In Silico Design, Optimization, and Evaluation of a Multi-Epitope Vaccine Targeting the Clostridium perfringens Collagen Adhesin Protein
Abstract
1. Introduction
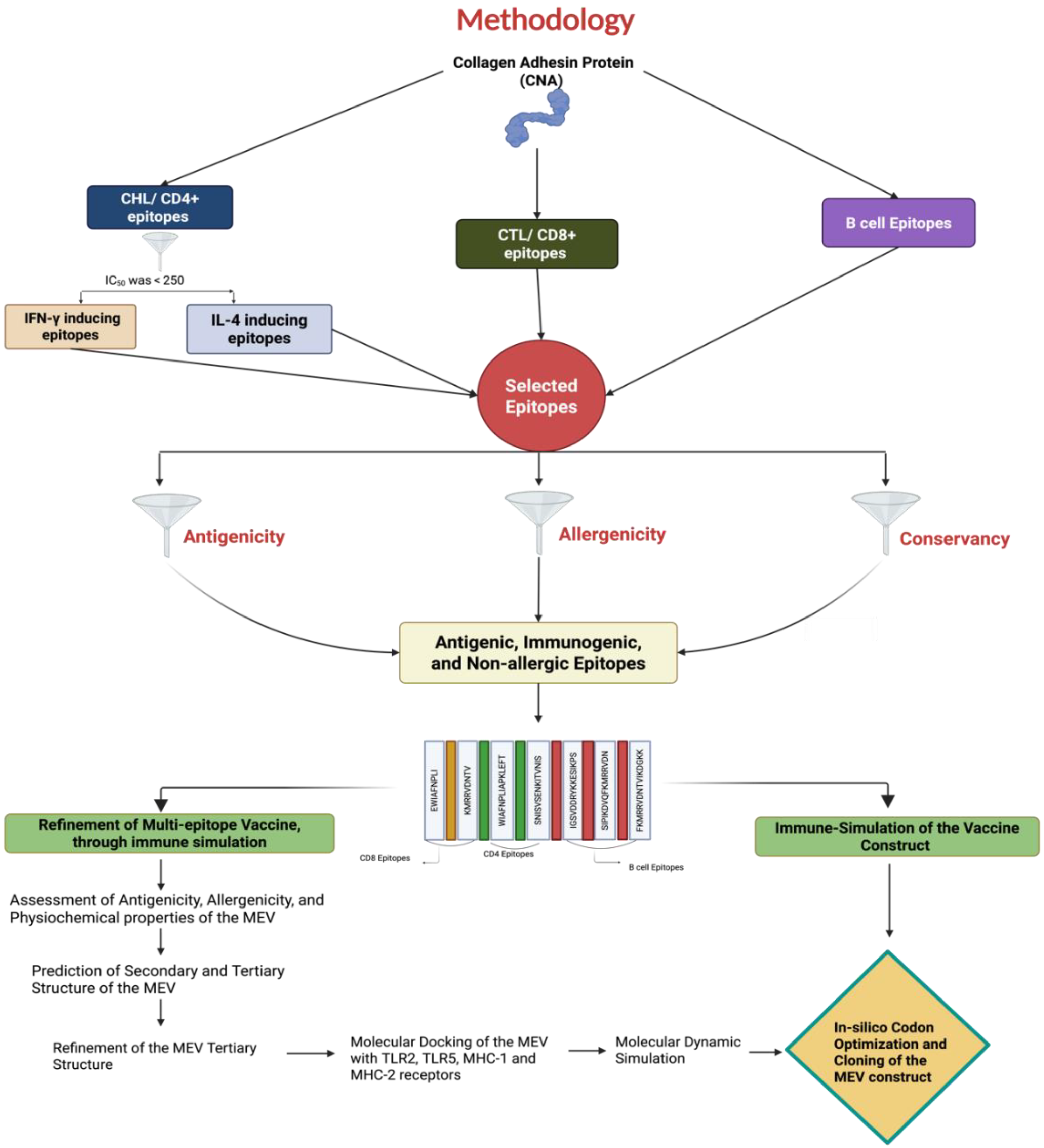
2. Materials and Methods
2.1. Raw Data Source and Sequence Retrieval
2.2. Prediction and Selection of Epitopes
2.2.1. Prediction of CD8+ CTL Epitopes
2.2.2. Prediction of CD4+ Th Epitopes
2.2.3. Prediction of B-Cell Epitopes
2.2.4. Antigenicity, Conservancy, and Allergenicity of the Selected Epitopes
2.3. Merging of Epitopes and Construction of the MEV
2.3.1. Assessment of Antigenicity, Allergenicity, and Physiochemical Properties of the MEV
2.3.2. Prediction of Secondary and Tertiary Structures of the MEV
2.3.3. Refinement of the MEV Tertiary Structure
2.4. Molecular Docking of the MEV with Toll-like Receptor (TLR) 2, TLR5, MHC-I, and MHC-II Receptors
2.5. Molecular Dynamic Simulation (MDS)
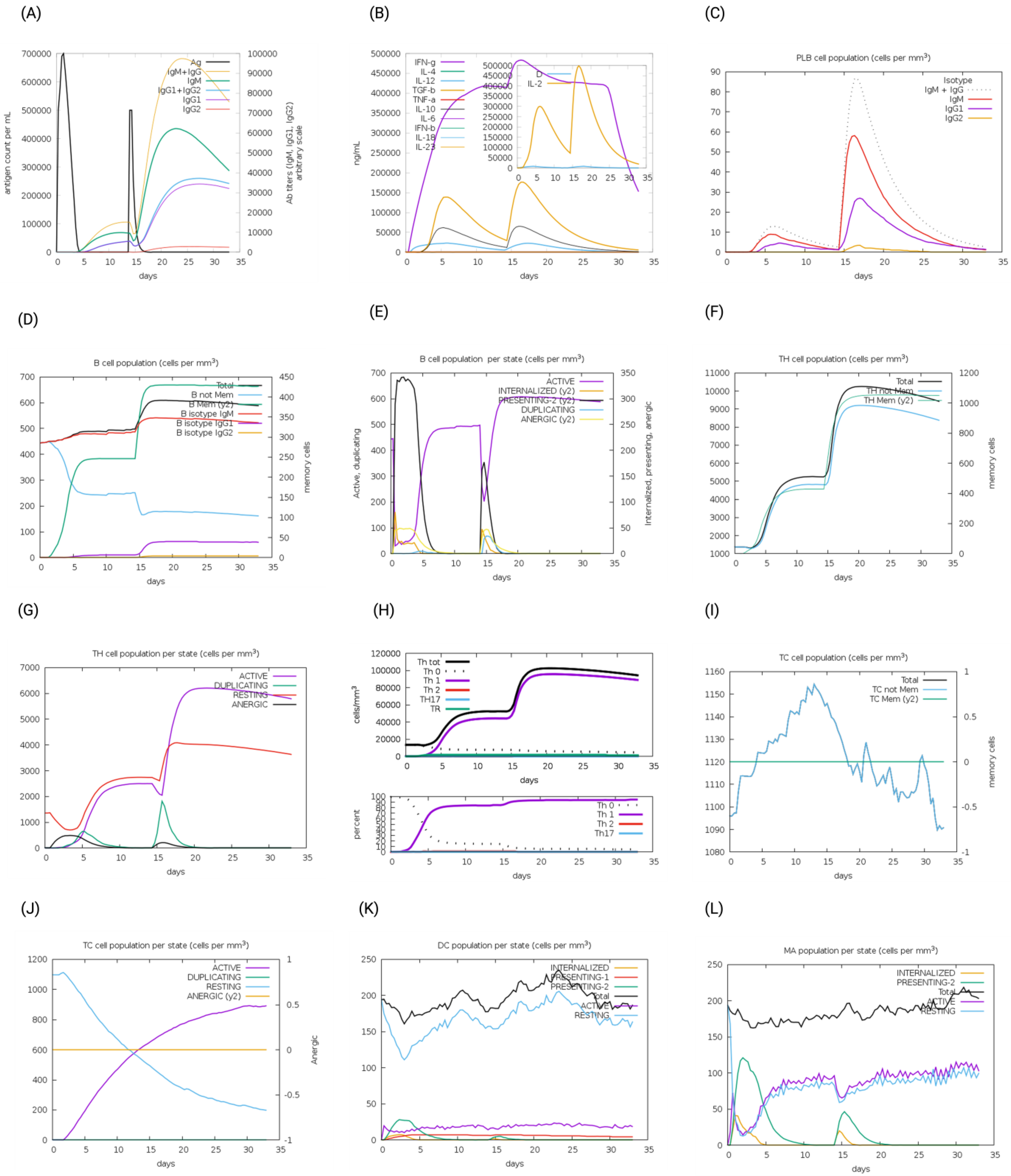
2.6. Immune Simulation of the MEV and Native CNA Protein
2.7. In Silico Codon Optimization and Cloning of the MEV Construct
3. Results
3.1. Prediction of T-Cell and B-Cell Epitopes
3.1.1. Cytotoxic T-Cell/CD8+ T-Cell Epitope Prediction
3.1.2. Helper T-Cell/CD4+ T-Cell Epitope Prediction
3.1.3. B-Cell Epitope Prediction
3.1.4. Antigenicity, Conservancy, and Allergenicity of Selected Epitopes
3.2. Construction, Evaluation, and Refinement of the MEV
3.3. Assessment of Antigenicity, Allergenicity, and Physiochemical Properties of the MEV
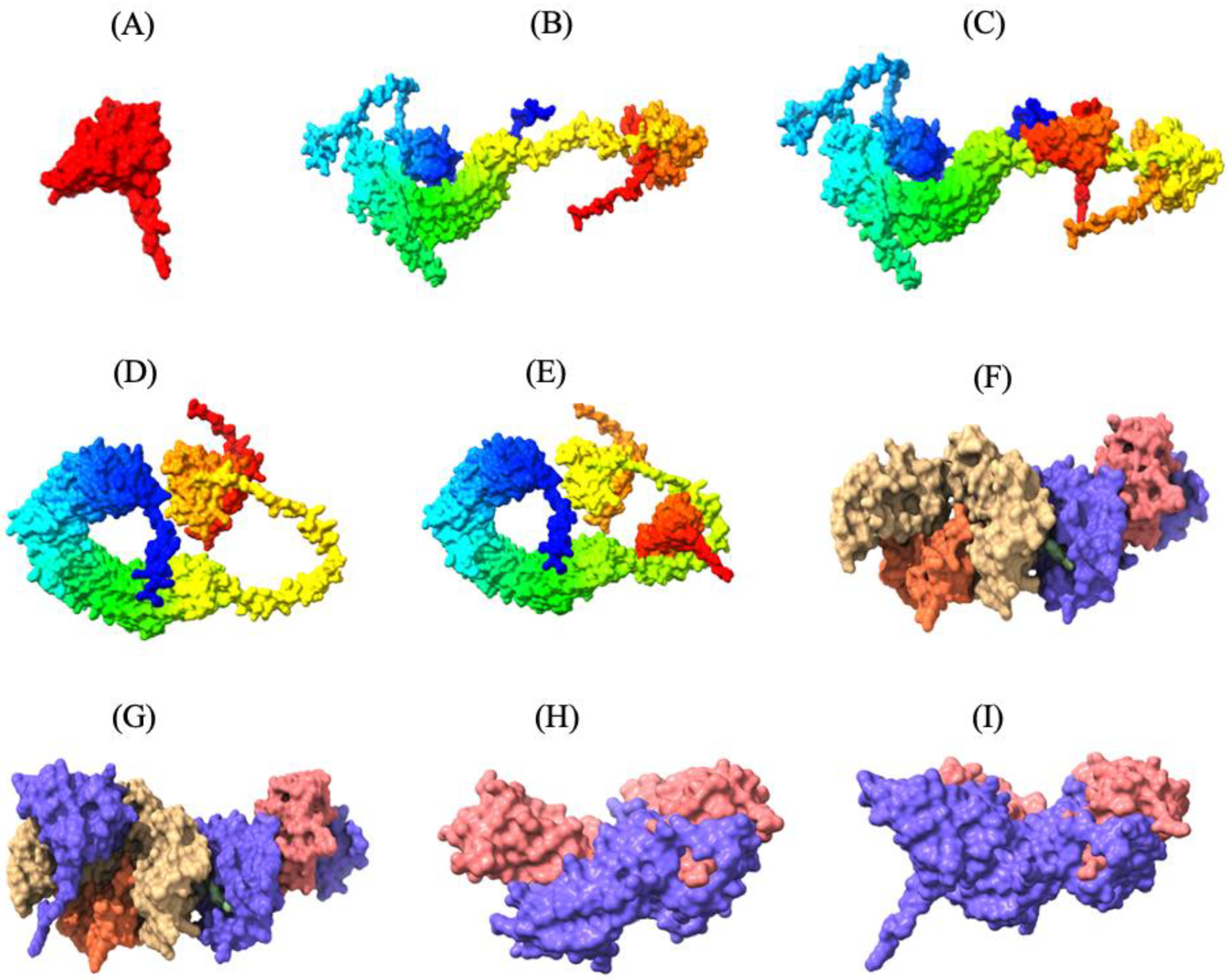
3.4. Secondary and Tertiary Structures of the MEV
3.5. Molecular Refinement of the Tertiary Structure of the MEV
3.6. Molecular Docking of the MEV with TLR2, TLR5, MHC-I, and MHC-II Receptors
3.7. Molecular Dynamic Simulation
3.8. Immune Simulation of the MEV and Native CNA Protein
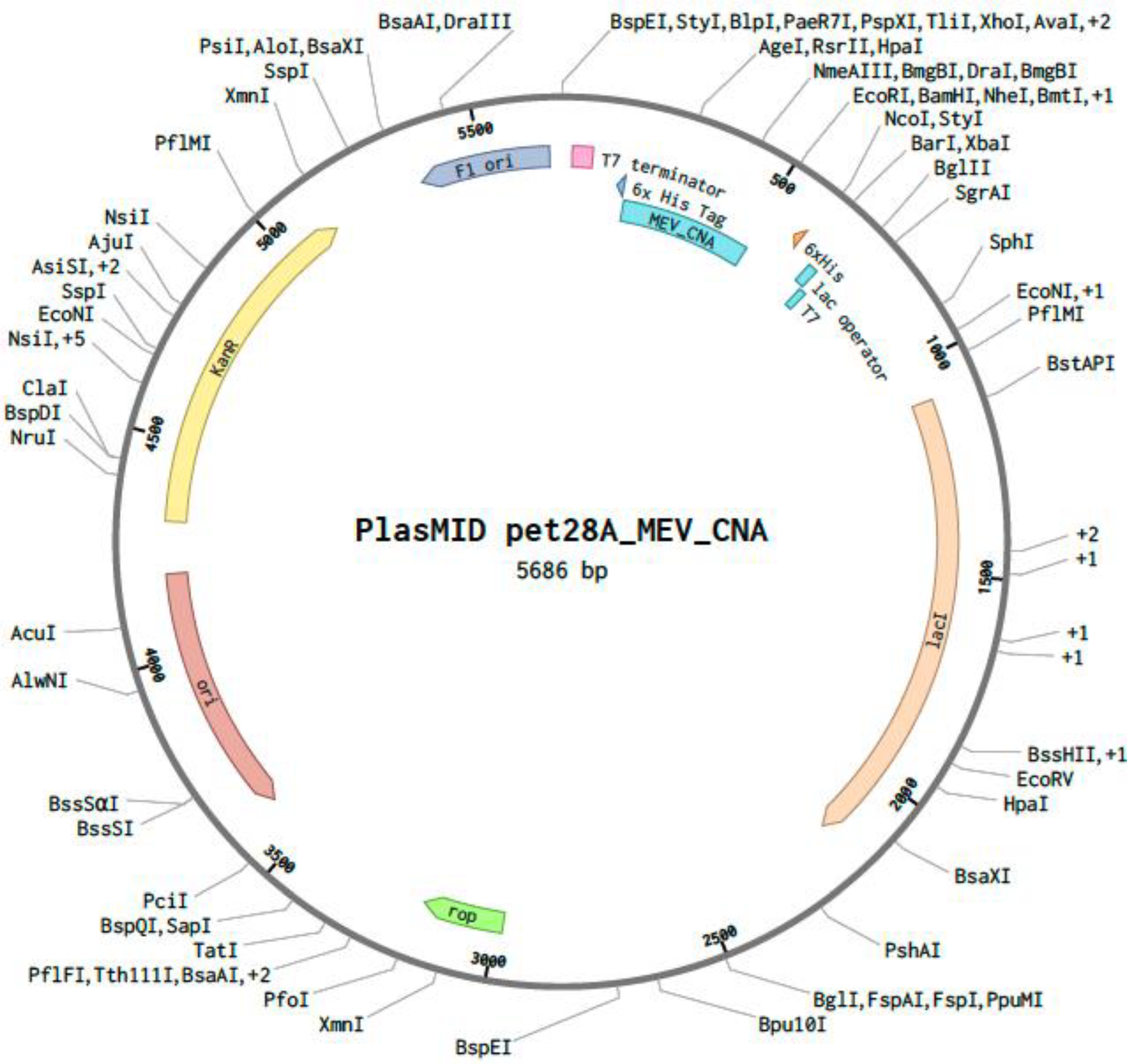
3.9. In Silico Codon Optimization and Cloning of the MEV Construct
