Widespread Detection of Fowl Adenovirus Serotype 2/11 Species D Among Cases of Inclusion Body Hepatitis–Hydropericardium Syndrome in Chickens in Egypt
Abstract
1. Introduction
2. Materials and Methods
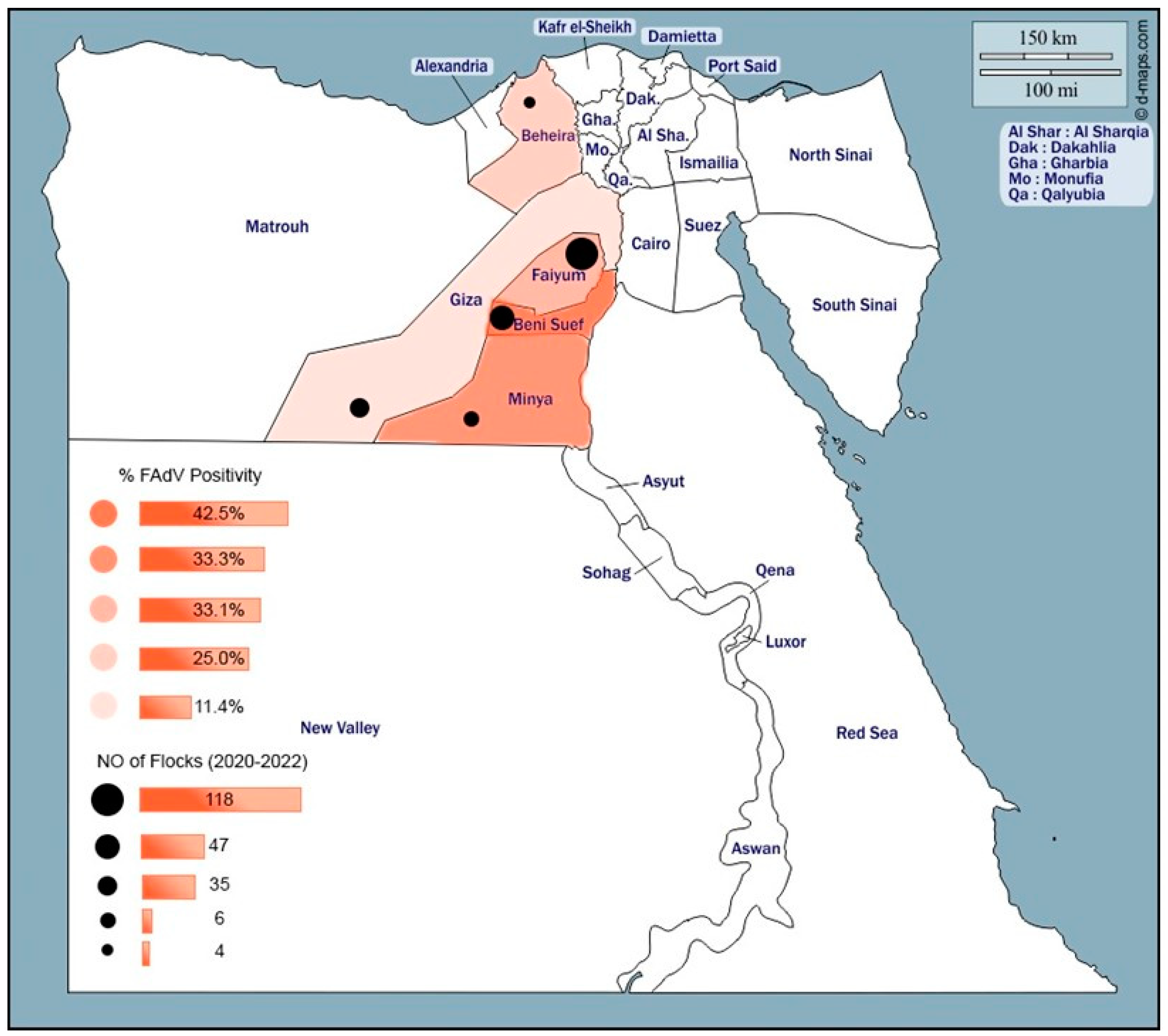
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Molecular Detection of FAdVs
2.4. Sequencing and Sequence Analysis of the L1 Region of the Hexon Gene
3. Results
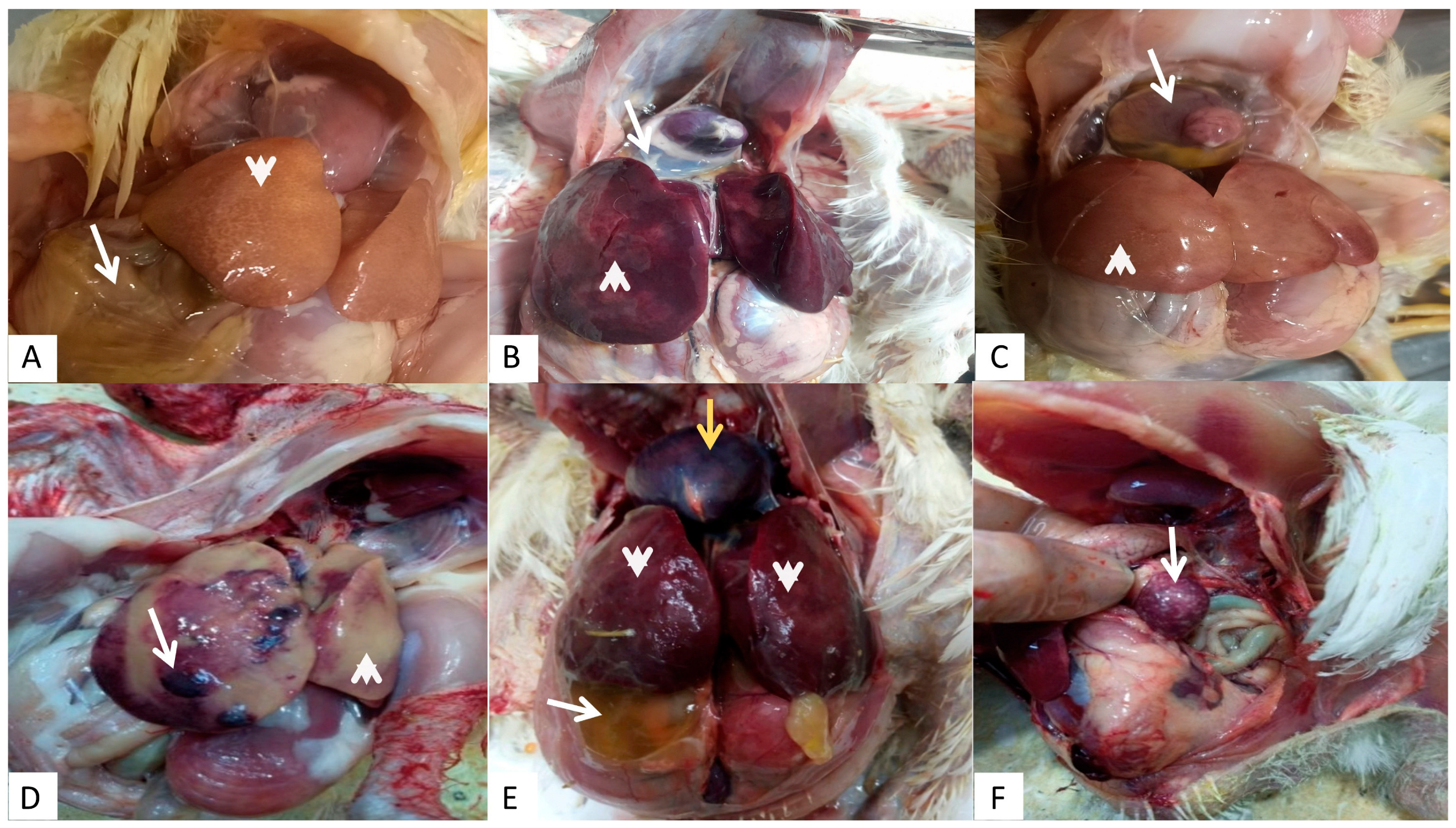
3.1. Clinical Cases and Gross Lesions
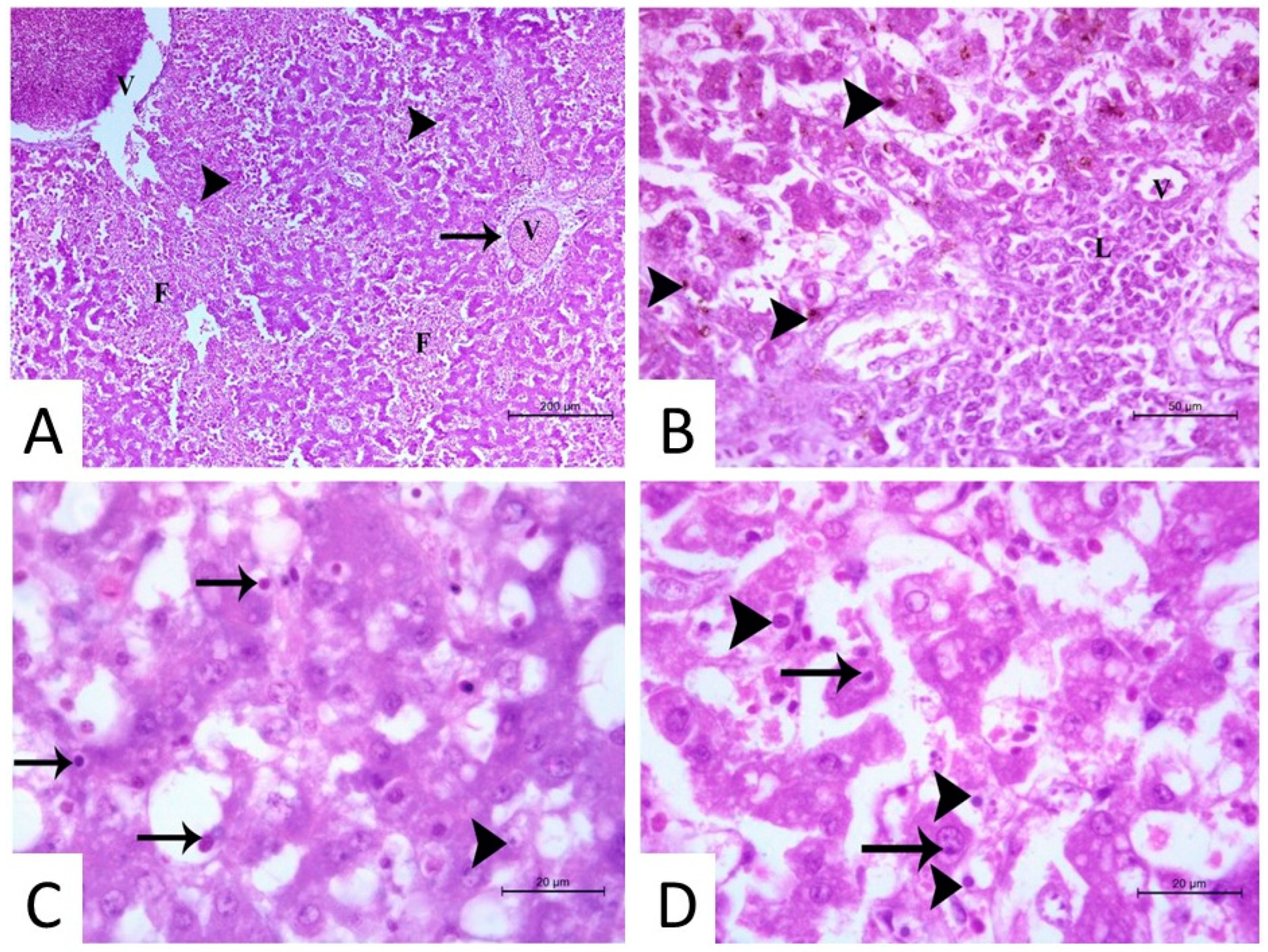
3.2. Histopathological Findings
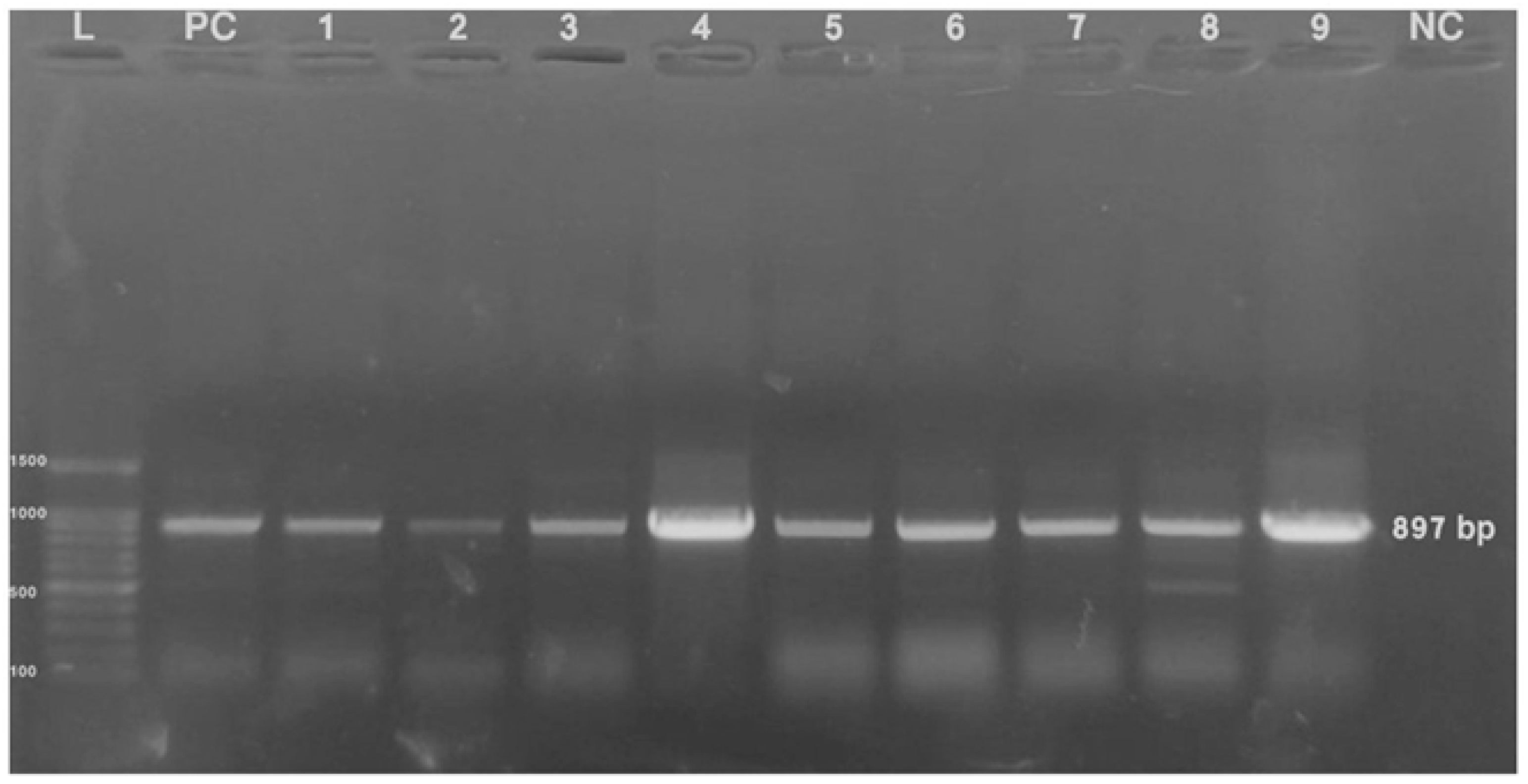
3.3. Virus Detection via PCR
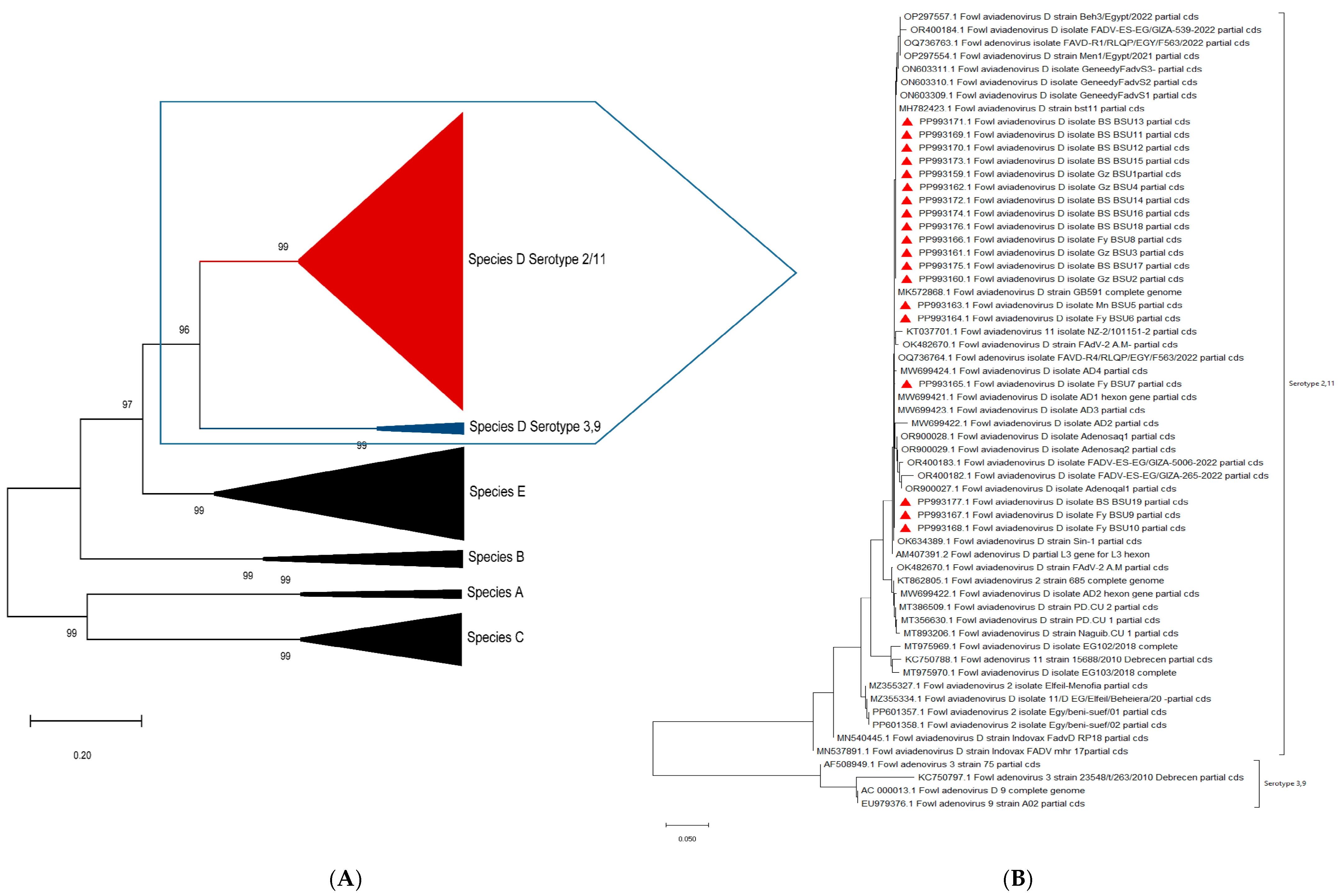
3.4. Gene Sequencing and Phylogenetic Analysis
3.5. Mutation Analysis of Amino Acid Residues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, M. Commensal or pathogen—A challenge to fulfil Koch’s Postulates. Br. Poult. Sci. 2017, 58, 1–12. [Google Scholar] [CrossRef]
- Safwat, M.M.; Sayed, A.S.R.; Ali Elsayed, M.F.; Ibrahim, A. Genotyping and pathogenicity of fowl adenovirus isolated from broiler chickens in Egypt. BMC Vet. Res. 2022, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Faustini, G.; Tucciarone, C.M.; Pasotto, D.; Legnardi, M.; Cecchinato, M. Conflicting Evidence between Clinical Perception and Molecular Epidemiology: The Case of Fowl Adenovirus D. Animals 2023, 13, 3851. [Google Scholar] [CrossRef] [PubMed]
- El-Shall, N.A.; El-Hamid, H.S.A.; Elkady, M.F.; Ellakany, H.F.; Elbestawy, A.R.; Gado, A.R.; Geneedy, A.M.; Hasan, M.E.; Jaremko, M.; Selim, S.; et al. Epidemiology, pathology, prevention, and control strategies of inclusion body hepatitis and hepatitis-hydropericardium syndrome in poultry: A comprehensive review. Front. Vet. Sci. 2022, 9, 963199. [Google Scholar] [CrossRef]
