Acetate and Butyrate: Interplay Between Substrate, C:N Ratio and pH. Front. Microbiol. 2021, 12, 703614. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Magyar, T.; Németh, B.; Tamás, J.; Nagy, P.T. Improvement of N and P ratio for enhanced biomass productivity and sustainable cultivation of Chlorella vulgaris microalgae. Heliyon 2024, 10, e23238. [Google Scholar] [CrossRef]
- Almomani, F. Kinetic modeling of microalgae growth and CO2 bio-fixation using central composite design statistical approach. Sci. Total Environ. 2020, 720, 137594. [Google Scholar] [CrossRef]
- Bulynina, S.S.; Ziganshina, E.E.; Ziganshin, A.M. Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater. BioTech 2023, 12, 53. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Yue, L.; Yang, Z.; Fu, Q.; Ye, Q. Enhancement effect of ethanol on lipid and fatty acid accumulation and composition of Scenedesmus sp. Bioresour. Technol. 2013, 140, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.P.; Matsudo, M.C.; Pérez Mora, L.S.; Sato, S.; De Carvalho, J.C.M. Ethanol effect on batch and fed-batch Arthrospira platensis growth. J. Ind. Microbiol. Biotechnol. 2014, 41, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Abate, R.; Bi, Y.; Song, G.; Mi, W.; Cheng, F.; Zhu, Y. Exogenous ethanol induces cell giantism accompanied by enhanced accumulation of lipid and carbohydrates in Chlorella sorokiniana. J. Appl. Phycol. 2024, 36, 1635–1651. [Google Scholar] [CrossRef]
- Jay, A.E. Toxic Effects of Organic Solvents on the Growth of Chlorella vulgaris and Selenastrum capricornutum. Bull. Environ. Contam. Toxicol. 1996, 57, 191–198. [Google Scholar] [CrossRef]
- Okumura, Y.; Koyama, J.; Takaku, H.; Satoh, H. Influence of organic solvents on the growth of marine microalgae. Arch. Environ. Contam. Toxicol. 2001, 41, 123–128. [Google Scholar] [CrossRef]
- Aruoja, V.; Moosus, M.; Kahru, A.; Sihtmäe, M.; Maran, U. Measurement of baseline toxicity and QSAR analysis of 50 non-polar and 58 polar narcotic chemicals for the alga Pseudokirchneriella subcapitata. Chemosphere 2014, 96, 23–32. [Google Scholar] [CrossRef]
- Gupta, S.P.; Siddiqi, N.J.; Khan, H.A.; Alrokayan, S.H.; Alhomida, A.S.; Singh, R.K.; Verma, P.K.; Kumar, S.; Acharya, A.; Sharma, B. Phytochemical Profiling of Microalgae Euglena tuba and Its Anticancer Activity in Dalton’s Lymphoma Cells. Front. Biosci.-Landmark 2022, 27, 120. [Google Scholar] [CrossRef]
- Federal, U.; Maria, D.S.; Vieira, K.R.; Federal, U.; Maria, D.S.; Pinheiro, P.N.; Paulino, B.N. Flavour generation from microalgae in mixotrophic cultivation. In Proceedings of the Weurman Flavour Research Symposium 2017, Graz, Austria, 18–22 September 2017; pp. 87–90. [Google Scholar]
- Meier, L.; Vilchez, C.; Cuaresma, M.; Torres-Aravena, Á.; Jeison, D. Effect of pH Change on the Microalgae-Based Biogas Upgrading Process. Appl. Sci. 2022, 12, 12194. [Google Scholar] [CrossRef]
- Lacroux, J.; Trably, E.; Bernet, N.; Steyer, J.P.; van Lis, R. Mixotrophic growth of microalgae on volatile fatty acids is determined by their undissociated form. Algal Res. 2020, 47, 101870. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; De Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Chandra, R.; Arora, S.; Rohit, M.V.; Venkata Mohan, S. Lipid metabolism in response to individual short chain fatty acids during mixotrophic mode of microalgal cultivation: Influence on biodiesel saturation and protein profile. Bioresour. Technol. 2015, 188, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Fu, R.; Shang, L.; Brigham, C.J.; Chang, H.N. Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess. Biosyst. Eng. 2015, 38, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Baroukh, C.; Turon, V.; Bernard, O. Dynamic metabolic modeling of heterotrophic and mixotrophic microalgal growth on fermentative wastes. PLoS Comput. Biol. 2017, 13, e1005590. [Google Scholar] [CrossRef] [PubMed]
