The Impact of Litter from Different Belowground Organs of Phragmites australis on Microbial-Mediated Soil Organic Carbon Accumulation in a Lacustrine Wetland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Preparation
2.3. Measurement Methods
2.3.1. Determination of Plant Basic Parameters
- (1)
- Mass Loss (R):
- (2)
- Decomposition Rate (SL):
- (3)
- Non-Additive Effects (YME):
2.3.2. Determination of Soil Basic Parameters
2.3.3. Determination of Soil Microbial Biomass
2.3.4. Bacterial and Fungal Community Structure
2.4. Statistical Analysis
3. Results
3.1. Litter Decomposition Dynamics
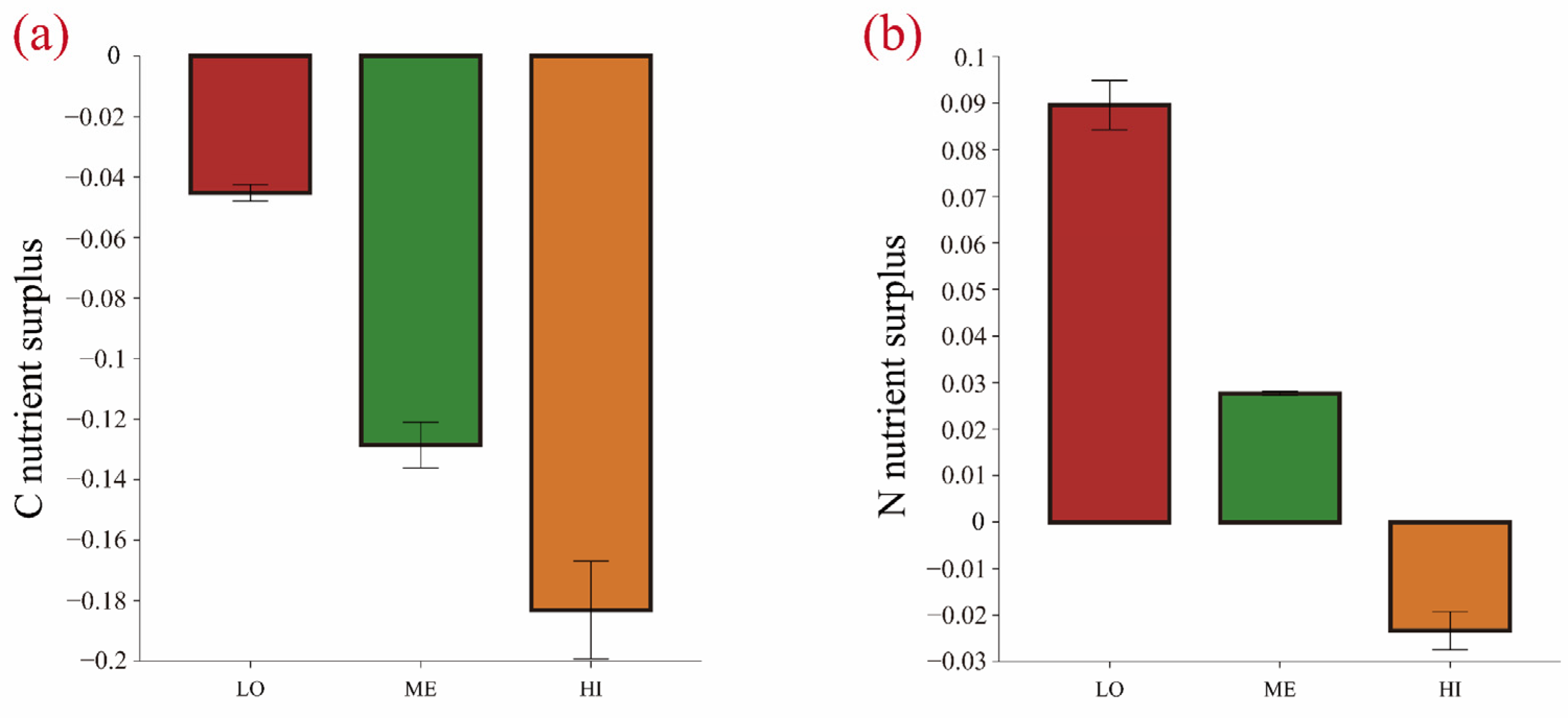
3.2. Effects of Litter Input on Soil Organic Carbon and Physicochemical Properties
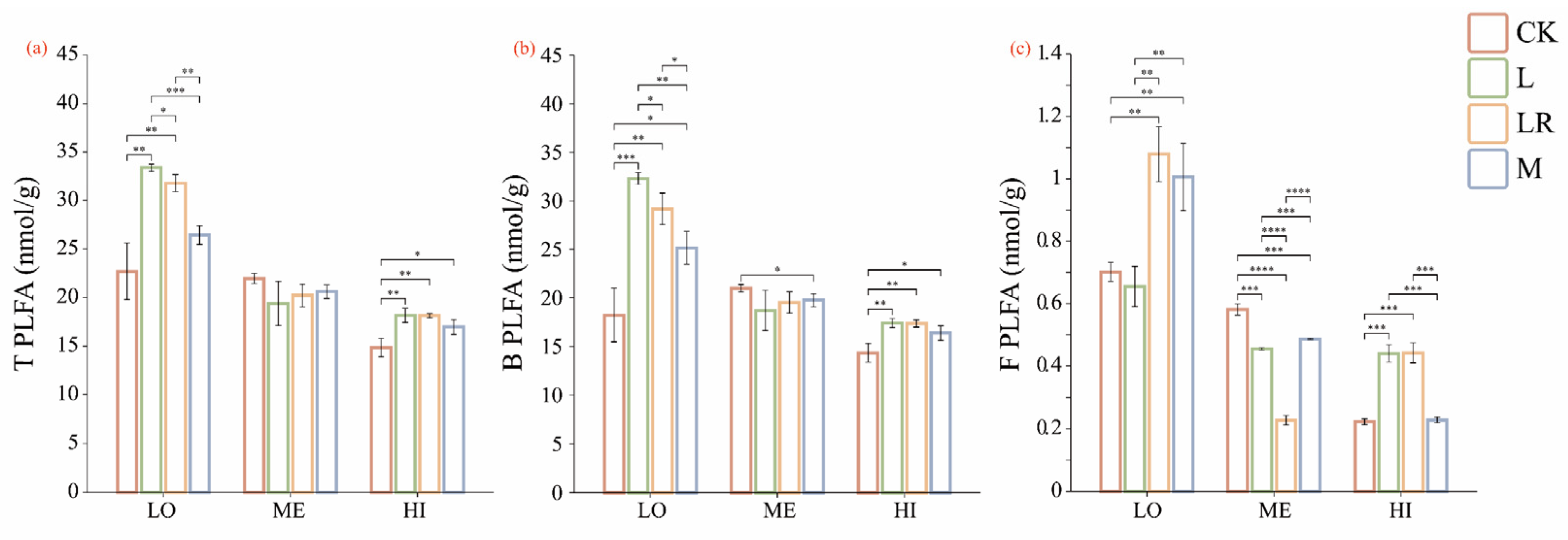
3.3. Effects of Litter Input on Soil Microbial Biomass
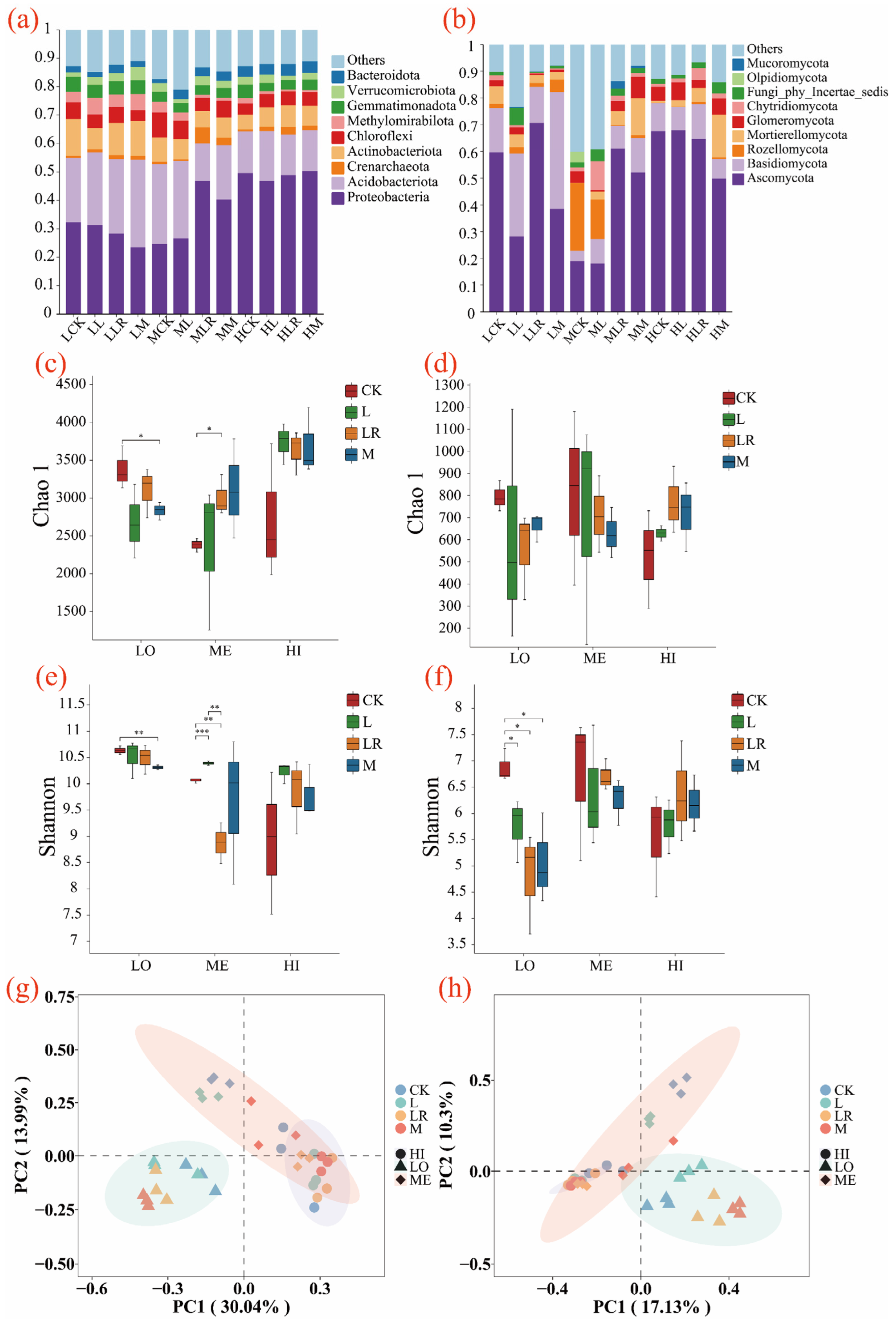
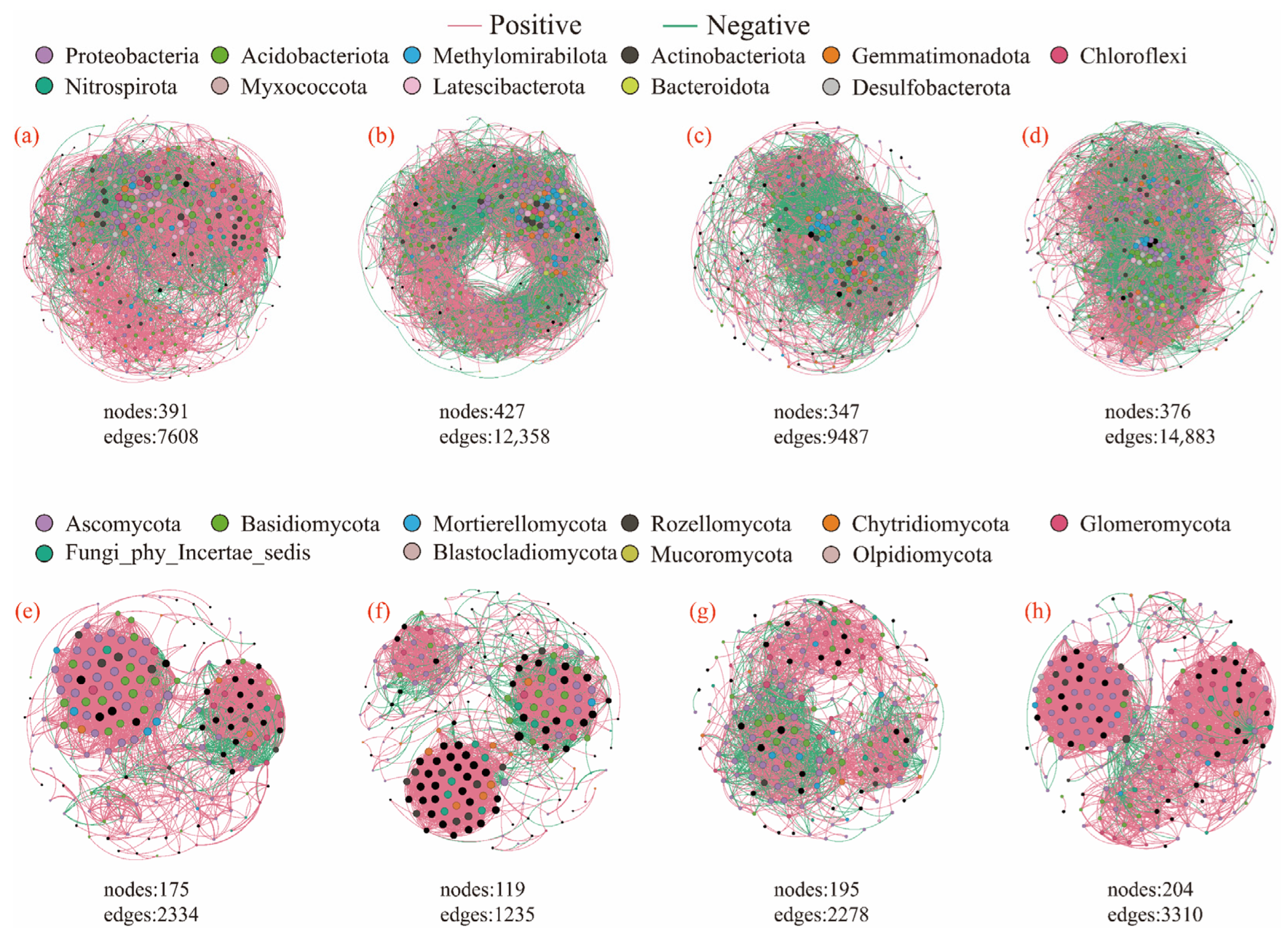
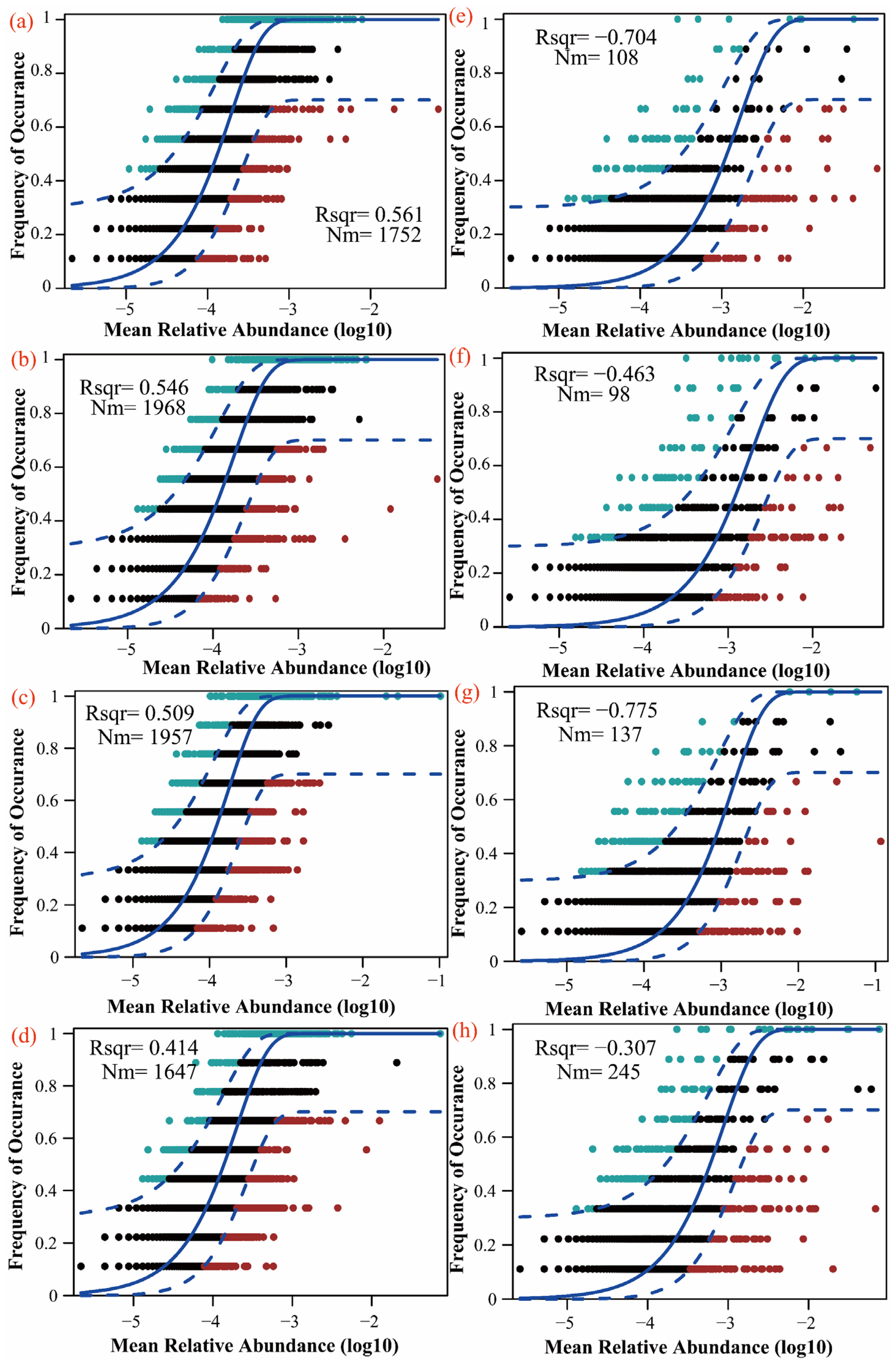
3.4. Effects of Litter Input on Soil Microbial Community Characteristics
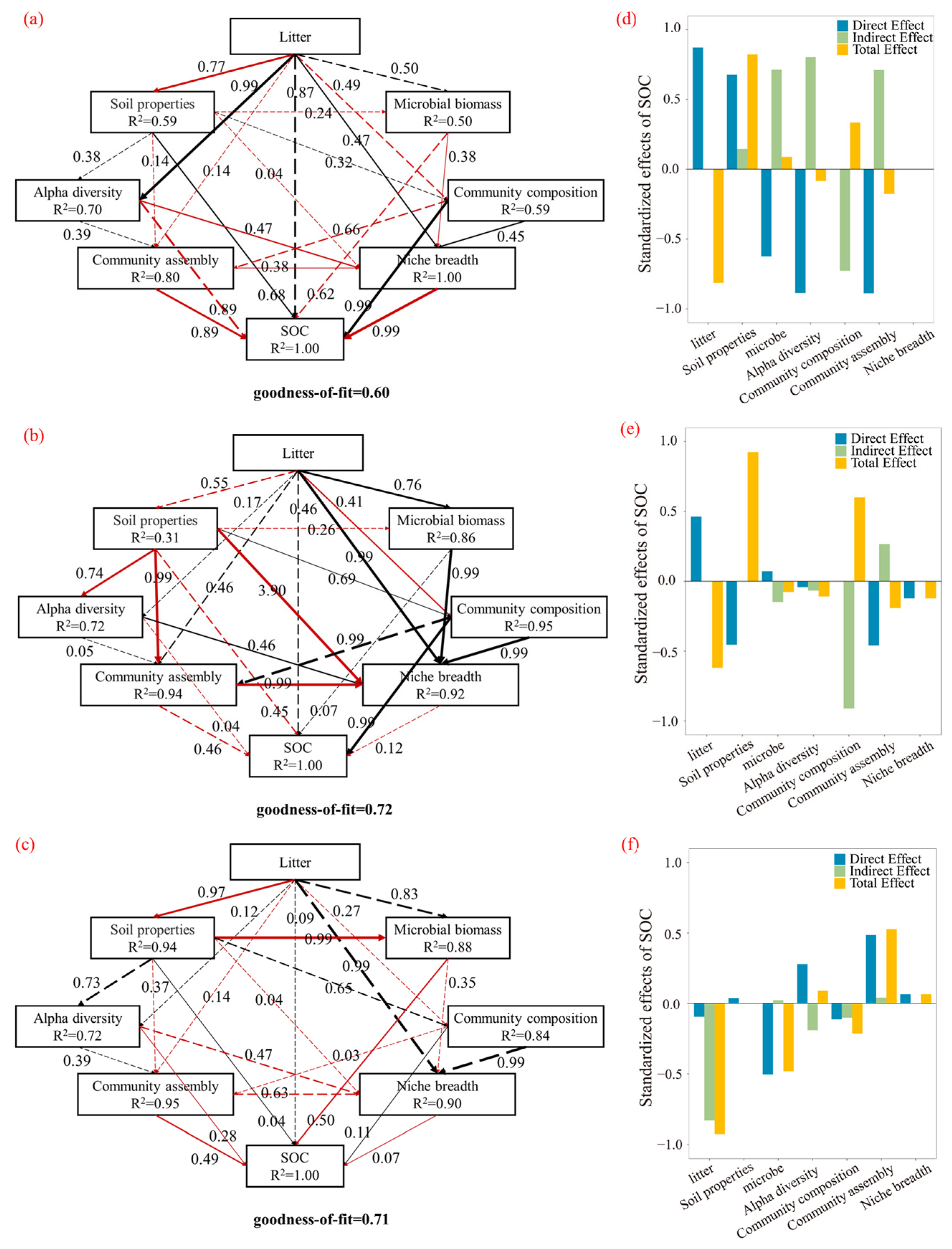
3.5. Pathways in Which Different Subsurface Organ Litter Inputs Affect SOC
4. Discussion
4.1. Litter Decomposition Characteristics
4.2. Litter Addition Alters Soil Organic Carbon Content and Physicochemical Properties
4.3. Litter Addition Alters Soil Microbial Biomass
4.4. Litter Addition Modifies Microbial Survival Strategies and Community Assembly
4.5. Pathways of Different Litter Inputs Affecting SOC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.J.; Niu, Z.G.; Chen, Y.F.; Li, L.F.; Zhang, H.Y. Global wetlands: Potential distribution, wetland loss, and status. Sci. Total Environ. 2017, 586, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, U.; Zhang, L.; Anderson, C.J.; Jorgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Roulet, N.T. Peatlands, carbon storage, greenhouse gases, and the Kyoto Protocol: Prospects and significance for Canada. Wetlands 2000, 20, 605–615. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Megonigal, J.P.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916. [Google Scholar] [CrossRef]
- Nie, X.Q.; Wang, D.; Yang, L.C.; Zhou, G.Y. Storage and Climatic Controlling Factors of Litter Standing Crop Carbon in the Shrublands of the Tibetan Plateau. Forests 2019, 10, 987. [Google Scholar] [CrossRef]
- Li, S.T.; Jiang, Y.M.; Lyu, M.; Deng, C.; Deng, W.; Wang, M.; Liu, J.L.; Lu, Y.M.; Xie, J.S. High-quality litter exerts a greater effect on soil carbon gain in unrestored than restored pine plantations. Sci. Total Environ. 2024, 952, 175975. [Google Scholar] [CrossRef]