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.A.; Martin, T.G. Disease producing capability of netB positive isolates of C. perfringens recovered from normal chickens and a cow, and netB positive and negative isolates from chickens with necrotic enteritis. Vet. Microbiol. 2010, 146, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Bannam, T.L.; Moore, R.J.; Rood, J.I. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2010, 2, 1913–1927. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Shamshirgaran, M.A.; Golchin, M. Necrotic enteritis in chickens: A comprehensive review of vaccine advancements over the last two decades. Avian Pathol. 2025, 54, 1–26. [Google Scholar] [CrossRef]
- Jiang, Y.; Kulkarni, R.R.; Parreira, V.R.; Prescott, J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins. Avian Dis. 2009, 53, 409–415. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, Z.; Lu, M.; Lillehoj, H.; Lee, Y.; Liu, L.; Yan, X.; Yang, D.A.; Li, C. Immunization with Pooled Antigens for Clostridium perfringens Conferred Partial Protection against Experimental Necrotic Enteritis in Broiler Chickens. Vaccines 2022, 10, 979. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Pu, X.; Xu, L.; Song, X.; Yan, R.; Li, X.; Li, C.; Yuan, C.; Lu, M. Vaccination with formulations targeting Eimeria maxima and Clostridium perfringens conferred comprehensive protection using a dual-infection challenge model of necrotic enteritis. Poult. Sci. 2025, 104, 104687. [Google Scholar] [CrossRef]
- Wang, S.; Hofacre, C.L.; Wanda, S.Y.; Zhou, J.; Callum, R.A.; Nordgren, B.; Curtiss, R., 3rd. A triple-sugar regulated Salmonella vaccine protects against Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 2022, 101, 101592. [Google Scholar] [CrossRef]
- Wilde, S.; Jiang, Y.; Tafoya, A.M.; Horsman, J.; Yousif, M.; Vazquez, L.A.; Roland, K.L. Salmonella-vectored vaccine delivering three Clostridium perfringens antigens protects poultry against necrotic enteritis. PLoS ONE 2019, 14, e0197721. [Google Scholar] [CrossRef] [PubMed]
