Community Structure, Assembly and Interactions of Nitrospira Nitrite-Oxidizing Bacteria in Sediments of the Eastern China Marginal Seas
Abstract
1. Introduction
2. Materials and Methods
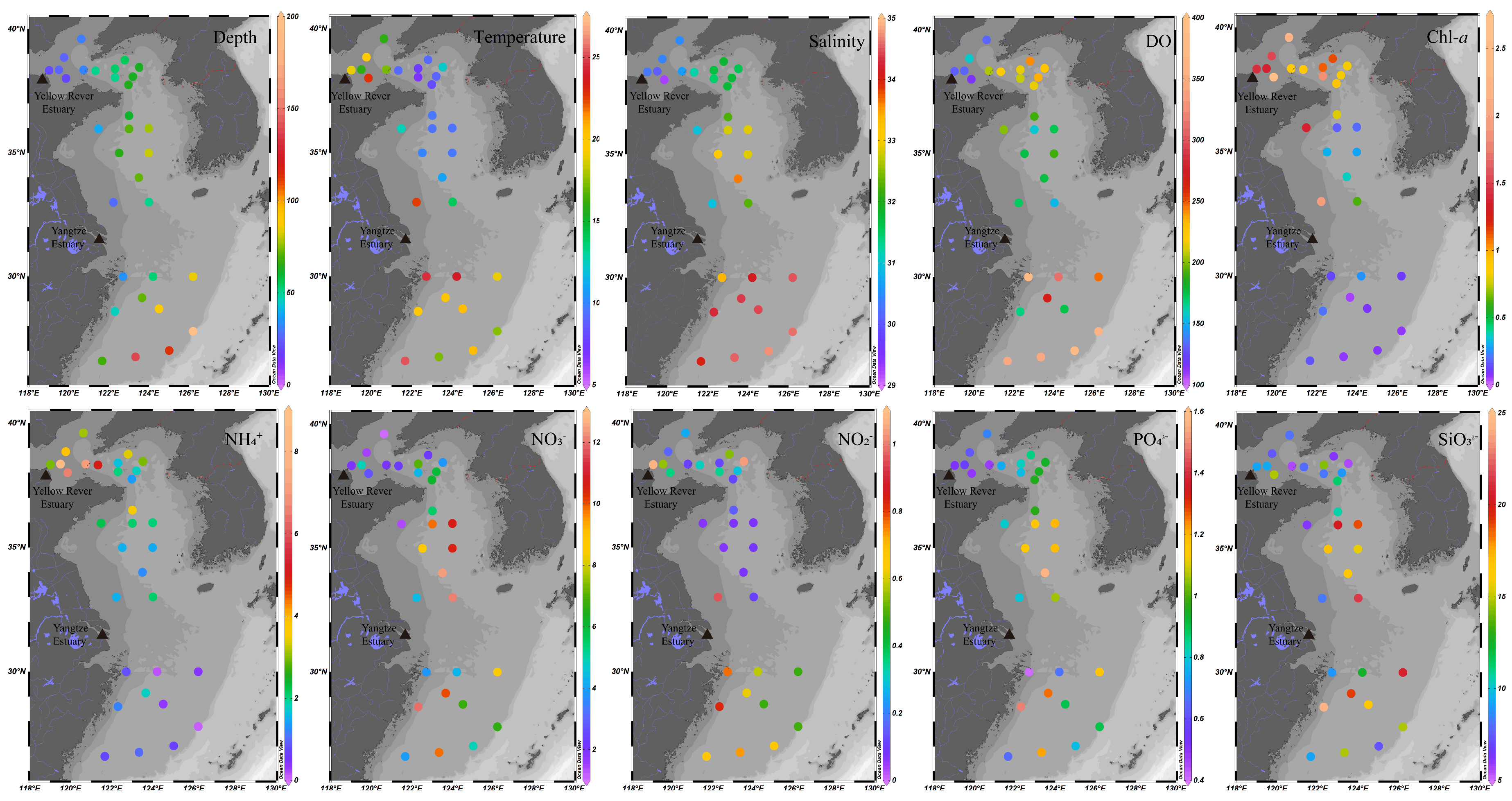
2.1. Sediment Sample Collection and Environmental Characterization
2.2. DNA Extraction and High-Throughput Sequencing Analysis
2.3. Sequence Data Processing and Statistical Analysis
2.4. Community Assembly, Co-Occurrence Network and Stability Analysis
3. Results
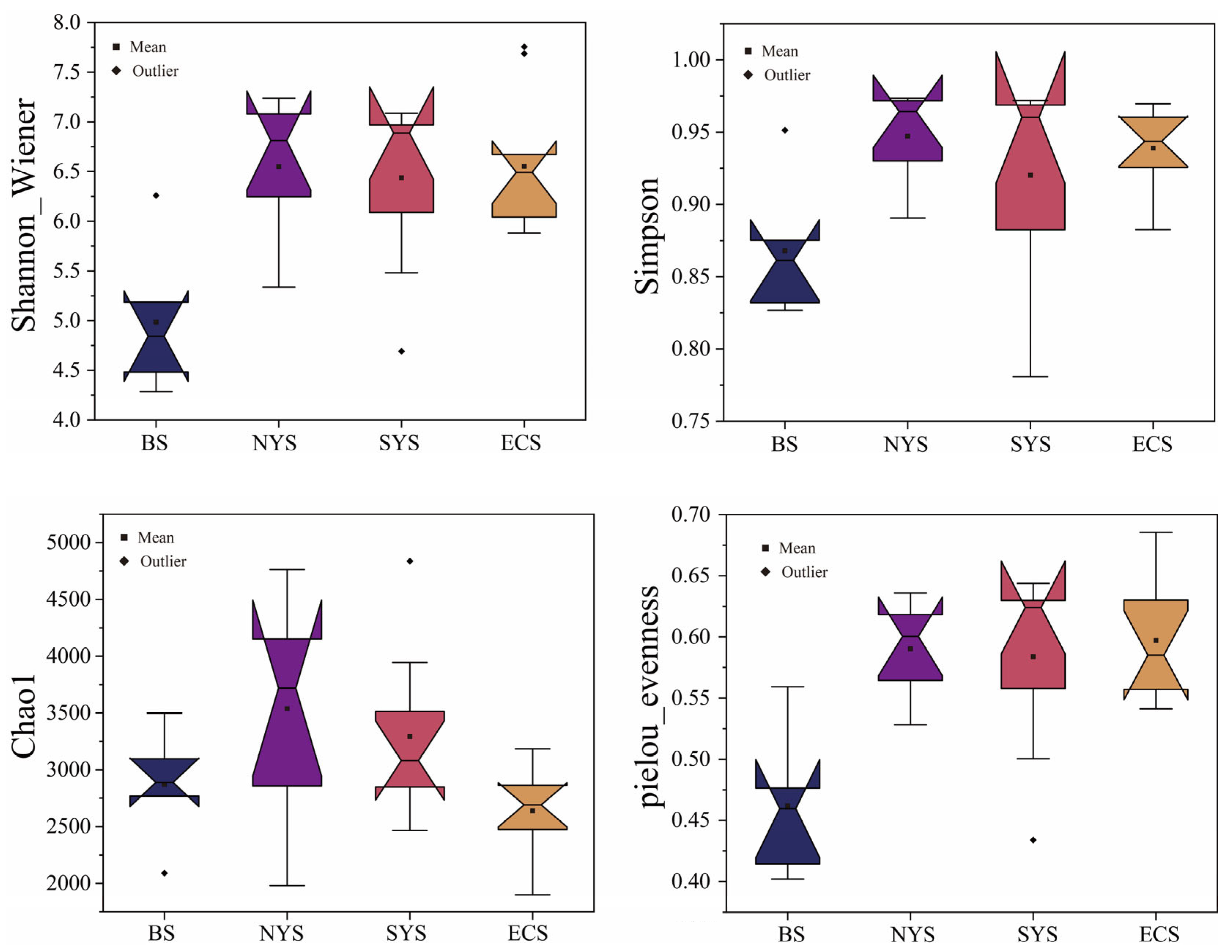
3.1. Nitrospira Community Characteristics
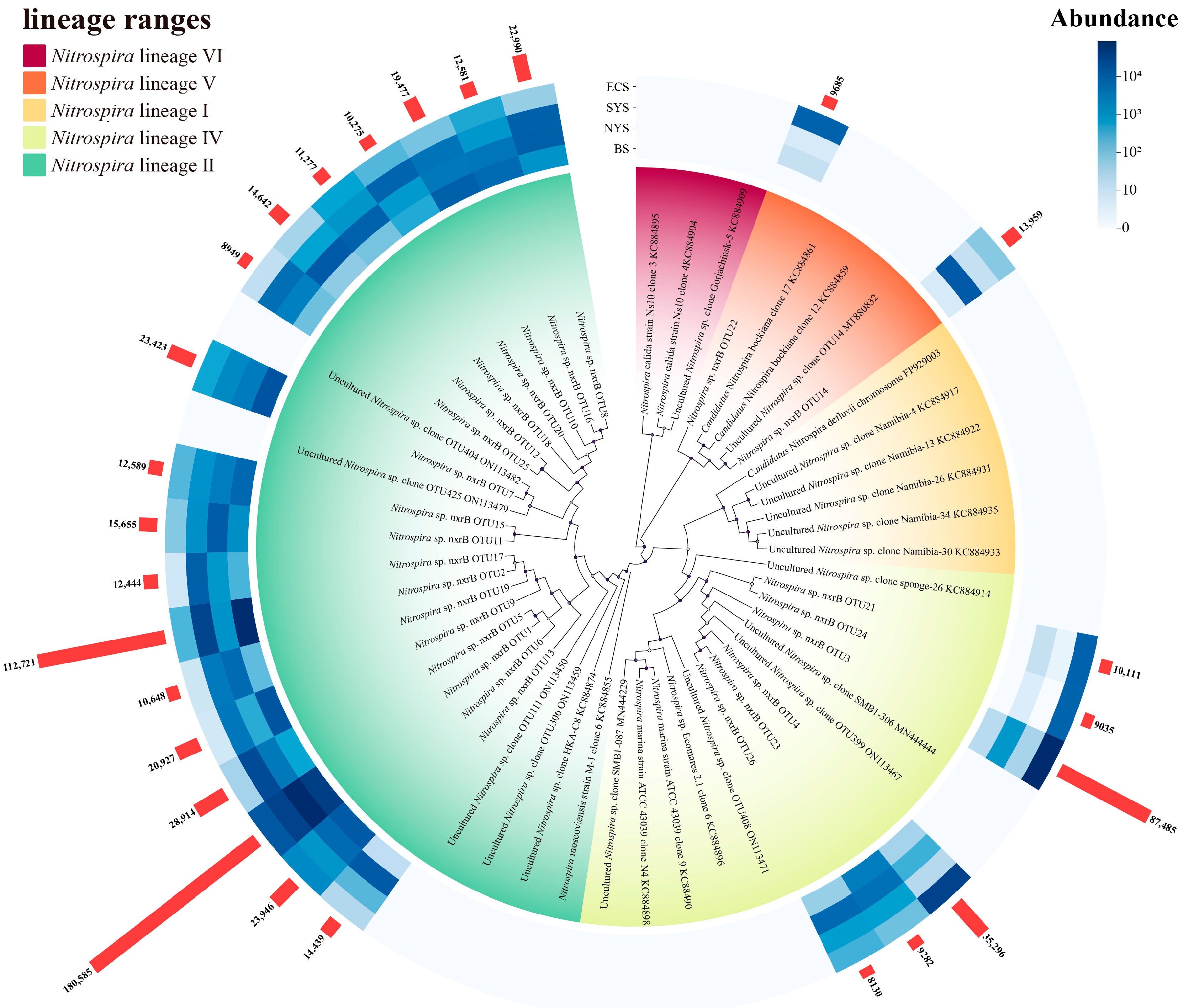
3.2. Phylogenetic Characteristics of Nitrospira
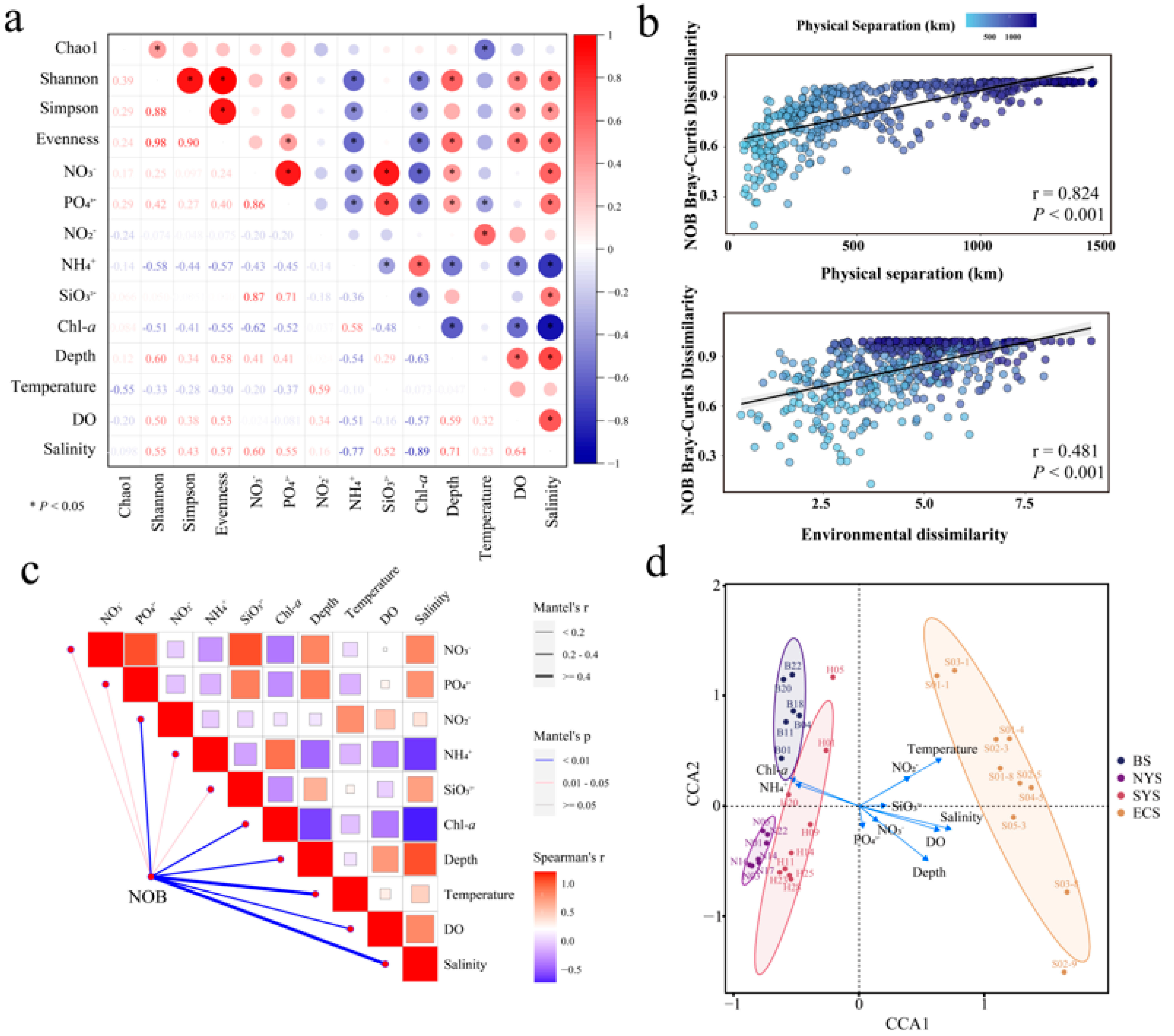
3.3. Effects of Environmental Factors on Nitrospira Communities
3.4. Community Assembly of Nitrospira
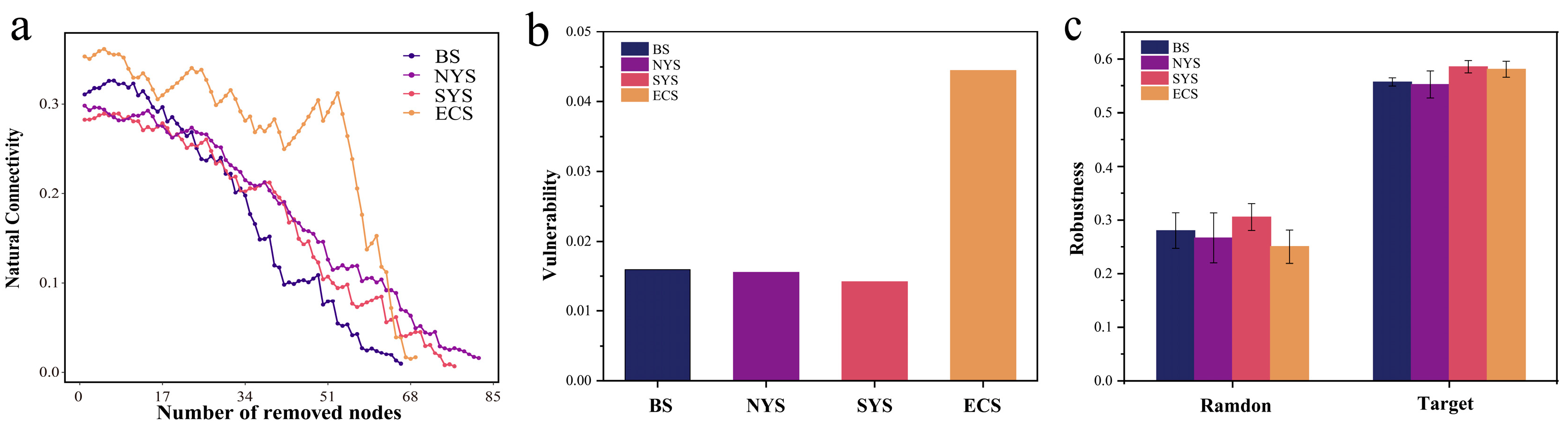
3.5. Community Interaction and Stability of Nitrospira
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Teske, A.; Alm, E.; Regan, J.M.; Toze, S.; Rittmann, B.E.; Stahl, D.A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J. Bacteriol. 1994, 176, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Env. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Treusch, A.H.; Leininger, S.; Kletzin, A.; Schuster, S.C.; Klenk, H.P.; Schleper, C. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 2005, 7, 1985–1995. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, X.; Shi, X.; Guo, W.; Luo, X. Niche differentiation and influencing factors of nitrite oxidation bacteria Nitrospira in sediments of the Luan River estuary in China. Environ. Sci. Pollut. Res. 2023, 30, 103313–103323. [Google Scholar] [CrossRef]
- Kouba, V.; Vejmelkova, D.; Proksova, E.; Wiesinger, H.; Concha, M.; Dolejs, P.; Hejnic, J.; Jenicek, P.; Bartacek, J. High-Rate Partial Nitritation of Municipal Wastewater after Psychrophilic Anaerobic Pretreatment. Environ. Sci. Technol. 2017, 51, 11029–11038. [Google Scholar] [CrossRef]
