Diversity of Antifungal Properties in Bacterial Isolates from Different Plant Species Growing Across Uzbekistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Location, Plant Materials, and Fungal Pathogens
2.2. Isolation and Purification of Bacteria from the Plant
2.3. DNA Extraction and Molecular Identification
2.4. Determination of Enzymatic Activities
2.5. Data Analysis
3. Result
3.1. Research Site and Plant Collection
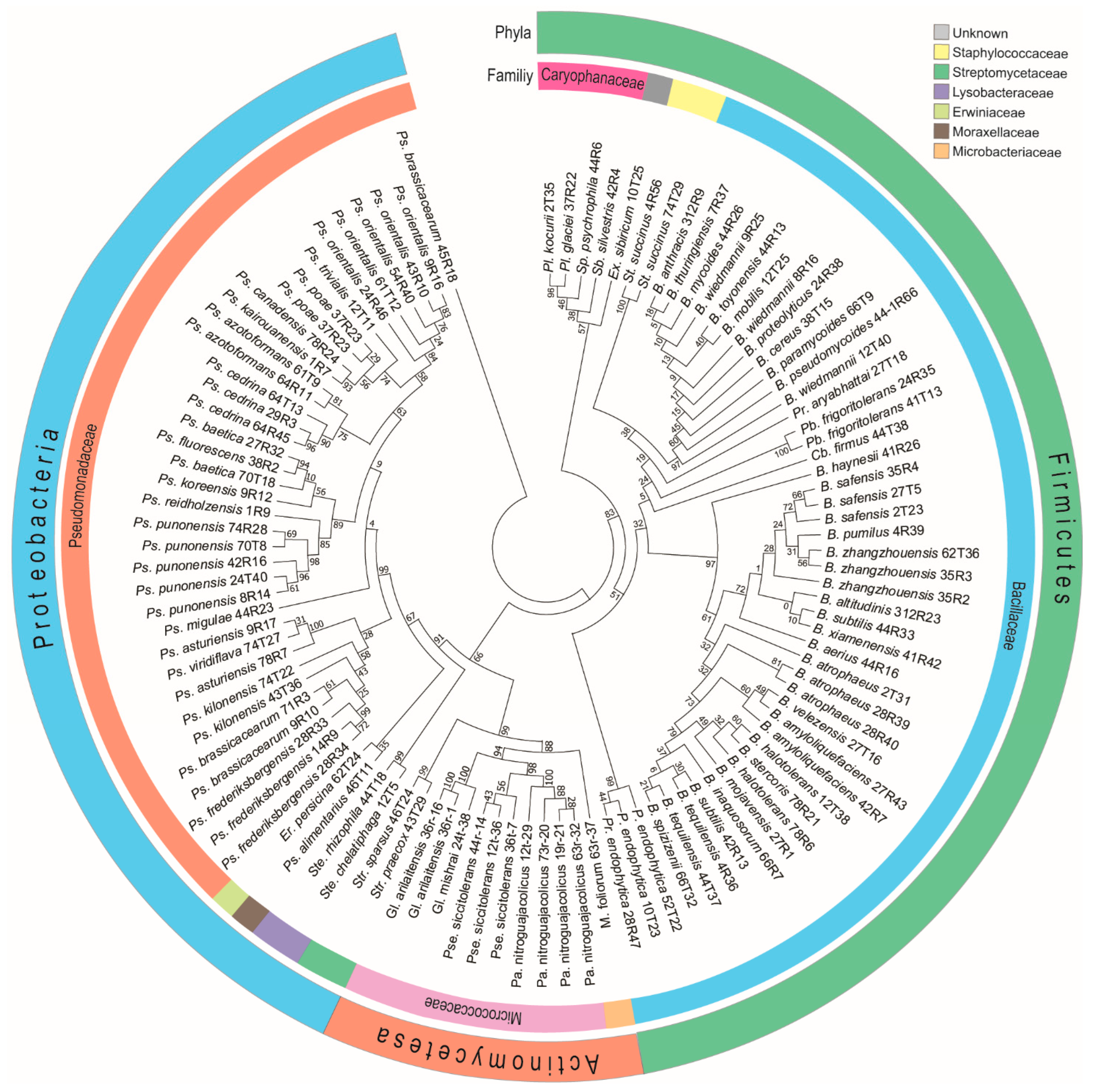
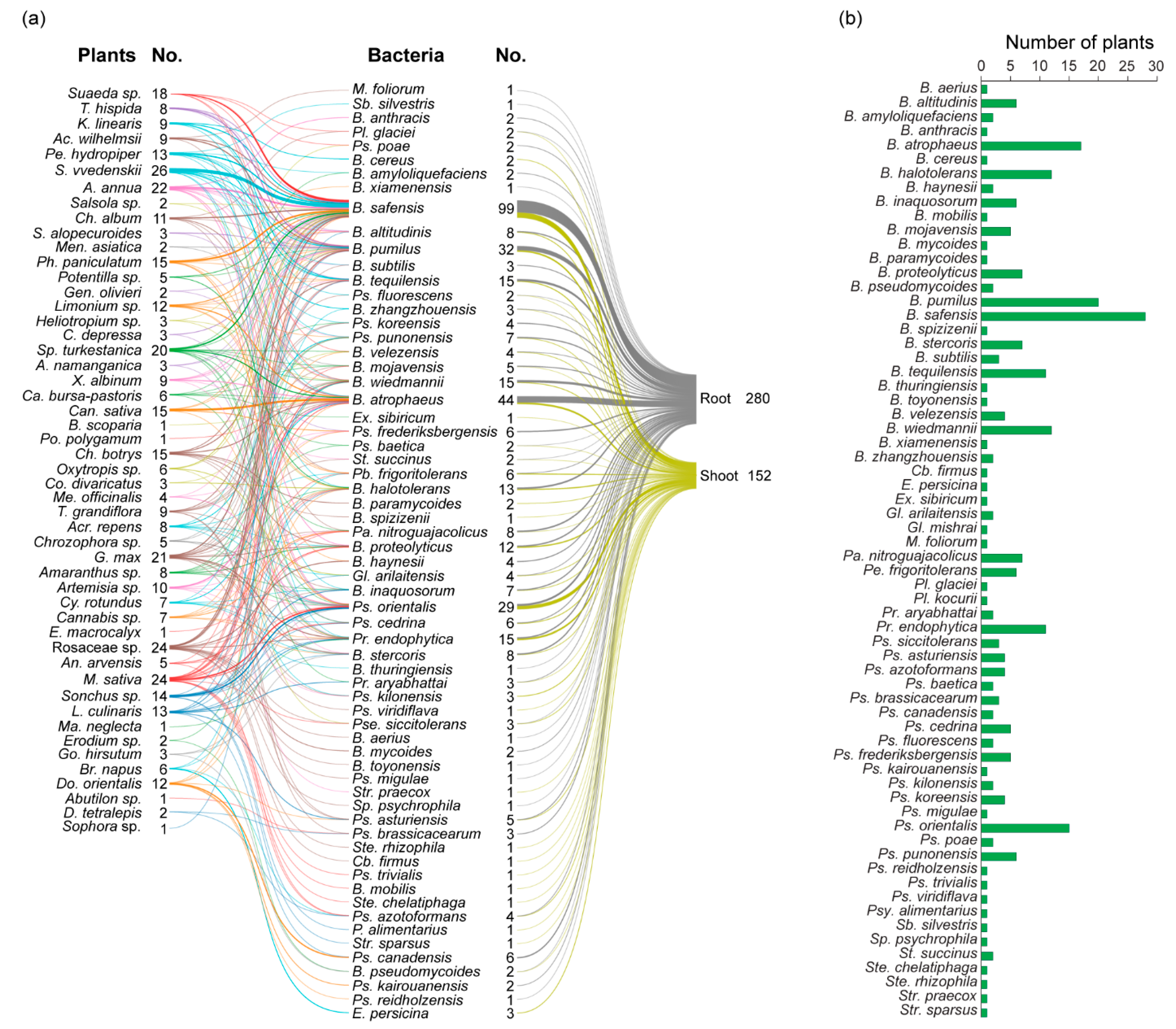
3.2. Identification of Cultivable Plant Associated Antagonistic Bacteria
3.3. Diversity of Antagonistic Bacteria
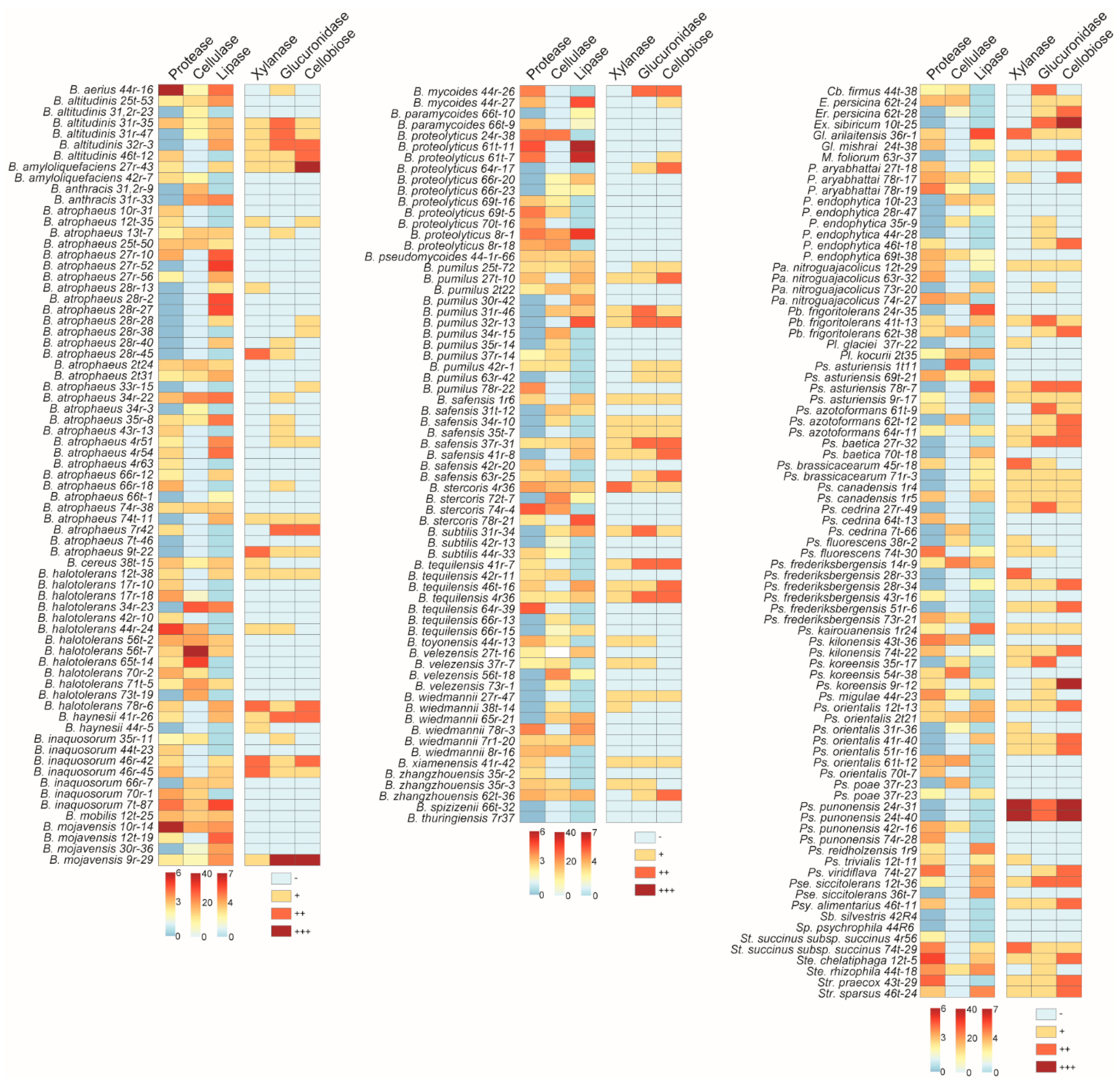
3.4. Determination of the Enzymatic Activities of the Antagonistic Bacteria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K.D. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere bacterial interactions and impact on plant health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Kohl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Bakker, P.A.; Doornbos, R.F.; Zamioudis, C.; Berendsen, R.L.; Pieterse, C.M. Induced systemic resistance and the rhizosphere microbiome. Plant Pathol. J. 2013, 29, 136–143. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Poelman, E.H.; Van Wees, S.C.; Dicke, M. Induced plant responses to microbes and insects. Front. Plant Sci. 2013, 4, 475. [Google Scholar] [CrossRef]
- Rajkumar, M.; Lee, K.J.; Freitas, H. Effects of chitin and salicylic acid on biological control activity of Pseudomonas spp. against damping off of pepper. S. Afr. J. Bot. 2008, 74, 268–273. [Google Scholar] [CrossRef]
- Srikamwang, C.; Onsa, N.E.; Sunanta, P.; Sangta, J.; Chanway, C.P.; Thanakkasaranee, S.; Sommano, S.R. Role of Microbial Volatile Organic Compounds in Promoting Plant Growth and Disease Resistance in Horticultural Production. Plant Signal Behav. 2023, 18, 2227440. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Haddoudi, I.; Cabrefiga, J.; Mora, I.; Mhadhbi, H.; Montesinos, E.; Mrabet, M. Biological control of Fusarium wilt caused by Fusarium equiseti in Vicia faba with broad spectrum antifungal plant-associated Bacillus spp. Biol. Control 2021, 160, 104671. [Google Scholar] [CrossRef]