- Lacroux, J.; Jouannais, P.; Atteia, A.; Bonnafous, A.; Trably, E.; Steyer, J.P.; van Lis, R. Microalgae screening for heterotrophic and mixotrophic growth on butyrate. Algal Res. 2022, 67, 102843. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.P. Growth of Chlorella sorokiniana on a mixture of volatile fatty acids: The effects of light and temperature. Bioresour. Technol. 2015, 198, 852–860. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kabra, A.N.; Kim, J.R.; Jeon, B.H. Photoheterotrophic microalgal hydrogen production using acetate- and butyrate-rich wastewater effluent. Energy 2014, 78, 887–894. [Google Scholar] [CrossRef]
- Bellou, S.; Aggelis, G. Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J. Biotechnol. 2012, 164, 318–329. [Google Scholar] [CrossRef]
- Prathima Devi, M.; Venkata Mohan, S. CO 2 supplementation to domestic wastewater enhances microalgae lipid accumulation under mixotrophic microenvironment: Effect of sparging period and interval. Bioresour. Technol. 2012, 112, 116–123. [Google Scholar] [CrossRef]
- Zhao, F.; Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Understanding the complexity of wastewater: The combined impacts of carbohydrates and sulphate on the performance of bioelectrochemical systems. Water Res. 2020, 176, 115737. [Google Scholar] [CrossRef]
- Seguel Suazo, K.; Dobbeleers, T.; Dries, J. Bacterial community and filamentous population of industrial wastewater treatment plants in Belgium. Appl. Microbiol. Biotechnol. 2024, 108, 43. [Google Scholar] [CrossRef]
- Banerjee, S.; Dasgupta, S.; Atta, A.; Das, D.; Dayal, D.; Malik, S.; Kumar, H.; Kishore, S.; Rustagi, S.; Almutary, A.G. Flow Rate Optimization in a Flat-Panel Photobioreactor for the Cultivation of Microalgae for Mitigating Waste Gas. Water 2023, 15, 2824. [Google Scholar] [CrossRef]
- Papapolymerou, G.; Gougoulias, N.; Metsoviti, M.N.; Kokkalis, A.; Kasiteropoulou, D.; Spiliotis, X.; Mpesios, A.; Papadopoulou, A. FAME and bio-oil properties and carbon yield coefficients of Chlorella sorokiniana cultivated heterotrophically with industrial effluents. Sustain. Chem. Pharm. 2022, 30, 100879. [Google Scholar] [CrossRef]
- Papapanagiotou, G.; Charisis, A.; Samara, C.; Kalogianni, E.P.; Chatzidoukas, C. Linking Cultivation Conditions to the Fatty Acid Profile and Nutritional Value of Chlorella sorokiniana Lipids. Processes 2024, 12, 2770. [Google Scholar] [CrossRef]
- Toumi, A.; Politaeva, N.A. Impact of the nitrate concentration on the biomass growth and the fatty acid profiles of microalgae Chlorella sorokiniana. IOP Conf. Ser. Earth Env. Sci. 2021, 689, 012026. [Google Scholar] [CrossRef]
- Matsui, H.; Shiozaki, K.; Okumura, Y.; Ishikawa, M.; Waqalevu, V.; Hayasaka, O.; Honda, A.; Kotani, T. Effects of phosphorous deficiency of a microalga Nannochloropsis oculata on its fatty acid profiles and intracellular structure and the effectiveness in rotifer nutrition. Algal Res. 2020, 49, 101905. [Google Scholar] [CrossRef]