- Córdova, S.C.; Olk, D.C.; Dietzel, R.N.; Mueller, K.E.; Archontouilis, S.V.; Castellano, M.J. Plant litter quality affects the accumulation rate, composition, and stability of mineral-associated soil organic matter. Soil Biol. Biochem. 2018, 125, 115–124. [Google Scholar] [CrossRef]
- Lyu, M.; Homyak, P.M.; Xie, J.S.; Penuelas, J.; Ryan, M.G.; Xiong, X.L.; Sardans, J.; Lin, W.S.; Wang, M.H.; Chen, G.S.; et al. Litter quality controls tradeoffs in soil carbon decomposition and replenishment in a subtropical forest. J. Ecol. 2023, 111, 2181–2193. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Mason, K.E.; Goodall, T.; Taylor, A.; Zhao, P.; Otero-Farina, A.; Chen, H.; Peacock, C.L.; Ostle, N.J.; Griffiths, R.; et al. Microbial and mineral interactions decouple litter quality from soil organic matter formation. Nat. Commun. 2024, 15, 10063. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.D.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.P.; Cornelissen, J.H.C. Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Hu, Y.L.; Zeng, D.H.; Ma, X.Q.; Chang, S.X. Root rather than leaf litter input drives soil carbon sequestration after afforestation on a marginal cropland. For. Ecol. Manag. 2016, 362, 38–45. [Google Scholar] [CrossRef]
- Almeida, L.F.J.; Souza, I.F.; Hurtarte, L.C.C.; Teixeira, P.P.C.; Inagaki, T.M.; Silva, I.R.; Mueller, C.W. Forest litter constraints on the pathways controlling soil organic matter formation. Soil Biol. Biochem. 2021, 163, 108447. [Google Scholar] [CrossRef]
- Xie, Y.J.; Xie, Y.H.; Xiao, H.Y.; Deng, Z.M.; Pan, Y.; Pan, B.H.; Hu, J.Y. Inhibition of litter decomposition of two emergent macrophytes by addition of aromatic plant powder. Sci. Rep. 2017, 7, 16685. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Perez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Guo, L.L.; Deng, M.F.; Yang, S.; Liu, W.X.; Wang, X.; Wang, J.; Liu, L.L. The coordination between leaf and fine root litter decomposition and the difference in their controlling factors. Glob. Ecol. Biogeogr. 2021, 30, 2286–2296. [Google Scholar] [CrossRef]
- Trogisch, S.; He, J.S.; Hector, A.; Scherer-Lorenzen, M. Impact of species diversity, stand age and environmental factors on leaf litter decomposition in subtropical forests in China. Plant Soil 2016, 400, 337–350. [Google Scholar] [CrossRef]
- Osono, T. Functional diversity of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2020, 35, 30–43. [Google Scholar] [CrossRef]
- Dove, N.C.; Stark, J.M.; Newman, G.S.; Hart, S.C. Carbon control on terrestrial ecosystem function across contrasting site productivities: The carbon connection revisited. Ecology 2019, 100, e02695. [Google Scholar] [CrossRef]
