Oral Microbiota Dysbiosis in Firefighters and the Potential Contributing Environmental and Lifestyle Factors Based on a Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Metadata Collection
2.3. Processing of Oral Microbiota Samples and Isolation of Microbial DNA
2.4. Analysis of the Microbiome
2.5. Statistical Analyses
3. Results
3.1. Population Characteristics
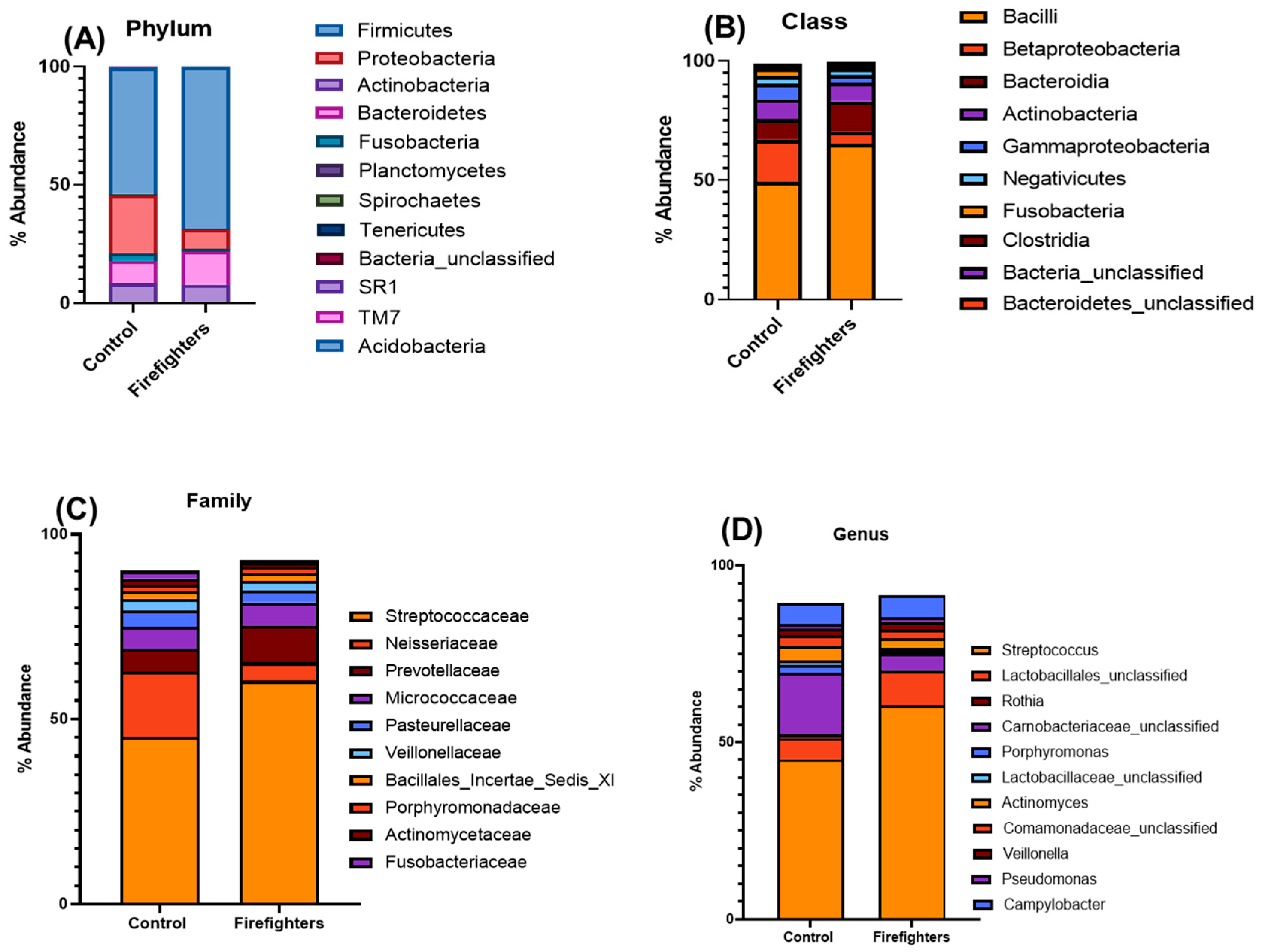
3.2. Alterations in the Oral Microbiota of Firefighters
3.3. Genus/Species-Level Alterations in the Oral Microbiota of Firefighters
3.4. Microbial Diversity in the Oral Microbiome in Firefighters
3.5. Factors Contributing to Oral Dysbiosis and Potential Occupational Health Risks in Firefighters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, B.; Mohammed, A.N.; Graham, B.; Bhattacharya, A.; Yadav, J.S. Chronic Heat Exposure Modulates Innate and Adaptive Immune Responses in Firefighters. Environments 2024, 11, 131. [Google Scholar] [CrossRef]
- Stec, A.A.; Dickens, K.E.; Salden, M.; Hewitt, F.E.; Watts, D.P.; Houldsworth, P.E.; Martin, F.L. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Elevated Cancer Incidence in Firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef]
- Woodall, C.A.; Hammond, A.; Cleary, D.; Preston, A.; Muir, P.; Pascoe, B.; Sheppard, S.K.; Hay, A.D. Oral and gut microbial biomarkers of susceptibility to respiratory tract infection in adults: A feasibility study. Heliyon 2023, 9, e18610. [Google Scholar] [CrossRef]
- Gustavsson, P.; Jakobsson, R.; Johansson, H.; Lewin, F.; Norell, S.; Rutkvist, L.E. Occupational exposures and squamous cell carcinoma of the oral cavity, pharynx, larynx, and oesophagus: A case-control study in Sweden. Occup. Environ. Med. 1998, 55, 393–400. [Google Scholar] [CrossRef]
- Chen, K.M.; Guttenplan, J.B.; Zhang, S.M.; Aliaga, C.; Cooper, T.K.; Sun, Y.W.; DelTondo, J.; Kosinska, W.; Sharma, A.K.; Jiang, K.; et al. Mechanisms of oral carcinogenesis induced by dibenzo[apyrene: An environmental pollutant and a tobacco smoke constituent. Int. J. Cancer 2013, 133, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Bouaoun, L.; Schüz, J.; Vermeulen, R.; Behrens, T.; Ge, C.; Kromhout, H.; Siemiatycki, J.; Gustavsson, P.; Boffetta, P.; et al. Lung Cancer Risks Associated with Occupational Exposure to Pairs of Five Lung Carcinogens: Results from a Pooled Analysis of Case-Control Studies (SYNERGY). Environ. Health Persp. 2024, 132, 017005. [Google Scholar] [CrossRef]
- Chieng, C.Y.; Dalal, A.; Ilankovan, V. Occupational exposure and risk of oral and oropharyngeal squamous cell carcinoma: Systematic review and 25-year retrospective cohort study of patients. Brit. J. Oral Max Surg. 2023, 61, 39–48. [Google Scholar] [CrossRef]