- Alattas, H.; Glick, B.R.; Murphy, D.V.; Scott, C. Harnessing Pseudomonas spp. for sustainable plant crop protection. Front. Microbiol. 2024, 15, 1485197. [Google Scholar] [CrossRef]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a promising biological control agents for plant pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 02048. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Ji, C.; Chen, Z.; Kong, X.; Xin, Z.; Sun, F.; Xing, J.; Li, C.; Li, K.; Liang, Z.; Cao, H. Biocontrol and plant growth promotion by combined Bacillus spp. inoculation affecting pathogen and AMF communities in the wheat rhizosphere at low salt stress conditions. Front. Plant Sci. 2022, 13, 1043171. [Google Scholar] [CrossRef]
- Peel, M.F.; Finlayson, B.L.; McMahon, B.T. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- Aytenov, I.S.; Bozorov, T.A.; Zhang, D.; Samadiy, S.A.; Muhammadova, D.A.; Isokulov, M.Z.; Murodova, S.M.; Zakirova, O.R.; Chinikulov, B.K.; Sherimbetov, A.G. Uncovering the Antifungal Potential of Plant-Associated Cultivable Bacteria from the Aral Sea Region against Phytopathogenic Fungi. Pathogens 2024, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.N.; Rekik, I.; Bełka, M.; Ibrahim, A.F.; Luptakova, L.; Jaspars, M.; Woodward, S.; Belbahri, L. Strain-level diversity of secondary metabolism in the biocontrol species Aneurinibacillus migulanus. Microbiol. Res. 2016, 182, 116–124. [Google Scholar] [CrossRef]
- Bozorov, T.A.; Rasulov, B.A.; Zhang, D. Characterization of the gut microbiota of invasive Agrilus mali Matsumara (Coleoptera: Buprestidae) using high-throughput sequencing: Uncovering plant cell-wall degrading bacteria. Sci. Rep. 2019, 9, 4923. [Google Scholar] [CrossRef]