- Bartosch, S.; Wolgast, I.; Spieck, E.; Bock, E. Identification of Nitrite-Oxidizing Bacteria with Monoclonal Antibodies Recognizing the Nitrite Oxidoreductase. Appl. Env. Microbiol. 1999, 65, 4126–4133. [Google Scholar] [CrossRef]
- Pachiadaki, M.G.; Sintes, E.; Bergauer, K.; Brown, J.M.; Record, N.R.; Swan, B.K.; Mathyer, M.E.; Hallam, S.J.; Hallam, S.J.; Takaki, Y.; et al. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation. Science 2017, 358, 1046–1051. [Google Scholar] [CrossRef]
- Su, Z.; Liu, T.; Guo, J.; Zheng, M. Nitrite Oxidation in Wastewater Treatment: Microbial Adaptation and Suppression Challenges. Environ. Sci. Technol. 2023, 57, 12557–12570. [Google Scholar] [CrossRef]
- Chisholm, C.; Di, H.J.; Cameron, K.; Podolyan, A.; Shah, A.; Hsu, L.; Shen, J. Soil moisture is a primary driver of comammox Nitrospira abundance in New Zealand soils. Sci. Total Environ. 2023, 858, 159961. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, H.; Chen, L.; Wang, J.; Zheng, M.; Liu, S.; Chen, Q.; Ni, J. Comammox Nitrospira within the Yangtze River continuum: Community, biogeography, and ecological drivers. ISME J. 2020, 14, 2488–2504. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiao, L.; Wu, J.; Ye, J.; Wei, M.; Hong, Y. Existence and distribution of novel phylotypes of Nitrospira in water columnsof the South China Sea. iScience 2022, 25, 104895. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Lücker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef]
- Watson, S.W.; Bock, E.; Valois, F.W.; Waterbury, J.B.; Schlosser, U. Nitrospira marina gen. nov. sp. nov.: A chemolithotrophic nitrite-oxidizing bacterium. Arch. Microbiol. 1986, 144, 1–7. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Pester, M.; Kitzinger, K.; Savio, D.F.; Loy, A.; Rattei, T.; Wagner, M.; Daims, H. Functionally relevant diversity of closely related Nitrospira in activated sludge. ISME J. 2015, 9, 643–655. [Google Scholar] [CrossRef]