- Kang, A.W.; Lui, N.S. Factors associated with lung cancer among firefighters: A systematic literature review. BMC Public Health 2025, 25, 281. [Google Scholar] [CrossRef] [PubMed]
- Paget-Bailly, S.; Guida, F.; Carton, M.; Menvielle, G.; Radoï, L.; Cyr, D.; Schmaus, A.; Cénée, S.; Papadopoulos, A.; Févotte, J.; et al. Occupation and Head and Neck Cancer Risk in Men. J. Occup. Environ. Med. 2013, 55, 1065–1073. [Google Scholar] [CrossRef]
- Eliot, M.N.; Michaud, D.S.; Langevin, S.M.; McClean, M.D.; Kelsey, K.T. Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer Cause Control 2013, 24, 1315–1322. (In English) [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Yoshida, H.; Takahashi, H.; Kawai, M. Policemen and firefighters have increased risk for type-2 diabetes mellitus probably due to their large body mass index: A follow-up study in Japanese men. Am. J. Ind. Med. 2006, 49, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of Firefighters to Particulates and Polycyclic Aromatic Hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M. Changes in Abundance of Oral Microbiota Associated with Oral Cancer. PLoS ONE 2014, 9, e106297. (In English) [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, A.A.H.S. A Positive or Negative Connection of Diabetes Mellitus to the Oral Microbiota. Eurasian J. Med. 2023, 55, 83–89. [Google Scholar] [CrossRef]
- Kwak, S.; Wang, C.; Usyk, M.; Wu, F.; Freedman, N.D.; Huang, W.Y.; McCullough, M.L.; Um, C.Y.; Shrubsole, M.J.; Cai, Q.Y.; et al. Oral Microbiome and Subsequent Risk of Head and Neck Squamous Cell Cancer. JAMA Oncol. 2024, 10, 1537–1547. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Wu, Z.N.; Han, Y.L.; Wan, Y.H.; Hua, X.; Chill, S.S.; Teshome, K.; Zhou, W.Y.; Liu, J.; Wu, D.J.; Hutchinson, A.; et al. Oral microbiome and risk of incident head and neck cancer: A nested case-control study. Oral Oncol. 2023, 137, 106305. [Google Scholar] [CrossRef]
- Czerniuk, M.R.; Surma, S.; Romanczyk, M.; Nowak, J.M.; Wojtowicz, A.; Filipiak, K.J. Unexpected Relationships: Periodontal Diseases: Atherosclerosis-Plaque Destabilization? From the Teeth to a Coronary Event. Biology 2022, 11, 272. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Di Spirito, F.; Di Palo, M.P.; Folliero, V.; Cannatà, D.; Franci, G.; Martina, S.; Amato, M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers 2023, 15, 5540. (In English) [Google Scholar] [CrossRef]