- Vargas-Asensio, G.; Pinto-Tomas, A.; Rivera, B.; Hernandez, M.; Hernandez, C.; Soto-Montero, S.; Murillo, C.; Sherman, D.H.; Tamayo-Castillo, G. Uncovering the cultivable microbial diversity of costa rican beetles and its ability to break down plant cell wall components. PLoS ONE 2014, 9, e113303. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef]
- Mayerhofer, H.J.; Marshall, R.T.; White, C.H.; Lu, M. Characterization of a heat-stable protease of Pseudomonas fluorescens P26. Appl. Microbiol. 1973, 25, 44–48. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, L.; Nagar, S.; Raina, C.; Parshad, R.; Gupta, V.K. Screening, isolation and production of lipase/esterase producing Bacillus sp. strain DVL2 and its potential evaluation in esterification and resolution reactions. Arch. Appl. Sci. Res. 2012, 4, 1763–1770. [Google Scholar]
- Bozorov, T.A.; Toshmatov, Z.O.; Kahar, G.; Zhang, D.; Shao, H.; Gafforov, Y. Wild apple-associated fungi and bacteria compete to colonize the larval gut of an invasive wood-borer Agrilus mali in Tianshan forests. Front. Microbiol. 2021, 12, 743831. [Google Scholar] [CrossRef]
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Zalila-Kolsi, I.; Ben Mahmoud, A.; Ali, H.; Sellami, S.; Nasfi, Z.; Tounsi, S.; Jamoussi, K. Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2016, 192, 148–158. [Google Scholar] [CrossRef]
- Bozorov, T.A.; Toshmatov, Z.O.; Kahar, G.; Muhammad, S.M.; Liu, X.; Zhang, D.; Aytenov, I.S.; Turakulov, K.S. Uncovering the antifungal activities of wild apple-associated bacteria against two canker-causing fungi, Cytospora mali and C. parasitica. Sci. Rep. 2024, 14, 6307. [Google Scholar] [CrossRef]
- Kamilari, E.; O’Connor, P.M.; Farias, F.M.d.; Johnson, C.N.; Buttimer, C.; Deliephan, A.; Hill, D.; Fursenko, O.; Wiese, J.; Stanton, C.; et al. Bacillus safensis APC 4099 has broad-spectrum antimicrobial activity against both bacteria and fungi and produces several antimicrobial peptides, including the novel circular bacteriocin safencin E. Appl. Environ. Microbiol. 2024, 91, e01942-24. [Google Scholar] [CrossRef]