- Alimolaei, M.; Golchin, M.; Abshenas, J.; Ezatkhah, M.; Bafti, M.S. A Recombinant Probiotic, Lactobacillus casei, Expressing the Clostridium perfringens alpha-toxoid, as an Orally Vaccine Candidate Against Gas Gangrene and Necrotic Enteritis. Probiotics Antimicrob. Proteins 2018, 10, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Portela, R.W.; Sproat, K.; Ford, M.E.; Bannam, T.L.; Yan, X.; Rood, J.I.; Moore, R.J. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet. Res. 2013, 44, 54. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F.; Parreira, V.R.; Mehdizadeh Gohari, I.; Lepp, D.; Gong, J. The pathogenesis of necrotic enteritis in chickens: What we know and what we need to know: A review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef]
- Lepp, D.; Zhou, Y.; Ojha, S.; Mehdizadeh Gohari, I.; Carere, J.; Yang, C.; Prescott, J.; Gong, J. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J. Bacteriol. 2021, 203, e00578-20. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, M.; Lillehoj, H.; Lee, Y.; Goo, D.; Yuan, B.; Yan, X.; Li, C. Characterization of collagen binding activity of Clostridium perfringens strains isolated from broiler chickens. Pathogens 2023, 12, 778. [Google Scholar] [CrossRef]
- Lepp, D.; Gong, J.; Songer, J.G.; Boerlin, P.; Parreira, V.R.; Prescott, J.F. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. J. Bacteriol. 2013, 195, 1152–1166. [Google Scholar] [CrossRef]
- Mandlik, A.; Swierczynski, A.; Das, A.; Ton-That, H. Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells. Mol. Microbiol. 2007, 64, 111–124. [Google Scholar] [CrossRef]
- Nielsen, H.V.; Guiton, P.S.; Kline, K.A.; Port, G.C.; Pinkner, J.S.; Neiers, F.; Normark, S.; Henriques-Normark, B.; Caparon, M.G.; Hultgren, S.J. The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. MBio 2012, 3, e00177-12. [Google Scholar] [CrossRef]
- Abbot, E.L.; Smith, W.D.; Siou, G.P.; Chiriboga, C.; Smith, R.J.; Wilson, J.A.; Hirst, B.H.; Kehoe, M.A. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell. Microbiol. 2007, 9, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Dramsi, S.; Caliot, E.; Bonne, I.; Guadagnini, S.; Prévost, M.C.; Kojadinovic, M.; Lalioui, L.; Poyart, C.; Trieu-Cuot, P. Assembly and role of pili in group B streptococci. Mol. Microbiol. 