- Zeng, X.T.; Deng, A.P.; Li, C.; Xia, L.Y.; Niu, Y.M.; Leng, W.D. Periodontal Disease and Risk of Head and Neck Cancer: A Meta-Analysis of Observational Studies. PLoS ONE 2013, 8, e79017. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.T.; Leng, W.D.; Zhang, C.; Liu, J.; Cao, S.Y.; Huang, W. Meta-analysis on the association between toothbrushing and head and neck cancer. Oral. Oncol. 2015, 51, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Lo, H.I.; Wong, T.Y.; Huang, C.C.; Lee, W.T.; Tsai, S.T.; Chen, K.C.; Yen, C.J.; Wu, Y.H.; Hsueh, W.T.; et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013, 49, 1010–1017. [Google Scholar] [CrossRef]
- Monteiro-da-Silva, F.; Sampaio-Maia, B.; Pereira, M.D.; Araujo, R. Characterization of the oral fungal microbiota in smokers and non-smokers. Eur. J. Oral Sci. 2013, 121, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef]
- Bang, E.; Oh, S.; Ju, U.; Chang, H.E.; Hong, J.S.; Baek, H.J.; Kim, K.S.; Lee, H.J.; Park, K.U. Factors influencing oral microbiome analysis: From saliva sampling methods to next-generation sequencing platforms. Sci. Rep. 2023, 13, 10086. (In English) [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. (In English) [Google Scholar] [CrossRef]
- Yadav, B.; Bhattacharya, S.S.; Rosen, L.; Nagpal, R.; Yadav, H.; Yadav, J.S. Oro-Respiratory Dysbiosis and Its Modulatory Effect on Lung Mucosal Toxicity during Exposure or Co-Exposure to Carbon Nanotubes and Cigarette Smoke. Nanomaterials 2024, 14, 314. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. Isme J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, Y.; Peng, X.; Qiu, W.; Kong, L.; Ren, B.; Li, M.; Cheng, G.; Zhou, X.; Cheng, L. Antibiotic-induced dysbiosis of the rat oral and gut microbiota and resistance to Salmonella. Arch. Oral Biol. 2020, 114, 104730. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.P.; Bassam, H.; Barnes, C.P.; Walker, A.S.; Klein, N.; Balloux, F. Modelling microbiome recovery after antibiotics using a stability landscape framework. ISME J. 2019, 13, 1845–1856. [Google Scholar] [CrossRef]
- Qiao, Y.A.; Wu, M.T.; Feng, Y.H.Z.; Zhou, Z.C.; Chen, L.; Chen, F.S. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci. Rep. 2018, 8, 1597. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Tai, W.-C.; Liang, C.-M.; Wu, C.-K.; Tsai, M.-C.; Hu, W.-H.; Huang, P.-Y.; Chen, C.-H.; Kuo, Y.-H.; Yao, C.-C.; et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Luo, E.L.; Wu, B.; Li, Z.; Guo, J.W.; Xia, Z.Y.; Zheng, C.Y.; Su, Q.Z.; Zeng, Y.; et al. Oral, Nasal, and Gut Microbiota in Parkinson’s Disease. Neuroscience 2022, 480, 65–78. [Google Scholar] [CrossRef]