- Rong, S.; Xu, H.; Li, L.; Chen, R.; Gao, X.; Xu, Z. Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic. Biochem. Physiol. 2020, 162, 69–77. [Google Scholar] [CrossRef]
- Bidima, M.G.S.; Chtaina, N.; Ezzahiri, B.; El Guilli, M.; Barakat, I.; El Kamli, T. Antifungal activity of bioactive compounds produced by the endophyte Bacillus velezensis NC318 against the soil borne pathogen Sclerotium rolfsii Sacc. J. Plant Prot. Res. 2022, 62, 326–333. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 inhibits Fusarium oxysporum growth and induces systemic resistance to cucumber mosaic virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Knežević, M.; Dervišević, M.; Jovković, M.; Jevđenović, G.; Maksimović, J.; Buntić, A. Versatile role of Bacillus velezensis: Biocontrol of Fusarium poae and wireworms and barley plant growth promotion. Biol. Control 2025, 206, 105789. [Google Scholar] [CrossRef]
- Dobrzynski, J.; Jakubowska, Z.; Kulkova, I.; Kowalczyk, P.; Kramkowski, K. Biocontrol of fungal phytopathogens by Bacillus pumilus. Front. Microbiol. 2023, 14, 1194606. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Z.Z.; Fan, J.B.; Liang, Y.P.; Bernier, M.C.; Gao, Y.; Zhao, L.J.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Tariq, A.; Liao, J.; Yang, A.; Jiang, X.; Yin, Y.; Shi, Y.; Li, C.; Pan, J.; Han, D.; et al. Identification and antagonistic potential of Bacillus atrophaeus against wheat crown rot caused by Fusarium pseudograminearum. Agronomy 2024, 14, 2135. [Google Scholar] [CrossRef]
- Harba, M.; Jawhar, M.; Arabi, M.I.E. In vitro antagonistic activity of diverse Bacillus species against Fusarium culmorum and F. solani pathogens. Open Agric. J. 2020, 14, 157–163. [Google Scholar] [CrossRef]
- Puopolo, G.; Masi, M.; Raio, A.; Andolfi, A.; Zoina, A.; Cimmino, A.; Evidente, A. Insights on the susceptibility of plant pathogenic fungi to phenazine-1-carboxylic acid and its chemical derivatives. Nat. Prod. Res. 2013, 27, 956–966. [Google Scholar] [CrossRef]