- Esperschutz, J.; Welzl, G.; Schreiner, K.; Buegger, F.; Munch, J.C.; Schloter, M. Incorporation of carbon from decomposing litter of two pioneer plant species into microbial communities of the detritusphere. Fems Microbiol. Lett. 2011, 320, 48–55. [Google Scholar] [CrossRef]
- Bray, S.R.; Kitajima, K.; Mack, M.C. Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol. Biochem. 2012, 49, 30–37. [Google Scholar] [CrossRef]
- Che, R.X.; Liu, D.; Qin, J.L.; Wang, F.; Wang, W.J.; Xu, Z.H.; Li, L.F.; Hu, J.M.; Tahmasbian, I.; Cui, X.Y. Increased litter input significantly changed the total and active microbial communities in degraded grassland soils. J. Soils Sediments 2020, 20, 2804–2816. [Google Scholar] [CrossRef]
- Jacob, M.; Leuschner, C.; Thomas, F.M. Productivity of temperate broad-leaved forest stands differing in tree species diversity. Ann. For. Sci. 2010, 67, 503. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Liu, Y.; An, S.S. Impact of litter quantity on the soil bacteria community during the decomposition of Quercus wutaishanica litter. PeerJ 2017, 5, e3777. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.L.; Yuan, M.T.; Wu, L.W.; Zhang, Y.; Guo, X.; Zhou, X.S.; Yang, Y.F.; Arkin, A.P.; Firestone, M.K.; Zhou, J.Z. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Yang, Y.F.; Xu, Y.Q.; Zhang, J.; Lu, Y.H. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.B.; Li, S.P.; Wang, X.; Zhang, Z.C.; Shen, M.W.; Yang, Q.S.; Lian, J.Y.; Wang, X.H.; et al. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Vályi, K.; Mardhiah, U.; Rillig, M.C.; Hempel, S. Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 2016, 10, 2341–2351. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Li, S.F.; Huang, X.B.; Shen, J.Y.; Xu, F.D.; Su, J.R. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- He, K.R.; Lai, Y.R.; Hu, S.R.; Song, M.Y.; Su, Y.; Li, C.Y.; Wu, X.L.; Zhang, C.Y.; Hua, Y.H.; Huang, J.Y.; et al. Assembly Characteristics and Influencing Factors of the Soil Microbial Community in the Typical Forest of Funiu Mountain. Microorganisms 2024, 12, 2355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Deng, Z.M.; Xie, Y.H.; Li, X.; Li, F.; Chen, X.S.; Zou, Y.A.; Zhang, C.Y.; Wang, W. Factors controlling Carex brevicuspis leaf litter decomposition and its contribution to surface soil organic carbon pool at different water levels. Biogeosciences 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, M.; Xu, J. Carbon reliance on dissolved inorganic carbon of junior and matured reed beds indicated by stable isotope analysis. J. Anim. Plant Sci. 2019, 29, 971–977. [Google Scholar]