- Ishag, H.Z.A.; Terab, A.M.A.; El Tigani-Asil, E.T.A.; Bensalah, O.K.; Khalil, N.A.H.; Khalafalla, A.I.; Al Hammadi, Z.; Shah, A.A.M.; Al Muhairi, S.S.M. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Vet. Sci. 2022, 9, 154. [Google Scholar] [CrossRef]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl adenovirus-induced diseases and strategies for their control—A review on the current global situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef]
- De Luca, C.; Hess, M. Vaccination strategies to protect chickens from fowl adenovirus (FAdV)-induced diseases: A comprehensive review. Vaccine 2025, 43 Pt 1, 126496. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, Q.; Shi, Y.; Ding, Y.; Li, Z.; Sun, Y.; Li, M.; Liu, S. Immunosuppressive potential of fowl adenovirus serotype 4. Poult. Sci. 2019, 98, 3514–3522. [Google Scholar] [CrossRef]
- Franzo, G.; Prentza, Z.; Paparounis, T.; Tsiouris, V.; Centonze, G.; Legnardi, M.; Catelli, E.; Tucciarone, C.M.; Koutoulis, K.; Cecchinato, M. Molecular epidemiology of fowl adenoviruses in Greece. Poult. Sci. 2020, 99, 5983–5990. [Google Scholar] [CrossRef]
- Abdellatif, D.M.; El-Sawah, A.A.; Elkady, M.F.; Ali, A.; Shany, S.A.S. Inclusion Body Hepatitis: A Comprehensive Overview of Disease Impact and Control in Poultry. Egypt. J. Vet. Sci. 2025, 1–17. [Google Scholar] [CrossRef]
- Radwan, M.M.; El-Deeb, A.H.; Mousa, M.R.; El-Sanousi, A.A.; Shalaby, M.A. First report of fowl adenovirus 8a from commercial broiler chickens in Egypt: Molecular characterization and pathogenicity. Poult. Sci. 2019, 98, 97–104. [Google Scholar] [CrossRef] [PubMed]
- El-Basrey, Y.; Hamed, R.; Mohamed, M.; Lebdah, M. Detection of inclusion body hepatitis virus in broilers at Sharkia province, Egypt. J. Anim. Health Prod. 2020, 9, 84–89. [Google Scholar] [CrossRef]
- Adel, A.; Mohamed, A.A.E.; Samir, M.; Hagag, N.M.; Erfan, A.; Said, M.; Arafa, A.E.S.; Hassan, W.M.M.; El Zowalaty, M.E.; Shahien, M.A. Epidemiological and molecular analysis of circulating fowl adenoviruses and emerging of serotypes 1, 3, and 8b in Egypt. Heliyon 2021, 7, e08366. [Google Scholar] [CrossRef]