2006, 60, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Wade, B.; Keyburn, A.L.; Seemann, T.; Rood, J.I.; Moore, R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015, 180, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Wade, B.; Keyburn, A.L.; Haring, V.; Ford, M.; Rood, J.I.; Moore, R.J. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet. Microbiol. 2016, 197, 53–61. [Google Scholar] [CrossRef]
- Broughan, J.; Anderson, R.; Anderson, A.S. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev. Vaccines 2011, 10, 695–708. [Google Scholar] [CrossRef]
- Proctor, R.A. Is there a future for a Staphylococcus aureus vaccine? Vaccine 2012, 30, 2921–2927. [Google Scholar] [CrossRef]
- Tahir ul Qamar, M.T.; Ahmad, S.; Fatima, I.; Ahmad, F.; Shahid, F.; Naz, A.; Abbasi, S.W.; Khan, A.; Mirza, M.U.; Ashfaq, U.A.; et al. Designing multi-epitope vaccine against Staphylococcus aureus by employing subtractive proteomics, reverse vaccinology and immuno-informatics approaches. Comput. Biol. Med. 2021, 132, 104389. [Google Scholar] [CrossRef]
- María, R.A.R.; Arturo, C.V.J.; Alicia, J.A.; Paulina, M.L.G.; Gerardo, A.O. The impact of bioinformatics on vaccine design and development. In Vaccines; Farhat, A., Hassan, H., Hani, O., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Wang, Y. Bioinformatics analysis of NetF proteins for designing a multi-epitope vaccine against Clostridium perfringens infection. Infect. Genet. Evol. 2020, 85, 104461. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Abrar, A.; Munir, S.; Aslam, S.; Allemailem, K.S.; Khurshid, M.; Ashfaq, U.A. Proteome-wide mapping and reverse vaccinology approaches to design a multi-epitope vaccine against Clostridium perfringens. Vaccines 2021, 9, 1079. [Google Scholar] [CrossRef]
- Krachler, A.M.; Orth, K. Targeting the bacteria–host interface: Strategies in anti-adhesion therapy. Virulence 2013, 4, 284–294. [Google Scholar] [CrossRef]