- Araujo, P.I.; Yahdjian, L.; Austin, A.T. Do soil organisms affect aboveground litter decomposition in the semiarid Patagonian steppe, Argentina? Oecologia 2012, 168, 221–230. [Google Scholar] [CrossRef]
- Li, H.J.; Wang, B.R.; Zhou, Y.; Zhang, H.L.; Liu, C.H.; Yang, X.; Zhu, Z.L.; Bai, X.J.; Toor, G.S.; An, S.S. Soil organic carbon formation from plant and microbial residual carbon: Effects of home-field advantage and root litter quality. Catena 2025, 254, 108985. [Google Scholar] [CrossRef]
- Min, Y.E.; Gao, Q.; Wang, Y.; Yu, X.F.; Tong, L.; Su, P.C.; Bian, H.F. Flooding lowers the emissions of CO2 and CH4 during the freeze-thaw process in a lacustrine wetland. Catena 2023, 227, 107132. [Google Scholar] [CrossRef]
- Sakaria, D.; Maat, S.M.; Matore, M. Examining the Optimal Choice of SEM Statistical Software Packages for Sustainable Mathematics Education: A Systematic Review. Sustainability 2023, 15, 3209. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Heredia-Acuña, C.; Semchenko, M.; De Vries, F.T. Root litter decomposition is suppressed in species mixtures and in the presence of living roots. J. Ecol. 2023, 111, 2519–2531. [Google Scholar] [CrossRef]
- Wambsganss, J.; Freschet, G.T.; Beyer, F.; Bauhus, J.; Scherer-Lorenzen, M. Tree Diversity, Initial Litter Quality, and Site Conditions Drive Early-Stage Fine-Root Decomposition in European Forests. Ecosystems 2022, 25, 1493–1509. [Google Scholar] [CrossRef]
- Wang, X.P.; Ma, Y.S.; Zhang, S.T. Litter quality-dependent dynamics of bacteria communities in litter and soil layers during litter decomposition. Plant Soil 2025, 508, 663–675. [Google Scholar] [CrossRef]
- Cai, A.D.; Liang, G.P.; Yang, W.; Zhu, J.; Han, T.F.; Zhang, W.J.; Xu, M.G. Patterns and driving factors of litter decomposition across Chinese terrestrial ecosystems. J. Clean. Prod. 2021, 278, 123964. [Google Scholar] [CrossRef]
- Hong, J.T.; Lu, X.Y.; Ma, X.X.; Wang, X.D. Five-year study on the effects of warming and plant litter quality on litter decomposition rate in a Tibetan alpine grassland. Sci. Total Environ. 2021, 750, 142306. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, A.L.; Li, X.Y.; Kuang, W.N.; Islam, W. Litter decomposition rate response to multiple global change factors: A meta-analysis. Soil Biol. Biochem. 2024, 195, 109474. [Google Scholar] [CrossRef]
- Chen, W.J.; Zhao, X.Z.; Su, J.S.; Lu, X.M.; Bai, Y.F. Plant litter regulates the effect of nitrogen and water enrichment on ecosystem carbon sequestration in a semi-arid grassland. Agric. Ecosyst. Environ. 2024, 374, 109154. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.Y.; Jian, J.N.; Xing, L.H.; Li, Y.; Wang, X.; Zhang, Q.; Ren, C.J.; Yang, G.H.; Han, X.H. Differences in the regulation of soil carbon pool quality and stability by leaf-litter and root-litter decomposition. Environ. Res. 2024, 263, 120285. [Google Scholar] [CrossRef]