- Attard, E.; Poly, F.; Commeaux, C.; Laurent, F.; Terada, A.; Smets, B.F.; Recous, S.; Le Roux, X. Shifts between Nitrospira- and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ. Microbiol. 2010, 12, 315–326. [Google Scholar] [CrossRef]
- Latocheski, E.C.; da Rocha, M.C.V.; Braga, M.C.B. Nitrospira in wastewater treatment: Applications, opportunities and research gaps. Rev. Environ. Sci. Bio/Technol. 2022, 21, 905–930. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, Z.; Chai, Z.; Zhang, A.; Gu, A.; Mu, G.; Wu, D.; Guo, J. Ubiquitous occurrence and functional dominance of comammox Nitrospira in full-scale wastewater treatment plants. Water Res. 2023, 236, 119931. [Google Scholar] [CrossRef]
- Meng, S.; Liang, X.; Peng, T.; Liu, Y.; Wang, H.; Huang, T.; Gu, J.-D.; Hu, Z. Ecological distribution and function of comammox Nitrospira in the environment. Appl. Microbiol. Biotechnol. 2023, 107, 3877–3886. [Google Scholar] [CrossRef]
- Müller, R. The impact of the rise in atmospheric nitrous oxide on stratospheric ozone. Ambio 2020, 50, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bowe, H. Analysis of influence of large-scale water conservancy project on variation characteristics of water and sediment in middle and lower reaches of Yangtze River. J. Water Resour. Water Eng. 2015, 26, 139–144. [Google Scholar]
- Wang, C.; Hao, Z.; Gao, J.; Feng, Z.; Ding, Y.; Zhang, C.; Zou, X. Reservoir Construction Has Reduced Organic Carbon Deposition in the East China Sea by Half Since 2006. Geophys. Res. Lett. 2020, 47, e2020GL087357. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, Q.; Zhao, H.; Hou, M.; Xie, Q.; Chen, J. Occurrence, distribution, and air-water exchange of organophosphorus flame retardants in a typical coastal area of China. Chemosphere 2018, 211, 335–344. [Google Scholar] [CrossRef]