- Sawa, T.; Kinoshita, M.; Inoue, K.; Ohara, J.; Moriyama, K. Immunoglobulin for treating bacterial infections: One more mechanism of action. Antibodies 2019, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Schepers, K.; Arens, R.; Schumacher, T.N.M. Dissection of cytotoxic and helper T cell responses. Cell. Mol. Life Sci. 2005, 62, 2695–2710. [Google Scholar] [CrossRef]
- Llanco, L.A.; Nakano, V.; Moraes, C.T.P.; Piazza, R.M.F.; Avila-Campos, M.J. Adhesion and invasion of Clostridium perfringens type A into epithelial cells. Braz. J. Microbiol. 2017, 48, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mou, S.; Li, L.; Chen, Q.; Yang, R.; Guo, S.; Jin, Y.; Liu, L.; Li, T.; Chen, H.; et al. The Barrier Disruption and Pyroptosis of Intestinal Epithelial Cells Caused by Perfringolysin O (PFO) from Clostridium perfringens. Cells 2024, 13, 1140. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, A.; Kermanshahi, H.; Mohammed, J.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh, M.; Razmyar, J.; Kulkarni, R. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 2021, 101, 101652. [Google Scholar] [CrossRef]
- Boodhoo, N.; Shojadoost, B.; Alizadeh, M.; Kulkarni, R.R.; Sharif, S. Ex Vivo Differential Responsiveness to Clostridium perfringens and Lactococcus lactis by Avian Small Intestine Macrophages and T Cells. Front. Immunol. 2022, 13, 807343. [Google Scholar] [CrossRef]
- Yadalam, P.K.; Anegundi, R.V.; Munawar, S.; Ramadoss, R.; Rengaraj, S.; Ramesh, S.; Aljeldah, M.; Shammari, B.R.A.; Alshehri, A.A.; Alwashmi, A.S.; et al. Designing novel multi-epitope vaccine construct against Prevotella intermedia-Interpain A: An immunoinformatics approach. Medicina 2023, 59, 302. [Google Scholar] [CrossRef]
- Madlala, T.; Adeleke, V.T.; Fatoba, A.J.; Okpeku, M.; Adeniyi, A.A.; Adeleke, M.A. Designing multiepitope-based vaccine against Eimeria from immune mapped protein 1 (IMP-1) antigen using immunoinformatic approach. Sci. Rep. 2021, 11, 18295. [Google Scholar] [CrossRef]
- Yan, Z.; Kim, K.; Kim, H.; Ha, B.; Gambiez, A.; Bennett, J.; de Almeida Mendes, M.F.; Trevizani, R.; Mahita, J.; Richardson, E.; et al. Next-generation IEDB tools: A platform for epitope prediction and analysis. Nucleic Acids Res. 2024, 52, W526–W532. [Google Scholar] [CrossRef]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef]