- Bouma, K.; Douma, M.I.; Veen, G.F.; Cornelissen, P.; van Eerden, M.R.; Bakker, E.S. Water level drawdown and perennial vegetation impact litter decomposition in the sediment of a eutrophic wetland in the Netherlands. Wetl. Ecol. Manag. 2025, 33, 5. [Google Scholar] [CrossRef]
- Morelle, J.; Parlanti, E.; Lecarpentier, T.; Laverman, A.M. Impact of water level management on organic carbon availability and nitrogen transformations in wetland sediments. Sci. Total Environ. 2024, 947, 174643. [Google Scholar] [CrossRef]
- Naramabuye, F.X.; Haynes, R.J. Effect of organic amendments on soil pH and AI solubility and use of laboratory indices to predict their liming effect. Soil Sci. 2006, 171, 754–763. [Google Scholar] [CrossRef]
- Bai, X.J.; Zhai, G.Q.; Yan, Z.F.; An, S.S.; Liu, J.Z.; Huo, L.Q.; Dippold, M.A.; Kuzyakov, Y. Effects of microbial groups on soil organic carbon accrual and mineralization during high- and low-quality litter decomposition. Catena 2024, 241, 108051. [Google Scholar] [CrossRef]
- Bai, X.J.; Zhai, G.Q.; Wang, B.R.; An, S.S.; Liu, J.Z.; Xue, Z.J.; Dippold, M.A. Litter quality controls the contribution of microbial carbon to main microbial groups and soil organic carbon during its decomposition. Biol. Fertil. Soils 2024, 60, 167–181. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Griffiths, H.; Chamberlain, P.M.; Stott, A.W.; Tanner, E.V.J. Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl. Soil Ecol. 2009, 42, 183–190. [Google Scholar] [CrossRef]
- Wan, S.Z.; Fu, S.L.; Zhang, C.L.; Liu, J.; Zhang, Y.; Mao, R. Effects of understory removal and litter addition on leaf and twig decomposition in a subtropical Chinese fir plantation. Land Degrad. Dev. 2021, 32, 5004–5011. [Google Scholar] [CrossRef]
- Martinovic, T.; Masinova, T.; Lopez-Mondejar, R.; Jansa, J.; Stursova, M.; Starke, R.; Baldrian, P. Microbial utilization of simple and complex carbon compounds in a temperate forest soil. Soil Biol. Biochem. 2022, 173, 108786. [Google Scholar] [CrossRef]
- Dong, X.D.; Gao, P.; Zhou, R.; Li, C.; Dun, X.J.; Niu, X. Changing characteristics and influencing factors of the soil microbial community during litter decomposition in a mixed Quercus acutissima Carruth. and Robinia pseudoacacia L. forest in Northern China. Catena 2021, 196, 104811. [Google Scholar] [CrossRef]
- Jing, H.; Wang, G.L.; Liu, G.B. Response of fungal and bacterial communities in fine root litter with different diameters to nitrogen application. J. Soils Sediments 2023, 23, 1250–1264. [Google Scholar] [CrossRef]