- Mi, B.; Zhang, Y.; Mei, X. The sediment distribution characteristics and transport pattern in the eastern China seas. Quat. Int. 2022, 629, 44–52. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Wang, X.; Wang, H.; Sun, Y.; Zhang, J.; Li, H. Contrasting benthic bacterial and fungal communities in two temperate coastal areas affected by different levels of anthropogenic pressure. Mar. Env. Res 2024, 198, 106501. [Google Scholar] [CrossRef]
- Xu, L.-L.; McIlroy, S.E.; Ni, Y.; Guibert, I.; Chen, J.; Rocha, U.; Baker, D.M.; Panagiotou, G. Chemical pollution drives taxonomic and functional shifts in marine sediment microbiome, influencing benthic metazoans. ISME Commun. 2025, 13, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Yao, P.; Ge, T.; Qiao, Y.; Zhao, M.; Zhang, X.-H. Distribution patterns of ammonia-oxidizing archaea and bacteria in sediments of the eastern China marginal seas. Syst. Appl. Microbiol. 2018, 41, 658–668. [Google Scholar] [CrossRef]
- Yu, S.; Yao, P.; Liu, J.; Zhao, B.; Zhang, G.; Zhao, M.; Yu, Z.; Zhang, X.-H. Diversity, Abundance, and Niche Differentiation of Ammonia-Oxidizing Prokaryotes in Mud Deposits of the Eastern China Marginal Seas. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Gao, M.; Liu, J.; Qiao, Y.; Zhao, M.; Zhang, X.-H. Diversity and Abundance of the Denitrifying Microbiota in the Sediment of Eastern China Marginal Seas and the Impact of Environmental Factors. Microb. Ecol. 2017, 73, 602–615. [Google Scholar] [CrossRef]
- Shehzad, A.; Liu, J.; Yu, M.; Qismat, S.; Liu, J.; Zhang, X. Diversity, Community Composition and Abundance of Anammox Bacteria in Sediments of the North Marginal Seas of China. Microbes Environ. 2016, 31, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, M.; Zheng, M.; Liu, H.; Kuang, S.; Chen, H.; Li, X. Abundance, diversity, and community structure of comammox clade A in sediments of China’s offshore continental shelf. Sci. Total Environ. 2023, 889, 164290. [Google Scholar] [CrossRef] [PubMed]