- Sultan, H.; Arafa, A.E.; Adel, A.; Selim, K.; Hossiny, M.; Talaat, S. Molecular Detection of a Novel Fowl Adenovirus Serotype-4 (FadV-4) from an Outbreak of Hepatitis Hydropericardium Syndrome in Commercial Broiler Chickens in Egypt. Avian Dis. 2021, 65, 385–390. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. iii. [Google Scholar] [CrossRef]
- Meulemans, G.; Boschmans, M.; Berg, T.P.; Decaesstecker, M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001, 30, 655–660. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, e3923. [Google Scholar] [CrossRef]
- Mohamed, M.H.A.; El-Sabagh, I.M.; Abdelaziz, A.M.; Al-Ali, A.M.; Alramadan, M.; Lebdah, M.A.; Ibrahim, A.M.; Al-Ankari, A.S. Molecular characterization of fowl aviadenoviruses species D and E associated with inclusion body hepatitis in chickens and falcons indicates possible cross-species transmission. Avian Pathol. 2018, 47, 384–390. [Google Scholar] [CrossRef]
- Niczyporuk, J.S. Deep analysis of Loop L1 HVRs1-4 region of the hexon gene of adenovirus field strains isolated in Poland. PLoS ONE 2018, 13, e0207668. [Google Scholar] [CrossRef]
- Dar, A.; Gomis, S.; Shirley, I.; Mutwiri, G.; Brownlie, R.; Potter, A.; Gerdts, V.; Tikoo, S.K. Pathotypic and molecular characterization of a fowl adenovirus associated with inclusion body hepatitis in Saskatchewan chickens. Avian Dis. 2012, 56, 73–81. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Abou El-Azm, K.I. Molecular detection and characterization of fowl adenovirus associated with inclusion body hepatitis from broiler chickens in Egypt. Trop. Anim. Health Prod. 2019, 51, 1065–1071. [Google Scholar] [CrossRef]
- Gunes, A.; Marek, A.; Grafl, B.; Berger, E.; Hess, M. Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A to FAdV-E). J. Virol. Methods 2012, 183, 147–153. [Google Scholar] [CrossRef]
