Microbiological Quality and Presence of Salmonella spp. in Broiler Carcasses with and Without Visible Gastrointestinal Contamination During Industrial Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analyses
2.3. Identification of Salmonella Serotypes
2.4. Proteomic Identification of Microorganisms
2.5. Experimental Design
3. Results and Discussion
3.1. Counts of Microorganisms Indicating Sanitary and Hygienic Quality
3.2. Salmonella spp. Detection
3.3. Identification of Salmonella Serotypes
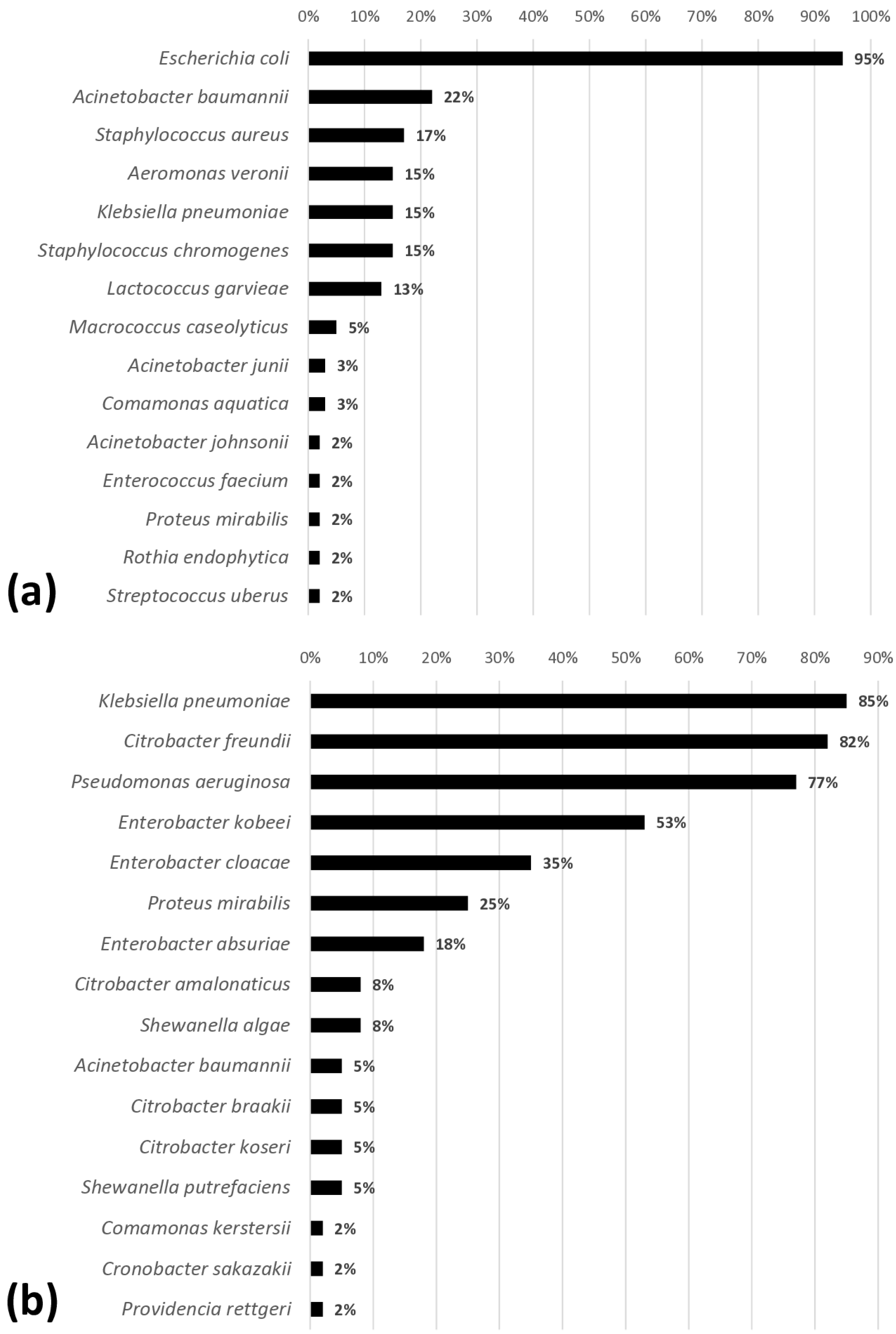
3.4. Identification of Contaminating Microorganisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Ai, H.; Li, S. Analysis of correlation between carcass and viscera for chicken eviscerating based on machine vision technology. J. Food Process Eng. 2020, 44, e13592. [Google Scholar] [CrossRef]
- Libera, K.; Lipman, L.; Berends, B.R. Small contaminations on broiler carcasses are more a quality matter than a food safety issue. Foods 2023, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50–66. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Line, J.E.; Oakley, B.B.; Stern, N.J. Comparison of cumulative drip sampling with whole carcass rinses for estimation of Campylobacter species and quality indicator organisms associated with processed broiler chickens. Poult. Sci. 2013, 92, 218–224. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Quantitative Analysis of Bacteria in Foods as Sanitary Indicators. MLG 3.02. 2015. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-03/MLG-3.pdf (accessed on 1 January 2025).