- Saha, S.; Raghava, G.P. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.; Ali, A.; Akbar, H.; Sayaf, A.M.; Khan, A.; Wei, D.Q. Immunoinformatics approaches to explore Helicobacter pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. Sci. Rep. 2019, 9, 13321. [Google Scholar] [CrossRef]
- Chao, P.; Zhang, X.; Zhang, L.; Yang, A.; Wang, Y.; Chen, X. Proteomics-based vaccine targets annotation and design of multi-epitope vaccine against antibiotic-resistant Streptococcus gallolyticus. Sci. Rep. 2024, 14, 4836. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Jones, D.T. The PSIPRED Protein Analysis Workbench: 20 years on. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef]
- Jones, D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999, 292, 195–202. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schutze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef]
- Lee, G.R.; Won, J.; Heo, L.; Seok, C. GalaxyRefine2: Simultaneous refinement of inaccurate local regions and overall protein structure. Nucleic Acids Res. 2019, 47, W451–W455. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Rullmannn, J.A.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Vajda, S. How good is automated protein docking? Proteins 2013, 81, 2159–2166. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Lopez-Blanco, J.R.; Aliaga, J.I.; Quintana-Orti, E.S.; Chacon, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef]
- Cheema, N.; Papamichail, G.; Papamichail, D. Computational tools for synthetic gene optimization. In New Frontiers and Applications of Synthetic Biology; Singh, V., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 171–189. [Google Scholar]
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef]
- FDA. Fact Sheet: Veterinary Feed Directive Final Rule and Next Steps. Available online: https://www.fda.gov/animal-veterinary/development-approval-process/fact-sheet-veterinary-feed-directive-final-rule-and-next-steps (accessed on 14 September 2024).