- Galvao-Moreira, L.V.; da Cruz, M.C.F.N. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Ioannou, P.; Alexakis, K.; Baliou, S.; Kofteridis, D.P. Infective Endocarditis by Moraxella Species: A Systematic Review. J. Clin. Med. 2022, 11, 1854. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Zhu, Y.; Li, D.; Wang, J.; Chi, W. Diversity and Biogeography of Human Oral Saliva Microbial Communities Revealed by the Earth Microbiome Project. Front. Microbiol. 2022, 13, 931065. [Google Scholar] [CrossRef]
- McCune, E.; Sharma, A.; Johnson, B.; O’Meara, T.; Theiner, S.; Campos, M.; Heditsian, D.; Brain, S.; Gilbert, J.A.; Esserman, L.; et al. Gut and oral microbial compositional differences in women with breast cancer, women with ductal carcinoma in situ, and healthy women. mSystems 2024, 9, e01237-24. [Google Scholar] [CrossRef]
- Wesolowski, S.R.; El Kasmi, K.C.; Jonscher, K.R.; Friedman, J.E. Developmental origins of NAFLD: A womb with a clue. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef]
- Khatoon, J.; Rai, R.P.; Prasad, K.N. Role of Helicobacter pylori in gastric cancer: Updates. World J. Gastro Oncol. 2016, 8, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Golombos, D.M.; Ayangbesan, A.; O’Malley, P.; Lewicki, P.; Barlow, L.; Barbieri, C.E.; Chan, C.; DuLong, C.; Abu-Ali, G.; Huttenhower, C.; et al. The Role of Gut Microbiome in the Pathogenesis of Prostate Cancer: A Prospective, Pilot Study. Urology 2018, 111, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, M.R.; Rosa, E.F.; Lourençao, D.S.; Inoue, G.; Gomes, E.F.; De Micheli, G.; Mendes, F.M.; Hirata, R.D.C.; Hirata, M.H.; Pannuti, C.M. Detection and Quantification of Periodontal Pathogens in Smokers and Never-Smokers With Chronic Periodontitis by Real-Time Polymerase Chain Reaction. J. Periodontol. 2014, 85, 1450–1457. [Google Scholar] [CrossRef]
- Fan, X.Z.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.Y.; Pei, Z.H.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 59. [Google Scholar] [CrossRef]
- Wang, L.; Ganly, I. The Oral Microbiome and Oral Cancer. Clin. Lab. Med. 2014, 34, 711. [Google Scholar] [CrossRef]
- Leung, W.K.; Jin, L.J.; Samaranayake, L.P.; Chiu, G.K.C. Subgingival microbiota of shallow periodontal pockets in individuals after head and neck irradiation. Oral. Microbiol. Immunol. 1998, 13, 1–10. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Vanhaecke, L.; Boeckaert, C.; Peru, K.; Headley, J.; Verstraete, W.; Siciliano, S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ. Health Persp. 2005, 113, 6–10. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Jelski, W.; Zalewska, A.; Szulc, A.; Szmitkowski, M.; Zwierz, K.; Szajda, S.D. Salivary alcohol dehydrogenase in non-smoking and smoking alcohol-dependent persons. Alcohol. 2014, 48, 611–616. [Google Scholar] [CrossRef]