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- Association of Official Agricultural Chemists (AOAC). AOAC 998.08-2002: Confirmed Escherichia coli Counts in Poultry, Meats, and Sea Food; AOAC Official Methods 998.08; AOAC International: Rockville, MD, USA, 2002. [Google Scholar]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. International Organization for Standardization: Geneva, Switzerland, 2017.
- Grimont, P.A.; Weil, F.X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; Institut Pasteur: Paris, France, 2007. [Google Scholar]
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; Guibourdenche, M.; Pinna, E.; Nair, S.; Fields, P.I.; Weill, F.X. Supplement 2008–2010 (no. 48) to the White-Kauffmann-Le Minor scheme. Microbiol. Res. 2014, 165, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanayjia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Cibin, V.; Mancin, M.; Pedersen, K.; Barruci, F.; Belluco, S.; Rocatto, A.; Cocola, F.; Ferrarini, S.; Sandri, A.; Baggesen, D.L.; et al. Usefulness of Escherichia coli and Enterobacteriaceae as process hygiene criteria in poultry: Experimental study. EFSA Support. Publ. 2014, 11, 635E. [Google Scholar] [CrossRef]
- Jimenez, S.M.; Salsi, M.S.; Tiburzi, M.C.; Pirovani, M.E. A Comparison between broiler chicken carcasses with and without visible faecal contamination during the slaughtering process on hazard identification of Salmonella spp. J. Appl. Microbiol. 2002, 93, 593–598. [Google Scholar] [CrossRef]
- Mendonça, E.P.; Melo, R.T.; Nalevaiko, P.C.; Monteiro, G.P.; Fonseca, B.B.; Galvão, N.N.; Giombelli, A.; Rossi, D.A. Spread of the serotypes and antimicrobial resistance in strains of Salmonella spp. isolated from broiler. Food Microbiol. 2019, 50, 515–522. [Google Scholar] [CrossRef]
- Habib, I.; Elbediwi, M.; Ghazawi, A.; Mohamed, M.Y.I.; Lakshmi, G.B.; Khan, M. First report from supermarket chicken meat and genomic characterization of colistin resistance mediated by mcr-1.1 in ESBL-producing, multidrug-resistant Salmonella Minnesota. Int. J. Food Microbiol. 2022, 379, 109835. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Chen, M.C.; Feng, Y.; Su, L.H.; Li, H.C.; Yang, H.P.; Yu, M.J.; Chen, C.L.; Chiu, C.H. Highly antimicrobial-resistant nontyphoidal Salmonella from retail meats and clinical impact in children, Taiwan. Pediatr. Neonatol. 2020, 61, 432–438. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chen, C.L.; Yang, H.P.; Chiu, C.H. Prevalence, serotypes, and antimicrobial resistance patterns of non-typhoid Salmonella in food in Northern Taiwan. Pathogens 2022, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.B.B.E.; Silva, R.L.; Menezes, J.; Machado, S.C.A.; Rodrigues, D.P.; Pomba, C.; Abreu, D.L.C.; Nascimento, E.R.; Aquino, M.H.C.; Pereira, V.L.A. High prevalence of multidrug-resistant nontyphoidal Salmonella recovered from broiler chickens and chicken carcasses in Brazil. Braz. J. Poult. Sci. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Lin, C.H.; Huang, J.F.; Sun, Y.F.; Adams, P.J.; Lin, J.H.; Robertson, I.D. Detection of chicken carcasses contaminated with Salmonella enterica serovar in the abattoir environment of Taiwan. Int. J. Food Microbiol. 2020, 325, 108640. [Google Scholar] [CrossRef]
- Boubendir, S.; Arsenault, J.; Quessy, S.; Thibodeau, A.; Fravalo, P.; Theriault, W.P.; Fournaise, S.; Gaucher, M.L. Salmonella contamination of broiler chicken carcasses at critical steps of the slaughter process and in the environment of two slaughter plants: Prevalence, genetic profiles, and association with the final carcass status. J. Food Prot. 2021, 84, 321–332. [Google Scholar] [CrossRef]
- Villegas, K.J.L.; Barragán, I.S.R. Virulence and antimicrobial-resistant gene profiles of Salmonella spp. isolates from chicken carcasses markets in Ibague City, Colombia. Int. J. Microbiol. 2024, 2024, 4674138. [Google Scholar] [CrossRef]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Elshebrawy, H.A.; Abdel-Naeem, N.H.S.; Mahros, M.A.; Elsayed, H.H.; Imre, K.; Herman, V.; Morar, A.; Sallam, K.I. Multidrug-resistant Salmonella enterica serovars isolated from frozen chicken carcasses. LWT 2022, 164, 113647. [Google Scholar] [CrossRef]