- Maltsev, Y.; Gusev, E.; Maltseva, I.; Kulikovskiy, M.; Namsaraev, Z.; Petrushkina, M.; Filimonova, A.; Sorokin, B.; Golubeva, A.; Butaeva, G.; et al. Description of a new species of soil algae, Parietochloris grandis sp. nov., and study of its fatty acid profiles under different culturing conditions. Algal Res. 2018, 33, 358–368. [Google Scholar] [CrossRef]
- Steinman, A.D.; Duhamel, S. Phosphorus Limitation, Uptake, and Turnover in Benthic Stream Algae. In Methods in Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 197–218. [Google Scholar]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76. [Google Scholar] [CrossRef]
- Álvarez-Díaz, P.D.; Ruiz, J.; Arbib, Z.; Barragán, J.; Garrido-Pérez, M.C.; Perales, J.A. Freshwater microalgae selection for simultaneous wastewater nutrient removal and lipid production. Algal Res. 2017, 24, 477–485. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Chandra, R.; Pradhan, S.; Patel, A.; Ghosh, U.K. An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. J. Environ. Manag. 2021, 297, 113210. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.; Moghazy, R.; Mansour, M.; Kerner, M. Growth of microalgae adapted to high-light temperature conditions in different types of wastewater. Water Sci. Technol. 2023, 88, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Osundeko, O.; Dean, A.P.; Davies, H.; Pittman, J.K. Acclimation of Microalgae to Wastewater Environments Involves Increased Oxidative Stress Tolerance Activity. Plant Cell Physiol. 2014, 55, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, L.; Gariépy, Y.; Bourdeau, N.; Barnabé, S.; Raghavan, G.S.V. Optimization of the proportions of four wastewaters in a blend for the cultivation of microalgae using a mixture design. Bioresour. Technol. 2019, 283, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, N.; Zamani, H. Potential of Chlorella sorokiniana Cultivated in Dairy Wastewater for Bioenergy and Biodiesel Production. Bioenergy Res. 2022, 15, 334–345. [Google Scholar] [CrossRef]
- Ramsundar, P.; Guldhe, A.; Singh, P.; Bux, F. Assessment of municipal wastewaters at various stages of treatment process as potential growth media for Chlorella sorokiniana under different modes of cultivation. Bioresour. Technol. 2017, 227, 82–92. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S.; Chand, N.; Mutiyar, P.K. Phycoremediation of milk processing wastewater and lipid-rich biomass production using Chlorella vulgaris under continuous batch system. Sci. Total Environ. 2022, 833, 155110. [Google Scholar] [CrossRef]
- Rossi, C.; Grossi, G.; Lacetera, N.; Vitali, A. Carbon Footprint and Carbon Sink of a Local Italian Dairy Supply Chain. Dairy 2024, 5, 201–216. [Google Scholar] [CrossRef]

| CHEMICAL | 3N BBM (mg L−1) | GFE (mg L−1) | DWW (mg L−1) | ANALYTICAL METHOD |
|---|---|---|---|---|
| TOTAL ORGANIC C | / | 2530 | 78.2 | UNI EN 15936:2012 |
| NH4+ | / | 219 | 370 | UNI 11669:2017 |
| NO3− | 547 | <4.5 | <4.5 | UNI EN ISO 10304-1:2009 |
| CHLORIDE | 27 | 1250 | 246 | UNI EN ISO 10304-1:2009 |
| SULFATE | 29 | 96.1 | 24.8 | UNI EN ISO 10304-1:2009 |
| PO4 | 153 | 65.8 | <60 | UNI EN ISO 10304-1:2009 |
| Ca | 12 | 14.1 | 46 | UNI EN ISO 15587-1:2002 (Annex A) + UNI EN ISO 11885:2009 |
| Fe | 0.12 | 0.67 | 0.196 | UNI EN ISO 15587-1:2002 (Annex A) + UNI EN ISO 11885:2009 |
| Mg | 7.4 | 22.8 | 11.7 | UNI EN ISO 15587-1:2002 (Annex A) + UNI EN ISO 11885:2009 |
| Mn | 0.067 | 2.94 | <0.01 | UNI EN ISO 15587-1:2002 (Annex A) + UNI EN ISO 11885:2009 |
| Mo | 0.011 | <0.01 | / | UNI EN ISO 15587-1:2002 (Annex A) + UNI EN ISO 11885:2009 |
| Zn | 0.008 | 0.121 | / | UNI EN ISO 15587-1:2002 (Annex A) + UNI EN ISO 11885:2009 |