- Fernández-Hidalgo, N.; Escolà-Vergé, L. Bacteremia Consider an Echocardiography, But Consult an Infectious Diseases Specialist. J. Am. Coll. Cardiol. 2019, 74, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Sowden, D.; Caffery, M.; Bint, M.; Broom, J. Rhodococcus equi infection: A diverse spectrum of disease. IDCases 2019, 15, e00487. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Salvachúa, D.; Thakur, I.S. Burkholderia: An Untapped but Promising Bacterial Genus for the Conversion of Aromatic Compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef]
- Ma, J.; Zhuang, Y.; Wang, Y.; Zhu, N.; Wang, T.; Xiao, H.; Chen, J. Update on new trend and progress of the mechanism of polycyclic aromatic hydrocarbon biodegradation by Rhodococcus, based on the new understanding of relevant theories: A review. Environ. Sci. Pollut. R. 2023, 30, 93345–93362. [Google Scholar] [CrossRef] [PubMed]


| Metadata Variables | Microbiome Family | Counts Changed (p-Value) | Proportion Changed (p-Value) |
|---|---|---|---|
| Firefighting experience (>5 years) | Gammaproteobacteria_unclassified | ↑ 0.08 | ↑ 0.08 |
| Carnobacteriaceae | ↓ 0.04 | ↓ 0.04 | |
| Peptococcaceae_1 | ↑ 0.07 | ↑ 0.08 | |
| Fire events (>20) | Comamonadaceae | ↑ 0.018 | ↑ 0.018 |
| ≥1 Number of fires of one hour or more duration within 3 months | Actinomycetaceae | ↓ 0.09 | ↓ 0.05 |
| Carnobacteriaceae | ↑ 0.05 | ↑ 0.09 | |
| Food PAH intake (within past 8 h) | Spirochaetaceae | ↑ 0.001 | ↑ 0.005 |
| Lactobacillaceae | ↑ 0.05 | ↑ 0.03 | |
| Peptostreptococcaceae | ↑ 0.036 | ↑ 0.033 | |
| Tobacco usage (within past 8 h) | Fusobacteriaceae | ↓ 0.02 | ↓ 0.04 |
| Comamonadaceae | ↓ 0.04 | ↓ 0.04 | |
| Eubacteriaceae | ↑ 0.045 | ↑ 0.029 | |
| ≥3 Tins/week of tobacco consumption | Spirochaetaceae | ↓ 0.02 | ↓ 0.01 |
| Peptostreptococcaceae | ↑ 0.09 | ↑ 0.06 | |
| Mouthwash (≥1 mouthwash daily) | Fusobacteriaceae | ↓ 0.051 | ↓ 0.051 |
| Comamonadaceae | ↓ 0.041 | ↓ 0.041 | |
| Spirochaetaceae | ↓ 0.044 | ↓ 0.022 | |
| Between Firefighters and Control | Bacteroidaceae | ↓ 0.0002 | ↓ 0.0001 |
| Fusobacteriaceae | ↓ 0.2 | ↓ 0.0001 | |
| Campylobacteraceae | ↓ 0.004 | ↓ 0.003 | |
| Enterobacteriaceae | ↓ 0.0003 | ↓ 0.0003 | |
| Shewanellaceae | ↓ 0.0005 | ↓ 0.0005 | |
| Pseudomonadaceae | ↓ 0.0018 | ↓ 0.0019 | |
| Staphylococcaceae | ↓ 0.086 | ↓ 0.093 | |
| Bacilli_unclassified | ↑ 0.002 | ↑ 0.011 | |
| Aerococcaceae | ↑ 0.064 | ↑ 0.1 | |
| Streptococcaceae | ↑ 0.0002 | ↑ 0.014 | |