- Ferreira, V.; Pazianoto, L.H.R.; Solla, A. Invasive forest pathogens affect the characteristics, microbial colonisation, and decomposition of leaf litter in streams. Freshw. Biol. 2022, 67, 416–429. [Google Scholar] [CrossRef]
- Wang, F.; Lin, D.M.; Li, W.; Dou, P.P.; Han, L.; Huang, M.F.; Qian, S.H.; Yao, J.M. Meiofauna promotes litter decomposition in stream ecosystems depending on leaf species. Ecol. Evol. 2020, 10, 9257–9270. [Google Scholar] [CrossRef]
- Lu, Y.P.; Gao, Z.; Zhu, Y.L.; Yao, D.L.; Wang, X.L. Microbial Community Structure, Diversity, and Succession During Decomposition of Kiwifruit Litters with Different Qualities. Microorganisms 2024, 12, 2498. [Google Scholar] [CrossRef]
- Prevost-Boure, N.C.; Maron, P.A.; Ranjard, L.; Nowak, V.; Dufrene, E.; Damesin, C.; Soudani, K.; Lata, J.C. Seasonal dynamics of the bacterial community in forest soils under different quantities of leaf litter. Appl. Soil Ecol. 2011, 47, 14–23. [Google Scholar] [CrossRef]
- Tanunchai, B.; Ji, L.; Schroeter, S.A.; Wahdan, S.F.M.; Thongsuk, K.; Hilke, I.; Gleixner, G.; Buscot, F.; Schulze, E.D.; Noll, M.; et al. Tree mycorrhizal type regulates leaf and needle microbial communities, affects microbial assembly and co-occurrence network patterns, and influences litter decomposition rates in temperate forest. Front. Plant Sci. 2023, 14, 1239600. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; He, S.Y.; Li, J.Q.; Hu, W.J.; Ma, Y.T.; Wu, L.; Gang, G. Co-occurrence patterns between bacterial and fungal communities in response to a vegetation gradient in a freshwater wetland. Can. J. Microbiol. 2019, 65, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, R.M.; Li, B.L.; Tian, K. Emerging Microplastics Alter the Influences of Soil Animals on the Fungal Community Structure in Determining the Litter Decomposition of a Deciduous Tree. Forests 2024, 15, 488. [Google Scholar] [CrossRef]
- Dehlin, H.; Nilsson, M.C.; Wardle, D.A. Aboveground and belowground responses to quality and heterogeneity of organic inputs to the boreal forest. Oecologia 2006, 150, 108–118. [Google Scholar] [CrossRef]
- Gundersen, M.J.S.; Vadstein, O. An exploration of the impact of phylogenetic tree structure on NTI and βNTI estimates of community assembly. Sci. Rep. 2024, 14, 23480. [Google Scholar] [CrossRef]
- Muller, E.E.L. Determining Microbial Niche Breadth in the Environment for Better Ecosystem Fate Predictions. mSystems 2019, 4, e00080-19. [Google Scholar] [CrossRef]