- Abghour, S.; Mouahid, M.; Darkaoui, S.; Berrada, J.; Zro, K.; Kichou, F. Pathogenicity of field strain of fowl aviadenovirus serotype 11 isolated from chickens with inclusion body hepatitis in Morocco. PLoS ONE 2021, 16, e0261284. [Google Scholar] [CrossRef]
- Wang, T.; Meng, F.; Chen, C.; Shen, Y.; Li, P.; Xu, J.; Feng, Z.; Qu, X.; Wang, F.; Li, B.; et al. Pathogenicity and epidemiological survey of fowl adenovirus in Shandong Province from 2021 to 2022. Front. Microbiol. 2023, 14, 1166078. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.; Gao, S.; Yang, J.; Tang, Y.; Diao, Y. Pathogenicity of fowl adenovirus serotype 4 (FAdV-4) in chickens. Infect. Genet. Evol. 2019, 75, 104017. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; Gonzalez, O.; Escobar, C.; Cerda, L.; Morales, M.A.; Gonzalez, C. Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Dis. 2001, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Chitradevi, S.; Sukumar, K.; Suresh, P.; Balasubramaniam, G.A.; Kannan, D. Molecular typing and pathogenicity assessment of fowl adenovirus associated with inclusion body hepatitis in chicken from India. Trop. Anim. Health Prod. 2021, 53, 412. [Google Scholar] [CrossRef]
- Elbestawy, A.R.; Ibrahim, M.; Hammam, H.; Noreldin, A.E.; Bahrawy, A.E.; Ellakany, H.F. Molecular characterization of fowl adenovirus D species in broiler chickens with inclusion body hepatitis in Egypt. Alex. J. Vet. Sci. 2020, 64, 110–117. [Google Scholar] [CrossRef]
- Rashid, F.; Xie, Z.; Wei, Y.; Xie, Z.; Xie, L.; Li, M.; Luo, S. Biological features of fowl adenovirus serotype-4. Front. Cell Infect. Microbiol. 2024, 14, 1370414. [Google Scholar] [CrossRef]
- Changjing, L.; Haiying, L.; Dongdong, W.; Jingjing, W.; Youming, W.; Shouchun, W.; Jida, L.; Ping, L.; Jianlin, W.; Shouzhen, X.; et al. Characterization of fowl adenoviruses isolated between 2007 and 2014 in China. Vet. Microbiol. 2016, 197, 62–67. [Google Scholar] [CrossRef]