- Simionato, A.S.; Navarro, M.O.P.; de Jesus, M.L.A.; Barazetti, A.R.; da Silva, C.S.; Simoes, G.C.; Balbi-Pena, M.I.; de Mello, J.C.P.; Panagio, L.A.; de Almeida, R.S.C.; et al. The effect of phenazine-1-carboxylic acid on mycelial growth of Botrytis cinerea produced by Pseudomonas aeruginosa LV strain. Front. Microbiol. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Tupe, S.G.; Kulkarni, R.R.; Shirazi, F.; Sant, D.G.; Joshi, S.P.; Deshpande, M.V. Possible mechanism of antifungal phenazine-1-carboxamide from Pseudomonas sp. against dimorphic fungi Benjaminiella poitrasii and human pathogen Candida albicans. J. Appl. Microbiol. 2015, 118, 39–48. [Google Scholar] [CrossRef]
- Goryluk-Salmonowicz, A.; Piorek, M.; Rekosz-Burlaga, H.; Studnicki, M.; Blaszczyk, M. Endophytic Detection in Selected European Herbal Plants. Pol. J. Microbiol. 2016, 65, 369–375. [Google Scholar] [CrossRef]
- Zachow, C.; Grosch, R.; Berg, G. Impact of biotic and a-biotic parameters on structure and function of microbial communities living on sclerotia of the soil-borne pathogenic fungus Rhizoctonia solani. Appl. Soil Ecol. 2011, 48, 193–200. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, S.; Su, X.; Zhang, X.; Wang, L.; Zhang, W.; Wang, Z.; Zeng, Q.; Wang, Q.; et al. Endogenous cell wall degrading enzyme LytD is important for the biocontrol activity of Bacillus subtilis. Front. Plant Sci. 2024, 15, 1381018. [Google Scholar] [CrossRef]
- Petkova, M.; Marcheva, M.; Petrova, A.L.; Slavova, V.; Shilev, S. Plant growth-promoting and biocontrol characteristics of four Bacillus strains and evaluation of their effects on eheat (Tr. aestivum L.). Int. J. Plant Biol. 2025, 16, 1. [Google Scholar] [CrossRef]