| Clostridiales_Incertae_Sedis_XI | ↓ 0.051 | ↓ 0.021 | |
| Clostridiales_unclassified | ↓ 0.068 | ↓ 0.072 | |
| Peptococcaceae_1 | ↓ 0.014 | ↓ 0.008 | |
| Caulobacteraceae | ↓ 0.037 | ↓ 0.037 | |
| Mycobacteriaceae | ↓ 0.0007 | ↓ 1 | |
| Nocardiaceae | ↑ 0.003 | ↑ 0.003 | |
| Thermotogales_incertae_sedis | ↑ 0.031 | ↑ 0.037 | |
| Oceanospirillaceae | ↑ 0.079 | ↑ 0.079 | |
| Burkholderiaceae | ↑ 0.0004 | ↑ 0.004 | |
| Rhizobiaceae | ↓ 0.07 | ↓ 0.0004 |
| Family | Genus | Fold Change * | OTU Count (FF/Control) | Family | Genus | Fold Change * | OTU Count (FF/Control) |
|---|---|---|---|---|---|---|---|
| Pseudomonadaceae | Pseudomonadaceae_unclassified | −1124.5 | 2/2249 | Eubacteriaceae | Eubacterium | −3.54 | 7.34/2597 |
| Pseudomonas | −26,370.5 | 2/5274 | Bacteroidales_incertae_sedis | Phocaeicola | −2.75 | 12/33 | |
| Bacteroidales_unclassified | Bacteroidales_unclassified | +2.56 | 34,201/13,308 | Cardiobacteriaceae | Cardiobacterium | −2.36 | 184/435 |
| Fusobacteriaceae | Fusobacterium | −2.33 | 24,860/57,774 | Leptotrichiaceae | Leptotrichiaceae_unclassified | +2.06 | 3442/1665 |
| Burkholderiales_unclassified | Burkholderiales_unclassified | −2.97 | 576/1711 | Campylobacteraceae | Arcobacter | −13 | 1/13 |
| Campylobacter | −7.03 | 1263/8883 | |||||
| Neisseriaceae | Bergeriella | −4.66 | 3/14 | Sulfurospirillum | --- | 0/1 | |
| Conchiformibius | +7.5 | 15/2 | Shewanellaceae | Shewanella | −25 | 2/50 | |
| Eikenella | −2.5 | 2/5 | |||||
| Neisseria | −3.98 | 141,957/564,929 | |||||
| Uruburuella | +++ | 3/0 | |||||
| Moraxellaceae | Moraxella | +35.87 | 287/8 | Gammaproteobacteria_unclassified | Gammaproteobacteria_unclassified | −14.04 | 44/618 |
| Enhydrobacter | --- | 0/3 | |||||
| Moraxellaceae_unclassified | +4.6 | 23/5 | |||||
| Psychrobacter | --- | 0/3 | |||||
| Spirochaetaceae | Treponema | −10.14 | 495/5021 | Mycoplasmataceae | Mycoplasma | −7.33 | 77/565 |
| Spirochaetaceae_unclassified | --- | 0/2 | Ureaplasma | --- | 0/3 | ||
| Bacteroidaceae | Bacteroides | −4.49 | 85/382 | Thermotogales_incertae_sedis | Oceanotoga | +++ | 3/0 |
| SR1_family_incertae_sedis | SR1_genus_incertae_sedis | −9.26 | 771/7141 | Staphylococcaceae | Staphylococcus | −3.93 | 15/59 |
| Jeotgalicoccus | --- | 0/1 | |||||
| Enterococcaceae | Enterococcaceae_unclassified | +2.67 | 24/9 | Bacilli_unclassified | Bacilli_unclassified | +2.09 | 4954/2369 |
| Enterococcus | +++ | 2/0 | |||||
| Lactobacillaceae | Lactobacillaceae_unclassified | −7.9 | 10/79 | Aerococcaceae | Abiotrophia | +2.39 | 8682/3626 |
| Lactobacillus | −13.93 | 113/1574 | Aerococcaceae_unclassified | +2.87 | 23/8 | ||