- Ojkic, D.; Martin, E.; Swinton, J.; Vaillancourt, J.P.; Boulianne, M.; Gomis, S. Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathol. 2008, 37, 95–100. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Pan, Y.; Chen, Z.; Huang, Y.; Li, L.; Dong, J.; Xiang, Y.; Zhai, Q.; Li, X.; et al. Prevalence, genomic characteristics, and pathogenicity of fowl adenovirus 2 in Southern China. Poult. Sci. 2024, 103, 103177. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Kye, S.J.; Kim, J.Y.; Jeon, W.J.; Lee, E.K.; Park, K.Y.; Sung, H.W. Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poult. Sci. 2012, 91, 2502–2506. [Google Scholar] [CrossRef]
- Matos, M.; Grafl, B.; Liebhart, D.; Hess, M. The outcome of experimentally induced inclusion body hepatitis (IBH) by fowl aviadenoviruses (FAdVs) is crucially influenced by the genetic background of the host. Vet. Res. 2016, 47, 69. [Google Scholar] [CrossRef]
- Maartens, L.H.; Joubert, H.W.; Aitchison, H.; Venter, E.H. Inclusion body hepatitis associated with an outbreak of fowl adenovirus type 2 and type 8b in broiler flocks in South Africa. J. S. Afr. Vet. Assoc. 2014, 85, e1–e5. [Google Scholar] [CrossRef]
- Lim, T.H.; Lee, H.J.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Youn, H.N.; Kim, M.S.; Youn, H.S.; Lee, J.B.; Park, S.Y.; et al. Identification and virulence characterization of fowl adenoviruses in Korea. Avian Dis. 2011, 55, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Hess, M. Aviadenovirus infections. In Diseases of Poultry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 290–300. [Google Scholar] [CrossRef]
- Lebdah, M.; Alshaya, D.S.; Jalal, A.S.; Mousa, M.R.; Radwan, M.M.; Samir, M.; Adel, A.; Albaqami, N.M.; El-Saadony, M.T.; El-Tarabily, K.A.; et al. Molecular characterization of aviadenovirus serotypes and pathogenicity of the identified adenovirus in broiler chickens. Poult. Sci. 2022, 101, 101918. [Google Scholar] [CrossRef] [PubMed]