- Larrea-Murrell, J.A.; Rojas-Badia, M.M.; Garcia-Soto, I.; Romeu-Alvarez, B.; Bacchetti, T.; Gillis, A.; Boltes-Espinola, A.K.; Heydrich-Perez, M.; Lugo-Moya, D.; Mahillon, J. Diversity and enzymatic potentialities of Bacillus sp. strains isolated from a polluted freshwater ecosystem in Cuba. World J. Microbiol. Biotechnol. 2018, 34, 28. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Wolfenden, R.E.; Vicente, J.L.; Wolfenden, A.D.; Menconi, A.; Bielke, L.R.; Hargis, B.M.; Tellez, G. Evaluation and Selection of Bacillus Species Based on Enzyme Production, Antimicrobial Activity, and Biofilm Synthesis as Direct-Fed Microbial Candidates for Poultry. Front. Vet. Sci. 2016, 3, 95. [Google Scholar] [CrossRef]

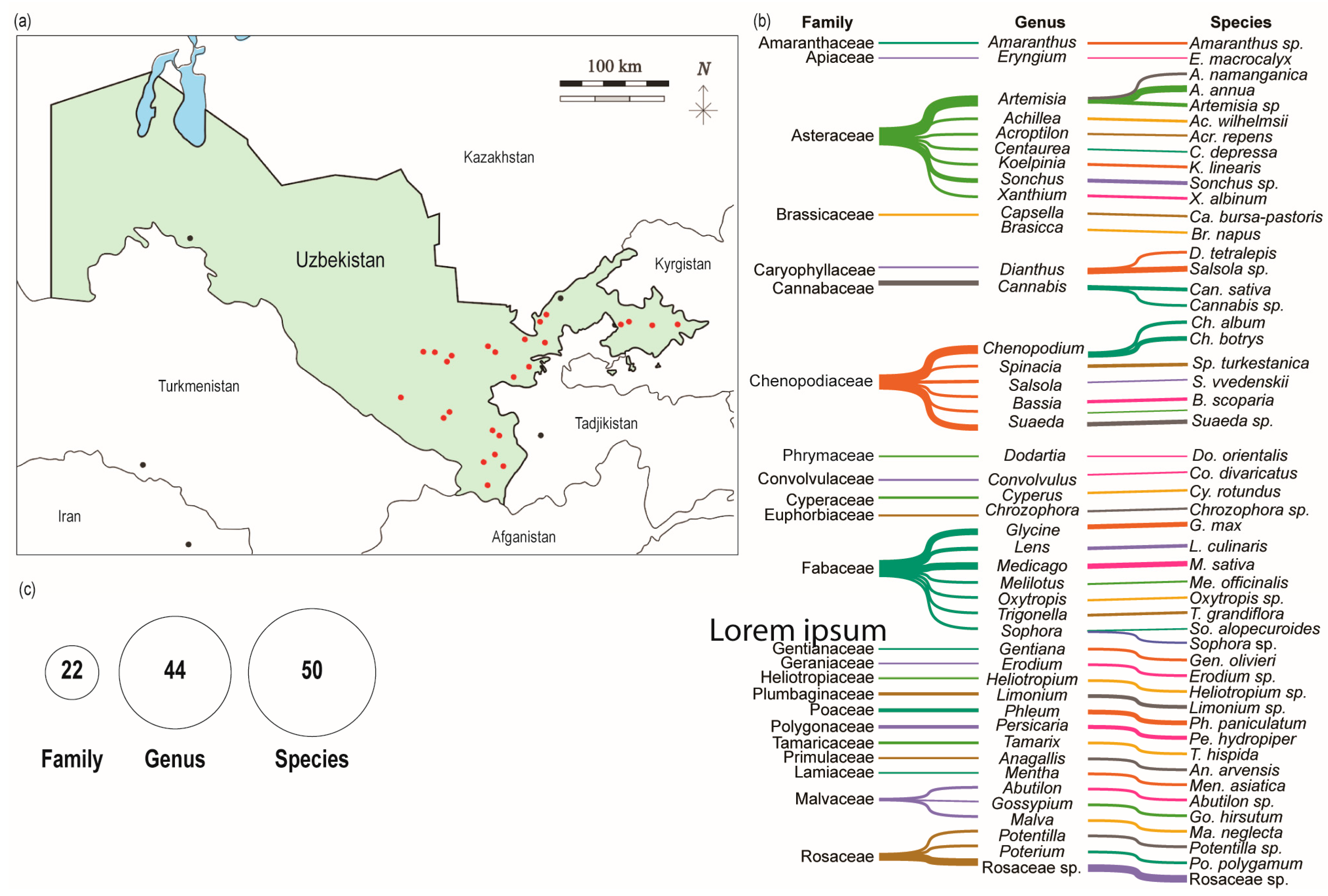


| Location | GPS Coordinates | Elevation (m) | Plant Species | |
|---|---|---|---|---|
| Region | District | |||
| Andijan | Andijan Ulugnor | 40°86’53.0 N 72°29′524 E 40°85′15.8 N 71°64′840 E | 455 408 | Lens culinaris Glycine max |
| Jizzakh | Zomin-1 Zomin-2 Zomin-3 Zomin-4 Zomin-5 Rovot-1 Rovot-2 Rovot-3 | 39°70’58.1 N 68°44’40.0 E 39°70’58.1 N 68°44’39.2 E 39°70’58.1 N 68°44’39.1 E 39°70’58.1 N 68°44’39.0 E 39°70’58.1 N 68°44’38.9 E 40°048’8.1 N 67°58’73.3 E 39°70’58.1 N 68°44’37.1 E 40°04’88.1 N 67°58’72.3 E | 1477 1477 1477 1477 1477 241 1477 241 | Eryngium macrocalyx Potentilla sp. Gentiana olivieri Poterium polygamum Capsella bursa-pastoris Medicago sativa Trigonella grandiflora |
| Namangan | Pop-1 Pop-2 | 40°85′61.0 N 70°95′97.2 E 40°91′56.5 N 71°15′44.5 E | 503 494 | Chenopodium botrys, Heliotropium sp., Artemisa namanganica, Abutilon sp., Amaranthus sp., Cannabis sp., Glycine max, Gossypium hirsutum |
| Navoiy | Xatirchi Paxtachi | 40°08′09.1 N 65°89′14.3 E 40°09′56.6 N 65°65′80 E | 419 372 | Mentha asiatica, Artemisia annua Tamarix hispida, Limonium sp., Bassia scoparia |
| Kashkadaria | Qamashi-1 Qamashi-2 Qamashi-3 Muborak | 38°91’17.9 N 66°66’67.0 E 38°90’738 N 66°66’20.5 E 38°91’57.9 N 66°67’21.2 E 39°30’88.7 N 65°12’01.6 E | 600 598 596 284 | Achillea wilhelmsii Anagallis arvensis, Brasicca napus Medicago sativa Koelpinia linearis, Oxytropis sp., Salsola vvedenskii, Suaeda sp. |