- Pinckney, J.; Papa, R.; Zingmark, R. Comparison of high-performance liquid chromatographic, spectrophotometric, and fluorometric methods for determining chlorophyll a concentrations in estaurine sediments. J. Microbiol. Methods 1994, 19, 59–66. [Google Scholar] [CrossRef]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lücker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2013, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bonin, A.; Guerrieri, A.; Ficetola, G.F. Optimal sequence similarity thresholds for clustering of molecular operational taxonomic units in DNA metabarcoding studies. Mol. Ecol. Resour. 2022, 23, 368–381. [Google Scholar] [CrossRef]
- Mamet, S.D.; Helgason, B.L.; Lamb, E.G.; McGillivray, A.; Stanley, K.G.; Robinson, S.J.; Aziz, S.U.; Vail, S.; Siciliano, S.D. Phenology-dependent root bacteria enhance yield of Brassica napus. Soil Biol. Biochem. 2022, 166, 108468. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Species divergence and the measurement of microbial diversity. FEMS Microbiol. Rev. 2008, 32, 557–578. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proc. Int. AAAI Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- Zelezniak, A.; Andrejev, S.; Ponomarova, O.; Mende, D.R.; Bork, P.; Patil, K.R. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. USA 2015, 112, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.H.; Eisen, J.A.; Penn, K.; Allison, S.D.; Horner-Devine, M.C. Drivers of bacterial β-diversity depend on spatial scale. Proc. Natl. Acad. Sci. USA 2011, 108, 7850–7854. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, X.; Zhang, Z. Material sources and transportation of sediments in the South Yellow Sea. Trans. Oceanol. Limnol. 2025, 4, 56–60. [Google Scholar] [CrossRef]