- Ma, J.M.; Ma, K.; Liu, J.L.; Chen, N.N. Rhizosphere Soil Microbial Community Under Ice in a High-Latitude Wetland: Different Community Assembly Processes Shape Patterns of Rare and Abundant Microbes. Front. Microbiol. 2022, 13, 783371. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Reed, S.C.; Keller, A.B.; Nemergut, D.R.; O’Neill, S.P.; Ostertag, R.; Vitousek, P.M. Litter quality versus soil microbial community controls over decomposition: A quantitative analysis. Oecologia 2014, 174, 283–294. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Fernández-Alonso, M.J.; Díaz-Pinés, E.; Kitzler, B.; Rubio, A. Tree species composition shapes the assembly of microbial decomposer communities during litter decomposition. Plant Soil 2022, 480, 457–472. [Google Scholar] [CrossRef]

| R | SL | N (g/kg) | C (g/kg) | C/N | ||
|---|---|---|---|---|---|---|
| LO | L | 0.3883 ± 0.0087 Cc | 0.0035 ± 0.0014 Cb | 6.7067 ± 0.1012 Ac | 498.5767 ± 1.5866 Aa | 74.3521 ± 1.1767 Aa |
| LR | 0.5049 ± 0.0032 Ca | 0.0065 ± 0.0003 Ca | 20.3000 ± 0.0985 Aa | 418.5333 ± 3.9845 Ac | 20.6171 ± 0.0974 Ac | |
| M | 0.4344 ± 0.0025 Cb | 0.0044 ± 0.0005 Cab | 13.5450 ± 0.1830 Ab | 453.5733 ± 1.6321 Ab | 33.4895 ± 0.3479 Ab | |
| ME | L | 0.5361 ± 0.0010 Ac | 0.0066 ± 0.0003 Ac | 7.1167 ± 0.1206 Ac | 471.8433 ± 24.3541 Aa | 66.2774 ± 2.3768 Aa |
| LR | 0.6488 ± 0.0008 Aa | 0.0103 ± 0.0004 Aa | 19.3433 ± 0.3089 Aa | 450.0800 ± 3.1980 Aa | 23.2703 ± 0.2416 Ac | |
| M | 0.5850 ± 0.0033 Ab | 0.0088 ± 0.0004 Ab | 12.5367 ± 0.3048 Ab | 405.2383 ± 2.1059 Ab | 32.3377 ± 0.8448 Ab | |
| HI | L | 0.4776 ± 0.0017 Bc | 0.0060 ± 0.0004 Bb | 8.5300 ± 0.3205 Ac | 441.1567 ± 4.9133 Ba | 51.7565 ± 1.5880 Aa |
| LR | 0.5450 ± 0.0033 Ba | 0.0077 ± 0.0005 Ba | 10.6800 ± 0.2972 Aa | 167.6767 ± 0.5554 Bc | 15.7091 ± 0.4857 Ac | |
| M | 0.5156 ± 0.0035 Bb | 0.0067 ± 0.0007 Bab | 9.3067 ± 0.0978 Ab | 294.605 ± 5.6638 Bb | 31.6617 ± 0.9371 Ab |
| pH | SOC (g/kg) | DOC (mg/kg) | TN (g/kg) | AP (mg/kg) | ||
|---|---|---|---|---|---|---|
| LO | CK | 7.05 ± 0.05 Ba | 15.62 ± 0.41 Ad | 285.19 ± 11.98 Ad | 0.95 ± 0.03 Ac | 13.23 ± 0.61 Ac |
| L | 6.56 ± 0.02 Bb | 18.39 ± 0.94 Ac | 315.55 ± 9.72 Ac | 1.62 ± 0.08 Aa | 15.62 ± 0.23 Ab | |
| LR | 6.52 ± 0.06 Bb | 23.20 ± 1.03 Ab | 351.16 ± 19.71 Ab | 1.72 ± 0.18 Aa | 16.37 ± 0.34 Aa | |
| M | 7.02 ± 0.06 Ba | 25.99 ± 2.78 Aa | 428.47 ± 14.12 Aa | 1.21 ± 0.05 Ab | 11.78 ± 0.44 Ad | |
| ME | CK | 6.88 ± 0.05 Ab | 10.91 ± 0.29 Bd | 105.23 ± 3.84 Bd | 0.47 ± 0.04 Bc | 16.54 ± 0.18 Aa |
| L | 7.03 ± 0.10 Aa | 11.82 ± 0.34 Bc | 118.21 ± 4.83 Bc | 0.59 ± 0.04 Ba | 9.01 ± 0.66 Ad | |
| LR | 6.92 ± 0.06 Aab | 13.09 ± 0.20 Bb | 135.58 ± 3.72 Bb | 0.60 ± 0.02 Ba | 12.85 ± 0.65 Ac | |