| VFAs | Concentration (g L−1) |
|---|---|
| Acetate | 0.21 |
| Butyrate | 0.16 |
| Caproate | 0.13 |
| Ethanol | 0.91 |
| Butanol | 1.04 |
| Hexanol | 0.76 |
| Compound | Pre (mg L−1) | Post (mg L−1) |
|---|---|---|
| Total Carbon | 4099.00 | 1591.60 |
| Total Nitrogen | 34.00 | <5.0 |
| Ammonium Nitrogen | 294.50 | <2.50 |
| Nitric Nitrogen | <4.50 | <1.50 |
| Chloride | 748.00 | 624.00 |
| Sulfate | 60.45 | 19.60 |
| Phosphate | 65.80 | <15.0 |
| Ca | 30.05 | 16.80 |
| Co | 0.21 | 0.18 |
| Fe | 0.43 | 0.08 |
| Mg | 17.25 | 15.80 |
| Mn | 2.94 | 1.35 |
| Cu | <0.01 | 0.02 |
| Zn | 0.06 | 0.06 |
| Compound | 50% DWW 50% GFE | Mixotrophy | Phototrophy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Saturation | Avg | SD | Avg | SD | p-Value | Avg | SD | p-Value |
| Pentadecanoic acid, methyl ester | C15:0 | 0.41 | 0.00 | / | / | ns | / | / | / |
| Methyl palmitoleate | C16:1 (Δ9, Z) | 2.89 | 0.30 | 2.80 | 1.15 | ns | 1.65 | 0.26 | ns |
| Methyl palmitate | C16:0 | 43.45 | 1.53 | 44.12 | 1.87 | ns | 48.39 | 2.55 | * |
| cis-10-Heptadecenoic acid methyl ester | C17:1 (Δ10, Z) | 1.41 | 0.32 | 1.26 | 0.14 | ns | 1.36 | 0.15 | ns |
| Heptadecanoic acid methyl ester | C17:0 | 0.73 | 0.10 | 0.73 | 0.06 | ns | 0.34 | 0.04 | *** |
| Linoleic acid methyl ester | C18:2 (Δ9, 12, Z, Z) | 32.70 | 1.39 | 31.29 | 2.47 | ns | 22.05 | 5.33 | ** |
| Elaidic acid methyl ester | C18:1 (Δ9, E) | 6.78 | 0.47 | 3.08 | 0.51 | *** | 3.93 | 0.63 | ** |
| Oleic acid methyl ester | C18:1 (Δ9, Z) | 3.58 | 0.34 | 1.56 | 0.37 | *** | 1.78 | 0.36 | *** |
| Stearic acid methyl ester | C18:0 | 7.70 | 0.96 | 13.25 | 1.05 | *** | 16.29 | 1.64 | **** |
| Cis-9-Pentacosenoic acid methyl ester | C24:1 (Δ15, Z) | 1.87 | 0.00 | 2.55 | 0.28 | ns | 2.56 | 0.39 | * |
| Tetracosanoic acid, methyl ester | C24:0 | / | / | / | / | ns | 2.81 | 0.28 | / |
| Arachidonic acid methyl ester | C20:4 (Δ5,8,11,14, all-Z) | / | / | / | / | ns | 0.91 | 0.00 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzocchi, E.; Usai, G.; Agostino, V.; Fraterrigo Garofalo, S.; Pinton, E.; Pirri, C.F.; Menin, B.; Cordara, A. Combination of Exhaust Gas Fermentation Effluent and Dairy Wastewater for Microalgae Production: Effect on Growth and FAME Composition of Chlorella sorokiniana. Microorganisms 2025, 13, 961. https://doi.org/10.3390/microorganisms13050961
Mazzocchi E, Usai G, Agostino V, Fraterrigo Garofalo S, Pinton E, Pirri CF, Menin B, Cordara A. Combination of Exhaust Gas Fermentation Effluent and Dairy Wastewater for Microalgae Production: Effect on Growth and FAME Composition of Chlorella sorokiniana. Microorganisms. 2025; 13(5):961. https://doi.org/10.3390/microorganisms13050961
Chicago/Turabian StyleMazzocchi, Elena, Giulia Usai, Valeria Agostino, Silvia Fraterrigo Garofalo, Eugenio Pinton, Candido Fabrizio Pirri, Barbara Menin, and Alessandro Cordara. 2025. "Combination of Exhaust Gas Fermentation Effluent and Dairy Wastewater for Microalgae Production: Effect on Growth and FAME Composition of Chlorella sorokiniana" Microorganisms 13, no. 5: 961. https://doi.org/10.3390/microorganisms13050961
APA StyleMazzocchi, E., Usai, G., Agostino, V., Fraterrigo Garofalo, S., Pinton, E., Pirri, C. F., Menin, B., & Cordara, A. (2025). Combination of Exhaust Gas Fermentation Effluent and Dairy Wastewater for Microalgae Production: Effect on Growth and FAME Composition of Chlorella sorokiniana. Microorganisms, 13(5), 961. https://doi.org/10.3390/microorganisms13050961