- Li, X.; Wan, W.; Zheng, L.; Wang, A.; Luo, X.; Huang, Q.; Chen, W. Community assembly mechanisms and co-occurrence patterns of nitrite-oxidizing bacteria communities in saline soils. Sci. Total Environ. 2021, 772, 145472. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, X.; Sun, D.; Hou, L.; Liu, M.; Zhao, Q.; Klümper, U.; Quan, Z.; Gu, J.-D.; Han, P. Salinity gradients shape the nitrifier community composition in Nanliu River Estuary sediments and the ecophysiology of comammox Nitrospira inopinata. Sci. Total Environ. 2021, 795, 148768. [Google Scholar] [CrossRef]
- Daebeler, A.; Kitzinger, K.; Koch, H.; Herbold, C.W.; Steinfeder, M.; Schwarz, J.; Zechmeister, T.; Karst, S.M.; Albertsen, M.; Nielsen, P.H.; et al. Exploring the upper pH limits of nitrite oxidation: Diversity, ecophysiology, and adaptive traits of haloalkalitolerant Nitrospira. ISME J. 2020, 14, 2967–2979. [Google Scholar] [CrossRef]
- Alawi, M.; Lipski, A.; Sanders, T.; Eva Maria, P.; Spieck, E. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 2007, 1, 256–264. [Google Scholar] [CrossRef]
- Kruse, M.; Zumbrägel, S.; Bakker, E.; Spieck, E.; Eggers, T.; Lipski, A. The nitrite-oxidizing community in activated sludge from a municipal wastewater treatment plant determined by fatty acid methyl ester-stable isotope probing. Syst. Appl. Microbiol. 2013, 36, 517–524. [Google Scholar] [CrossRef]
- Lian, K.; Liu, F.; Li, Y.; Wang, C.; Zhang, C.; McMinn, A.; Wang, M.; Wang, H. Environmental gradients shape microbiome assembly and stability in the East China sea. Environ. Res. 2023, 238, 117197. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; Cram, J.A.; Needham, D.M. Marine microbial community dynamics and their ecological interpretation. Nat. Rev. Microbiol. 2015, 13, 133–146. [Google Scholar] [CrossRef]