- Hossiny, M.; Abdelrazek, A.; Sultan, H. Molecular Characterization of Hydropericardium Hepatitis Syndrome in Broiler Chickens. J. Curr. Vet. Res. 2024, 6, 151–160. [Google Scholar] [CrossRef]
- Rashid, F.; Xie, Z.; Zhang, L.; Luan, Y.; Luo, S.; Deng, X.; Xie, L.; Xie, Z.; Fan, Q. Genetic characterization of fowl aviadenovirus 4 isolates from Guangxi, China, during 2017–2019. Poult. Sci. 2020, 99, 4166–4173. [Google Scholar] [CrossRef]
- Gupta, A. Pathogenesis and Control of Inclusion Body Hepatitis in Broiler Chickens. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Popowich, S.; Gupta, A.; Chow-Lockerbie, B.; Ayalew, L.; Ambrose, N.; Ojkic, D.; Gunawardana, T.; Kurukulasuriya, S.; Willson, P.; Tikoo, S.K.; et al. Broad spectrum protection of broiler chickens against inclusion body hepatitis by immunizing their broiler breeder parents with a bivalent live fowl adenovirus vaccine. Res. Vet. Sci. 2018, 118, 262–269. [Google Scholar] [CrossRef]
- Gupta, A.; Popowich, S.; Ojkic, D.; Kurukulasuriya, S.; Chow-Lockerbie, B.; Gunawardana, T.; Goonewardene, K.; Karunarathna, R.; Ayalew, L.E.; Ahmed, K.A.; et al. Inactivated and live bivalent fowl adenovirus (FAdV8b + FAdV11) breeder vaccines provide broad-spectrum protection in chicks against inclusion body hepatitis (IBH). Vaccine 2018, 36, 744–750. [Google Scholar] [CrossRef]
| Province | Positivity at Different Ages (Positive/Total Tested (%)) | Totals | |||
|---|---|---|---|---|---|
| 1–10 Days | 11–20 Days | 21–30 Days | >30 Days | ||
| Menia | 0/0 | 1/1 | 1/3 | 0/2 | 2/6 (33.3%) |
| Beni-Suef | 4/17 | 3/4 | 8/16 | 5/10 | 20/47 (42.5%) |
| ^ Fayoum ** | 1/22 | 7/28 | 13/36 | 18/32 | 39/118 (33.1%) |
| Giza *** | 0/10 | 0/9 | 1/11 | 3/5 | 4/35 (11.4%) |
| Behera | 0/1 | 0/1 | 0/1 | 1/1 | 1/4 (25%) |
| Subtotal | 5/50 (10%) | 11/43 (25.6%) | 23/67 (34.3%) | 27/50 (54%) | 66/210 (31.4%) |