- Zhou, H.; Lepp, D.; Pei, Y.; Liu, M.; Yin, X.; Ma, R.; Prescott, J.F.; Gong, J. Influence of pCP1NetB ancillary genes on the virulence of Clostridium perfringens poultry necrotic enteritis strain CP1. Gut Pathog. 2017, 9, 6. [Google Scholar] [CrossRef]
- Seib, K.L.; Zhao, X.; Rappuoli, R. Developing vaccines in the era of genomics: A decade of reverse vaccinology. Clin. Microbiol. Infect. 2012, 18, 109–116. [Google Scholar] [CrossRef]
- Depla, E.; Van der Aa, A.; Livingston, B.D.; Crimi, C.; Allosery, K.; De Brabandere, V.; Krakover, J.; Murthy, S.; Huang, M.; Power, S.; et al. Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections. J. Virol. 2008, 82, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Fikes, J.; Hoffman, S.; Franke, E.; Sacci, J.; Appella, E.; Chisari, F.V.; Guidotti, L.G.; Chesnut, R.W.; Livingston, B.; et al. The optimization of helper T lymphocyte (HTL) function in vaccine development. Immunol. Res. 1998, 18, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Beikzadeh, B. Immunoinformatics design of multi-epitope vaccine using OmpA, OmpD and enterotoxin against non-typhoidal salmonellosis. BMC Bioinform. 2023, 24, 63. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Panda, S.; Pashupathi, M.; Kumar, A.; Singh, R. Design of multiepitope vaccine construct against non-typhoidal Salmonellosis and its characterization using immunoinformatics approach. Int. J. Pept. Res. Ther. 2021, 27, 2333–2348. [Google Scholar] [CrossRef]
- Dey, J.; Mahapatra, S.R.; Lata, S.; Patro, S.; Misra, N.; Suar, M. Exploring Klebsiella pneumoniae capsule polysaccharide proteins to design multiepitope subunit vaccine to fight against pneumonia. Expert Rev. Vaccines 2022, 21, 569–587. [Google Scholar] [CrossRef]
- Baseer, S.; Ahmad, S.; Ranaghan, K.E.; Azam, S.S. Towards a peptide-based vaccine against Shigella sonnei: A subtractive reverse vaccinology based approach. Biologicals 2017, 50, 87–99. [Google Scholar] [CrossRef]
- Beikzadeh, B. Immunoinformatics design of novel multi-epitope vaccine against Trueperella pyogenes using collagen adhesion protein, fimbriae, and pyolysin. Arch. Microbiol. 2024, 206, 90. [Google Scholar] [CrossRef]
- Khatoon, N.; Pandey, R.K.; Prajapati, V.K. Exploring Leishmania secretory proteins to design B and T cell multi-epitope subunit vaccine using immunoinformatics approach. Sci. Rep. 2017, 7, 8285. [Google Scholar] [CrossRef]
- Feller, S.M.; Lewitzky, M. What’s in a loop? Cell Commun. Signal. 2012, 10, 31. [Google Scholar] [CrossRef]
- Ramakrishnan, C.; Ramachandran, G.N. Stereochemical criteria for polypeptide and protein chain conformations: II. Allowed conformations for a pair of peptide units. Biophys. J. 1965, 5, 909–933. [Google Scholar] [CrossRef]
- Bibi, S.; Ullah, I.; Zhu, B.; Adnan, M.; Liaqat, R.; Kong, W.-B.; Niu, S. In silico analysis of epitope-based vaccine candidate against tuberculosis using reverse vaccinology. Sci. Rep. 2021, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Lim, B.; Kim, D.-Y.; Kim, J.-M. Designing multi-epitope-based vaccine targeting surface immunogenic protein of Streptococcus agalactiae using immunoinformatics to control mastitis in dairy cattle. BMC Vet. Res. 2022, 18, 337. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol. 2008, 1, 460–469. [Google Scholar] [CrossRef] [PubMed]