- Hao, Z.; Xu, H.; Feng, Z.; Zhang, C.; Zhou, X.; Wang, Z.; Zheng, J.; Zou, X. Spatial distribution, deposition flux, and environmental impact of typical persistent organic pollutants in surficial sediments in the Eastern China Marginal Seas (ECMSs). J. Hazard. Mater. 2021, 407, 124343. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.; Shan, X.; Jin, X.; Yang, T. Marine litter on the seafloors of the Bohai Sea, Yellow Sea and northern East China Sea. Mar. Pollut. Bull. 2021, 169, 112516. [Google Scholar] [CrossRef]
- Pandey, R.S.; Liou, Y.-A. Sea surface temperature (SST) and SST anomaly (SSTA) datasets over the last four decades (1977–2016) during typhoon season (May to November) in the entire Global Ocean, North Pacific Ocean, Philippine Sea, South China sea, and Eastern China Sea. Data Brief 2022, 45, 108646. [Google Scholar] [CrossRef]
- Liu, Y.; Callaway, R.M.; Hart, M.M.; Pither, J.; Klironomos, J. Phylogenetic structure of arbuscular mycorrhizal community shifts in response to increasing soil fertility. Soil Biol. Biochem. 2015, 89, 196–205. [Google Scholar] [CrossRef]
- Han, S.; Xiong, X.; Luo, X.; Zeng, L.; Wei, D.; Chen, W.; Huang, Q. Fertilization rather than aggregate size fractions shape the nitrite-oxidizing microbial community in a Mollisol. Soil Biol. Biochem. 2018, 124, 179–183. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, Q.; Zou, D.; Zhang, S.; Zhang, C.; Quan, Z.; Li, M. Deterministic Factors Determine the Comammox Community Composition in the Pearl River Estuary Ecosystem. Microbiol. Spectr. 2022, 10, e0101622. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Zhao, S.; Cao, L.; Pan, Y.; Li, F.; Li, F.; Lu, J.; Li, Y.; Song, G.; et al. Uncovering the dynamic evolution of microbes and n-alkanes: Insights from the Kuroshio Extension in the Northwest Pacific Ocean. Sci. Total Environ. 2023, 875, 162418. [Google Scholar] [CrossRef]
- Yang, D.; Kato, H.; Kawatsu, K.; Osada, Y.; Azuma, T.; Nagata, Y.; Kondoh, M. Reconstruction of a Soil Microbial Network Induced by Stress Temperature. Microbiol. Spectr. 2022, 10, e02748-22. [Google Scholar] [CrossRef]