| Province | System of Housing (Positive /Total Tested (%)) | Totals | |
|---|---|---|---|
| Deep Litter | Cages | ||
| Menia | 2/6 | 0/0 | 2/6 |
| Beni-Suef | 13/38 | 7/9 | 20/47 (42.5%) |
| Fayoum | 26/90 | 13/28 | 39/118 (33.1%) |
| Giza | 4/35 | 0 | 4/35 (11.4%) |
| Behera | ¼ | 0 | 1/4 (25%) |
| Subtotal | 46/173 (26.6%) | 20/37 (54.1%) | 66/210 (31.4%) |
| Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide identity % | |||||||||||||||||||||
| 1. KT862805-FAdV 2 strain 685 UK | 96.7 | 97.6 | 97.8 | 97.8 | 97.4 | 97.7 | 97.8 | 97.4 | 97.7 | 97.9 | 97.9 | 97.7 | 97.6 | 97.4 | 97.6 | 97.7 | 97.6 | 97.8 | 97.6 | 97.8 | |
| 2. MT975968 FAdV D isolate EG101/2018 | 96.3 | 94.3 | 94.8 | 94.8 | 94.2 | 94.7 | 94.7 | 94.2 | 94.6 | 94.9 | 94.9 | 94.4 | 94.5 | 94.2 | 94.7 | 94.6 | 94.5 | 94.8 | 94.5 | 94.9 | |
| 3. PP993159 FAdV D isolate Gz BSU1 | 96.5 | 95 | 100 | 100 | 99.9 | 99.9 | 99.9 | 99.7 | 100 | 99.9 | 99.9 | 100 | 100 | 99.9 | 100 | 100 | 100 | 100 | 100 | 99.9 | |
| 4. PP993160 FAdV D isolate Gz BSU2 | 96.9 | 95.5 | 99.6 | 100 | 99.9 | 99.9 | 99.9 | 99.6 | 100 | 99.9 | 99.9 | 100 | 100 | 99.9 | 99.9 | 100 | 100 | 100 | 100 | 99.9 | |
| 5. PP993161 FAdV D isolate Gz BSU3 | 96.9 | 95.5 | 99.6 | 99.7 | 99.9 | 99.9 | 99.9 | 99.6 | 100 | 99.9 | 99.9 | 100 | 100 | 99.9 | 99.9 | 100 | 100 | 100 | 100 | 99.9 | |
| 6. PP993162 FAdV D isolate Gz BSU4 | 96.6 | 95 | 99.6 | 99.6 | 99.6 | 99.7 | 99.7 | 99.6 | 99.9 | 99.7 | 99.7 | 99.8 | 99.9 | 99.7 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.7 | |
| 7. PP993163 FAdV D isolate Gz BSU5 | 97.3 | 95.8 | 99.6 | 99.6 | 99.6 | 99.6 | 100 | 99.7 | 99.9 | 100 | 100 | 99.9 | 99.9 | 99.9 | 99.7 | 99.9 | 99.9 | 99.9 | 99.9 | 100 | |
| 8. PP993164 FAdV D isolate Gz BSU6 | 97.4 | 95.9 | 99.6 | 99.6 | 99.6 | 99.6 | 100 | 99.7 | 99.9 | 100 | 100 | 99.9 | 99.9 | 99.7 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 100 | |