| M | 6.83 ± 0.09 Ab | 13.78 ± 0.22 Ba | 176.27 ± 2.00 Ba | 0.55 ± 0.01 Bb | 14.64 ± 0.35 Ab | |
| HI | CK | 6.99 ± 0.05 Aa | 5.18 ± 0.15 Cd | 105.10 ± 2.12 Bd | 0.30 ± 0.02 Cc | 16.60 ± 0.33 Aa |
| L | 6.99 ± 0.04 Aa | 7.11 ± 0.20 Cc | 123.28 ± 3.11 Bc | 0.40 ± 0.01 Cb | 10.46 ± 0.25 Ac | |
| LR | 6.88 ± 0.05 Ab | 7.86 ± 0.20 Cb | 165.94 ± 3.20 Ba | 0.45 ± 0.02 Ca | 8.51 ± 0.33 Ad | |
| M | 6.84 ± 0.02 Ab | 8.50 ± 0.24 Ca | 143.08 ± 1.72 Bb | 0.47 ± 0.02 Ca | 13.51 ± 0.19 Ab |
| Litter Treatment | Node | Edge | Positive Link (%) | Negative Link (%) | Modularity | Average Clustering Coefficient | Average Degree | Average Path Distance | |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | CK | 391 | 7608 | 72.96 | 27.04 | 0.435 | 0.545 | 38.916 | 2.738 |
| L | 427 | 12,358 | 61.74 | 38.26 | 0.462 | 0.584 | 57.883 | 2.464 | |
| LR | 347 | 9487 | 54.74 | 45.26 | 0.277 | 0.582 | 54.68 | 2.697 | |
| M | 376 | 14,883 | 51.19 | 48.81 | 0.296 | 0.648 | 79.165 | 2.266 | |
| Fungi | CK | 184 | 2636 | 86.49 | 13.51 | 0.448 | 0.708 | 28.652 | 2.841 |
| L | 211 | 2584 | 81.85 | 18.15 | 0.607 | 0.704 | 24.493 | 3.519 | |
| LR | 195 | 2278 | 68.53 | 31.47 | 0.491 | 0.594 | 23.364 | 2.928 | |
| M | 204 | 3310 | 92.48 | 7.52 | 0.542 | 0.735 | 32.451 | 2.787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, Y.; Yang, L.; Min, Y.; Yue, K.; Lu, S.; Bian, H.; Wang, X.; Zhang, L. The Impact of Litter from Different Belowground Organs of Phragmites australis on Microbial-Mediated Soil Organic Carbon Accumulation in a Lacustrine Wetland. Microorganisms 2025, 13, 1146. https://doi.org/10.3390/microorganisms13051146
Chen C, Wang Y, Yang L, Min Y, Yue K, Lu S, Bian H, Wang X, Zhang L. The Impact of Litter from Different Belowground Organs of Phragmites australis on Microbial-Mediated Soil Organic Carbon Accumulation in a Lacustrine Wetland. Microorganisms. 2025; 13(5):1146. https://doi.org/10.3390/microorganisms13051146
Chicago/Turabian StyleChen, Chong, Yong Wang, Liu Yang, Yongen Min, Keming Yue, Sitong Lu, Hongfeng Bian, Xue Wang, and Leilei Zhang. 2025. "The Impact of Litter from Different Belowground Organs of Phragmites australis on Microbial-Mediated Soil Organic Carbon Accumulation in a Lacustrine Wetland" Microorganisms 13, no. 5: 1146. https://doi.org/10.3390/microorganisms13051146
APA StyleChen, C., Wang, Y., Yang, L., Min, Y., Yue, K., Lu, S., Bian, H., Wang, X., & Zhang, L. (2025). The Impact of Litter from Different Belowground Organs of Phragmites australis on Microbial-Mediated Soil Organic Carbon Accumulation in a Lacustrine Wetland. Microorganisms, 13(5), 1146. https://doi.org/10.3390/microorganisms13051146