- Hiyoshi, H.; Tiffany, C.R.; Bronner, D.N.; Bäumler, A.J. Typhoidal Salmonella serovars: Ecological opportunity and the evolution of a new pathovar. FEMS Microbiol. Rev. 2018, 42, 527–541. [Google Scholar] [CrossRef]
- Reta, G.G.; Lopes, S.M.; Aquino, N.S.M.; Tondo, E.C. Quantification of Salmonella transfer in cross-contamination scenarios found in chicken slaughterhouses. Food Microbiol. 2023, 116, 104347. [Google Scholar] [CrossRef]
- Lyu, C.; Li, D.; Wang, B.; Rao, W.; Han, M.; Deng, S.; Xu, X.; Wang, H. Risk investigation and diversity of microbial contamination during slaughter processing of yellow-feathered broiler. LWT 2024, 210, 116801. [Google Scholar] [CrossRef]
- Hussain, A.; Shaik, S.; Ranjan, A.; Nandanwar, N.; Tiwari, S.K.; Majid, M.; Baddam, R.; Qureshi, I.A.; Semmler, T.; Wieler, L.H.; et al. Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free-range retail chicken in India. Front. Microbiol. 2017, 8, 2120. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Gautam, N.; Poudel, R.; Lekhak, B.; Upreti, M.K. Antimicrobial susceptibility pattern of Gram-negative bacterial isolates from raw chicken meat samples. Tribhuvan Univ. J. Microbiol. 2019, 6, 89–95. [Google Scholar] [CrossRef]
- Adzitey, F.; Pepra, P.A.; Teye, G.A.; Somboro, A.M.; Kumalo, H.M.; Amoako, D.G. Prevalence and antimicrobial resistance of Escherichia coli isolated from various meat types in the Tamale metropolis of Ghana. Int. J. Food Sci. 2020, 2020, 8877196. [Google Scholar] [CrossRef]
- Wasfi, R.; Elkhatib, W.F.; Ashour, H.M. Molecular typing and virulence analysis of multidrug resistant Klebsiella pneumoniae clinical isolates recovered from Egyptian hospitals. Sci. Rep. 2016, 6, 38929. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Mourão, J.; Magalhães, M.; Almeida, M.R.; Rebelo, A.; Novais, C.; Peixe, L.; Novais, A.; Antunes, P. Decoding Klebsiella pneumoniae in poultry chain: Unveiling genetic landscape, antibiotic resistance, and biocide tolerance in non-clinical reservoirs. Front. Microbiol. 2024, 15, 1365011. [Google Scholar] [CrossRef]
- El-Ghany, W.A.A. Pseudomonas aeruginosa infection of avian origin: Zoonosis and one health implications. Vet. World 2021, 14, 2155–2159. [Google Scholar] [CrossRef]
| Counts of Bacterial Indicators | ||
|---|---|---|
| Type of Carcass | Aerobic Mesophilic Microorganisms | E. coli |
| Without visible gastrointestinal contamination | 5.42 ± 0.40 b | 3.74 ± 0.68 b |
| With visible gastrointestinal contamination | 6.01 ± 0.61 a | 4.70 ± 0.62 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, B.L.d.A.; Machado, R.A.; Jesus, J.L.B.d.; Reis, F.Y.T.; Zanon, I.P.; Casas, M.R.T.; Silva, R.O.S.; Figueiredo, H.C.P.; Figueiredo, T.C.d.; Souza, M.R.d.; et al. Microbiological Quality and Presence of Salmonella spp. in Broiler Carcasses with and Without Visible Gastrointestinal Contamination During Industrial Processing. Microorganisms 2025, 13, 1124. https://doi.org/10.3390/microorganisms13051124
Brito BLdA, Machado RA, Jesus JLBd, Reis FYT, Zanon IP, Casas MRT, Silva ROS, Figueiredo HCP, Figueiredo TCd, Souza MRd, et al. Microbiological Quality and Presence of Salmonella spp. in Broiler Carcasses with and Without Visible Gastrointestinal Contamination During Industrial Processing. Microorganisms. 2025; 13(5):1124. https://doi.org/10.3390/microorganisms13051124
Chicago/Turabian StyleBrito, Bruno Leandro de Almeida, Rafaela Assis Machado, João Luís Batista de Jesus, Francisco Yan Tavares Reis, Isabela Pádua Zanon, Monique Ribeiro Tiba Casas, Rodrigo Otávio Silveira Silva, Henrique César Pereira Figueiredo, Tadeu Chaves de Figueiredo, Marcelo Resende de Souza, and et al. 2025. "Microbiological Quality and Presence of Salmonella spp. in Broiler Carcasses with and Without Visible Gastrointestinal Contamination During Industrial Processing" Microorganisms 13, no. 5: 1124. https://doi.org/10.3390/microorganisms13051124
APA StyleBrito, B. L. d. A., Machado, R. A., Jesus, J. L. B. d., Reis, F. Y. T., Zanon, I. P., Casas, M. R. T., Silva, R. O. S., Figueiredo, H. C. P., Figueiredo, T. C. d., Souza, M. R. d., & Cançado, S. d. V. (2025). Microbiological Quality and Presence of Salmonella spp. in Broiler Carcasses with and Without Visible Gastrointestinal Contamination During Industrial Processing. Microorganisms, 13(5), 1124. https://doi.org/10.3390/microorganisms13051124