| Samarkand | Nurobod Nurbuloq | 39°77′00.2 N 66°42′03.3 E 39°75′39.0 N 66°40′26.8 E | 567 543 | Cannabis sativa, Centaurea depressa, Pseudosophora alopecuroides, Spinacia turkestanica Acroptilon repens |
| Surkhandaria | Khonguzar Sangardak Denov Oltin-Soy Shorchi Jarkurgan Angor | 38°60’77.9 N 67°57’12.9 E 38°51’07.9 N 67°65’21.2 E 38°21’57.2 N 67°54’76.4 E 38°048’35 N 67°37’79.9 E 38°03′87.9 N 67°82′80.5 E 37°49′77.5 N 67°42′03.7 E 37°51′52.0 N 67°37′60.7 E | 1702 996 793 538 783 348 361 | Dianthus tetralepis, Erodium sp., Rosaceae sp. Sonchus sp., Artemisia sp. Persicaria hydropiper Phleum paniculatum Melilotus officinalis Malva neglecta Chrozophora sp., Convolvulus divaricatus |
| Syrdaria | Boyovut Sardoba Ok-Oltin Syrdaria | 40°43’75.4 N 68°87’12.1 E 40°43’75.4 N 68°87’11.8 E 40°55’47.8 N 68°39’43.0 E 40°90’30.9 N 68°69’39.6 E | 279 279 266 253 | Cyperus rotundus Salsola sp. Artemisia annua Xanthium albinum |
| Tashkent | Yangiyo’l | 41°16′65.4 N 69°05′32.5 E | 361 | Chenopodium album, Dodartia orientalis |
| Plant Species | Number of Bacterial Isolates | Plant Species | Number of Bacterial Isolates | ||
|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | ||
| Abutilon sp. | 24 | 24 | Go. hirsutum | 24 | 24 |
| Ac. wilhelmsii | 48 | 21 | Heliotropium sp. | 24 | 24 |
| Acr. repens | 48 | 72 | K. linearis | 24 | 24 |
| Amaranthus sp. | 24 | 36 | L. culinaris | 24 | 24 |
| An. arvensis | 24 | 22 | Limonium sp. | 24 | 19 |
| A. namanganica | 48 | 18 | Ma. neglecta | 48 | 24 |
| A. annua | 48 | 15 | M. sativa | 24 | 48 |
| Artemisia sp. | 48 | 48 | Me. officinalis | 22 | 24 |
| B. scoparia | 36 | 24 | Men. asiatica | 48 | 25 |
| Br. napus | 48 | 48 | Oxytropis sp. | 24 | 24 |
| Can. sativa | 46 | 24 | Pe. hydropiper | 48 | 24 |
| Cannabis sp. | 24 | 24 | Ph. paniculatum | 48 | 24 |
| Ca. bursa-pastoris | 36 | 48 | Potentilla sp. | 62 | 24 |
| C. depressa | 24 | 24 | Po. polygamum | 24 | 41 |
| Ch. album | 24 | 38 | So. alopecuroides | 36 | 48 |
| Ch. botrys | 24 | 36 | Rosaceae sp. | 48 | 48 |
| Chrozophora sp. | 48 | 12 | Salsola sp. | 92 | 48 |