- Li, H.; Liu, P.-Q.; Luo, Q.-P.; Ma, J.-J.; Yang, X.-R.; Yan, Y.; Su, J.-Q.; Zhu, Y.-G. Spatiotemporal variations of microbial assembly, interaction, and potential risk in urban dust. Environ. Int. 2022, 170, 107577. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.W.; Shade, A. Dormancy dynamics and dispersal contribute to soil microbiome resilience. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190255. [Google Scholar] [CrossRef] [PubMed]
- Czárán, T.L.; Hoekstra, R.F.; Pagie, L. Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. USA 2002, 99, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Wang, H.; Yang, G.; Kan, J.; Yang, M.; Yu, X.; Guo, C.; Wang, M.; Wang, W.; et al. Assembly and Network Stability of Planktonic Microorganisms under the Influence of Salinity Gradient: An Arctic Case Study from the Lena River Estuary to the Laptev Sea. Microbiol. Spectr. 2023, 11, e02115–e02122. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; He, H.; Wang, M. Community Structure, Assembly and Interactions of Nitrospira Nitrite-Oxidizing Bacteria in Sediments of the Eastern China Marginal Seas. Microorganisms 2025, 13, 1112. https://doi.org/10.3390/microorganisms13051112
Dong H, He H, Wang M. Community Structure, Assembly and Interactions of Nitrospira Nitrite-Oxidizing Bacteria in Sediments of the Eastern China Marginal Seas. Microorganisms. 2025; 13(5):1112. https://doi.org/10.3390/microorganisms13051112
Chicago/Turabian StyleDong, Hao, Hui He, and Min Wang. 2025. "Community Structure, Assembly and Interactions of Nitrospira Nitrite-Oxidizing Bacteria in Sediments of the Eastern China Marginal Seas" Microorganisms 13, no. 5: 1112. https://doi.org/10.3390/microorganisms13051112
APA StyleDong, H., He, H., & Wang, M. (2025). Community Structure, Assembly and Interactions of Nitrospira Nitrite-Oxidizing Bacteria in Sediments of the Eastern China Marginal Seas. Microorganisms, 13(5), 1112. https://doi.org/10.3390/microorganisms13051112