| Number | Epitopes | ||
|---|---|---|---|
| Cytotoxic T Lymphocytes (CTL) | T Helper Lymphocytes (Th) | B Cells | |
| 1 | EWIAFNPLI | WIAFNPLIAPKLEFT | IGSVDDRYKKESIKPS |
| 2 | KMRRVDNTV | SNISVSENKITVNIS | SIPIKDVQFKMRRVDN |
| 3 | FKMRRVDNTVIKDGKK | ||
| Physiochemical Parameters | Values |
|---|---|
| Molecular weight | 12.98 kDa |
| Isoelectric point | 10.26 |
| Extinction coefficient | 13,980 |
| Half-life in mammalian reticulocytes, in vitro | 1 h |
| Yeast, in vivo | 30 min |
| Escherichia coli, in vivo | >10 h |
| Instability index | 30.28 |
| Aliphatic index | 77.91 |
| Grand average of hydropathicity (GRAVY) | −0.617 |
| Solubility | 0.848 |
| Parameters | Galaxyrefine Alphafold2 Model (Relaxed Rank2) | Original Alphafold2 Model (Relaxed Rank2) |
|---|---|---|
| Residues in core regions | 85.40% | 75.00% |
| Residues in allowed regions | 13.50% | 22.90% |
| Residues in generously allowed regions | 1.00% | 1.00% |
| Residues in disallowed regions | 0.00% | 1.00% |
| G-factors (dihedrals, covalent, overall) | −0.09, −0.13, −0.10 | −0.58, 0.18, −0.27 |
| Warnings | 5 | 5 |
| Errors | 0 | 2 |
| Parameters | CNA | Construct 1 | Construct 4 | Construct 6 | Construct 8 |
|---|---|---|---|---|---|
| IgG + IgM | ~75,000 | ~70,000 | ~80,000 | ~76,000 | ~90,000 |
| B-cell isotype IgM | ~325 | ~375 | ~375 | ~360 | ~350 |
| B-cell isotype IgG1 | ~50 | ~50 | ~60 | ~60 | ~100 |
| B-cell isotype IgG2 | ~0 | ~0 | ~0 | ~0 | ~0 |
| B-cell (not Memory) | ~100 | ~100 | ~100 | ~100 | ~100 |
| PLB (Plasma) cells | ~85 | ~80 | ~90 | ~80 | ~100 |
| Active B cells | ~300 | ~300 | ~310 | ~300 | ~275 |
| Th memory cells | ~1200 | ~1300 | ~1400 | ~1400 | ~1800 |
| Active CTL cells population | ~0.5 (almost 50 percent of total CTL cells are active) | ~0.4 | ~0.4 | ~0.5 | ~0.5 (but slightly greater than the rest) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chundru, D.; Bhattrai, S.; Timilsina, M.; Lillehoj, H.; Sun, Z.; Ghanem, M.; Li, C. In Silico Design, Optimization, and Evaluation of a Multi-Epitope Vaccine Targeting the Clostridium perfringens Collagen Adhesin Protein. Microorganisms 2025, 13, 1147. https://doi.org/10.3390/microorganisms13051147
Chundru D, Bhattrai S, Timilsina M, Lillehoj H, Sun Z, Ghanem M, Li C. In Silico Design, Optimization, and Evaluation of a Multi-Epitope Vaccine Targeting the Clostridium perfringens Collagen Adhesin Protein. Microorganisms. 2025; 13(5):1147. https://doi.org/10.3390/microorganisms13051147
Chicago/Turabian StyleChundru, Dhiraj, Shailes Bhattrai, Madhusudan Timilsina, Hyun Lillehoj, Zhifeng Sun, Mostafa Ghanem, and Charles Li. 2025. "In Silico Design, Optimization, and Evaluation of a Multi-Epitope Vaccine Targeting the Clostridium perfringens Collagen Adhesin Protein" Microorganisms 13, no. 5: 1147. https://doi.org/10.3390/microorganisms13051147
APA StyleChundru, D., Bhattrai, S., Timilsina, M., Lillehoj, H., Sun, Z., Ghanem, M., & Li, C. (2025). In Silico Design, Optimization, and Evaluation of a Multi-Epitope Vaccine Targeting the Clostridium perfringens Collagen Adhesin Protein. Microorganisms, 13(5), 1147. https://doi.org/10.3390/microorganisms13051147