| Clostridiales_Incertae_Sedis_XI | Anaerococcus | --- | 0/4 | Clostridiales_Incertae_Sedis_XIII | Anaerovorax | −53 | 1/53 |
| Clostridiales_In certae_Sedis_XI_unclassified | −2.25 | 4/9 | |||||
| Parvimonas | −5.48 | 517/2836 | Clostridiales_Incertae_Sedis_XIII_unclassified | −19 | 3/57 | ||
| Helcococcus | --- | 0/1 | |||||
| Peptoniphilus | +4 | 4/1 | |||||
| Peptococcaceae_1 | Peptococcus | −13.26 | 15/199 | Mogibacterium | −2.22 | 698/1548 | |
| Peptococcaceae_1_unclassified | --- | 0/1 | |||||
| Peptostreptococcaceae | Acetoanaerobium | +++ | 1/0 | Clostridiales_unclassified | Clostridiales_unclassified | −2.49 | 991/2473 |
| Filifactor | −10.18 | 220/2244 | |||||
| Peptostreptococcaceae_unclassified | −7.83 | 30/235 | |||||
| Enterobacteriaceae | Enterobacteriaceae_unclassified | −585 | 13/7605 | Mycobacteriaceae | Mycobacterium | --- | 0/7 |
| Escherichia_Shigella | --- | 0/1 | Nocardiaceae | Rhodococcus | +++ | 13/0 | |
| Salmonella | --- | 0/1 | Oceanospirillaceae | Nitrincola | +++ | 3/0 | |
| Serratia | --- | 0/5 | Oceanospirillaceae_unclassified | +++ | 1/0 | ||
| Yersinia | --- | 0/25 | Burkholderiaceae | Burkholderia | +++ | 30/0 | |
| Caulobacteraceae | Brevundimonas | --- | 0/11 | Burkholderiaceae_unclassified | +++ | 1/0 | |
| Rhizobiaceae | Rhizobium | --- | 0/5 | Ralstonia | +++ | 4/0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, S.S.; Yadav, B.; Jandarov, R.; Jetter, W.A.; Yadav, J.S. Oral Microbiota Dysbiosis in Firefighters and the Potential Contributing Environmental and Lifestyle Factors Based on a Case-Control Study. Microorganisms 2025, 13, 1154. https://doi.org/10.3390/microorganisms13051154
Bhattacharya SS, Yadav B, Jandarov R, Jetter WA, Yadav JS. Oral Microbiota Dysbiosis in Firefighters and the Potential Contributing Environmental and Lifestyle Factors Based on a Case-Control Study. Microorganisms. 2025; 13(5):1154. https://doi.org/10.3390/microorganisms13051154
Chicago/Turabian StyleBhattacharya, Sukanta S., Brijesh Yadav, Roman Jandarov, William A. Jetter, and Jagjit S. Yadav. 2025. "Oral Microbiota Dysbiosis in Firefighters and the Potential Contributing Environmental and Lifestyle Factors Based on a Case-Control Study" Microorganisms 13, no. 5: 1154. https://doi.org/10.3390/microorganisms13051154
APA StyleBhattacharya, S. S., Yadav, B., Jandarov, R., Jetter, W. A., & Yadav, J. S. (2025). Oral Microbiota Dysbiosis in Firefighters and the Potential Contributing Environmental and Lifestyle Factors Based on a Case-Control Study. Microorganisms, 13(5), 1154. https://doi.org/10.3390/microorganisms13051154