| Co. divaricatus | 24 | 24 | S. vvedenskii | 24 | 17 |
| Cy. rotundus | 22 | 44 | Sonchus sp. | 48 | 24 |
| D. tetralepis | 36 | 13 | Sp. turkestanica | 56 | 24 |
| Do. orientalis | 48 | 24 | Suaeda sp. | 48 | 24 |
| Erodium sp. | 72 | 24 | Ta. hispida | 24 | 24 |
| E. macrocalyx | 24 | 24 | T. grandiflora | 33 | 24 |
| Gen. olivieri | 24 | 24 | X. albinum | 72 | 46 |
| G. max | 48 | 48 | |||
| Total | 920 | 759 | Total | 949 | 700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shodmonova, M.K.; Muhammadova, D.A.; Aytenov, I.S.; Isokulov, M.Z.; Bozorov, T.A.; Zhang, D.; Abduraimov, O.S.; Murodova, S.M.; Melikuziev, F.A.; Ochilov, B.O.; et al. Diversity of Antifungal Properties in Bacterial Isolates from Different Plant Species Growing Across Uzbekistan. Microorganisms 2025, 13, 1161. https://doi.org/10.3390/microorganisms13051161
Shodmonova MK, Muhammadova DA, Aytenov IS, Isokulov MZ, Bozorov TA, Zhang D, Abduraimov OS, Murodova SM, Melikuziev FA, Ochilov BO, et al. Diversity of Antifungal Properties in Bacterial Isolates from Different Plant Species Growing Across Uzbekistan. Microorganisms. 2025; 13(5):1161. https://doi.org/10.3390/microorganisms13051161
Chicago/Turabian StyleShodmonova, Mukhlisa K., Dono A. Muhammadova, Ilkham S. Aytenov, Marufbek Z. Isokulov, Tohir A. Bozorov, Daoyuan Zhang, Ozodbek S. Abduraimov, Sojida M. Murodova, Fazliddin A. Melikuziev, Bekhruz O. Ochilov, and et al. 2025. "Diversity of Antifungal Properties in Bacterial Isolates from Different Plant Species Growing Across Uzbekistan" Microorganisms 13, no. 5: 1161. https://doi.org/10.3390/microorganisms13051161
APA StyleShodmonova, M. K., Muhammadova, D. A., Aytenov, I. S., Isokulov, M. Z., Bozorov, T. A., Zhang, D., Abduraimov, O. S., Murodova, S. M., Melikuziev, F. A., Ochilov, B. O., & Meliev, S. K. (2025). Diversity of Antifungal Properties in Bacterial Isolates from Different Plant Species Growing Across Uzbekistan. Microorganisms, 13(5), 1161. https://doi.org/10.3390/microorganisms13051161