| 9. PP993165 FAdV D isolate Gz BSU7 | 96.5 | 94.9 | 99.2 | 98.8 | 98.8 | 99.2 | 99.2 | 99.2 | 99.6 | 99.7 | 99.7 | 99.6 | 99.6 | 99.5 | 99.6 | 99.6 | 99.6 | 99.6 | 99.6 | 99.7 | |
| 10. PP993166 FAdV D isolate Gz BSU8 | 96.7 | 95.2 | 99.6 | 99.6 | 99.6 | 99.6 | 99.6 | 99.6 | 98.4 | 99.9 | 99.9 | 100 | 100 | 99.9 | 99.9 | 100 | 100 | 100 | 100 | 99.9 | |
| 11. PP993167 FAdV D isolate Gz BSU9 | 97.2 | 95.7 | 99.2 | 99.3 | 99.3 | 99.2 | 100 | 100 | 99.2 | 99.3 | 100 | 99.9 | 99.9 | 99.7 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 100 | |
| 12. PP993168 FAdV D isolate Gz BSU10 | 97.2 | 95.8 | 99.2 | 99.3 | 99.3 | 99.2 | 100 | 100 | 99.2 | 99.3 | 99.7 | 99.9 | 99.9 | 99.7 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 100 | |
| 13. PP993169 FAdV D isolate Gz BSU11 | 96.3 | 94.7 | 99.6 | 99.6 | 99.6 | 99.6 | 99.2 | 99.2 | 98.3 | 99.6 | 99.2 | 99.2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.9 | |
| 14. PP993170 FAdV D isolate Gz BSU12 | 96.5 | 94.9 | 99.6 | 99.6 | 99.6 | 99.6 | 99.2 | 99.2 | 98.3 | 99.6 | 99.2 | 99.2 | 99.6 | 100 | 100 | 100 | 100 | 100 | 100 | 99.9 | |
| 15. PP993171 FAdV D isolate Gz BSU13 | 96.2 | 94.7 | 99.2 | 99.3 | 99.3 | 99.2 | 99.6 | 98.9 | 98 | 99.3 | 98.9 | 98.9 | 99.6 | 99.6 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.7 | |
| 16. PP993172 FAdV D isolate Gz BSU14 | 96.5 | 95.1 | 99.6 | 99.3 | 99.3 | 99.6 | 99.2 | 99.6 | 98.8 | 99.3 | 99.3 | 99.3 | 99.6 | 99.6 | 99.3 | 100 | 100 | 99.9 | 100 | 99.9 | |
| 17. PP993173 FAdV D isolate Gz BSU15 | 97.1 | 95.6 | 100 | 100 | 100 | 100 | 99.6 | 99.6 | 98.8 | 99.6 | 99.6 | 99.6 | 99.6 | 99.6 | 99.3 | 100 | 100 | 100 | 100 | 99.9 | |
| 18. PP993174 FAdV D isolate Gz BSU16 | 97 | 95.5 | 100 | 100 | 100 | 100 | 99.6 | 99.6 | 98.8 | 100 | 99.6 | 99.6 | 99.6 | 99.6 | 99.3 | 100 | 100 | 100 | 100 | 99.9 | |
| 19. PP993175 FAdV D isolate Gz BSU17 | 96.8 | 95.4 | 99.6 | 99.7 | 99.7 | 99.6 | 99.6 | 99.6 | 98.8 | 99.6 | 99.3 | 99.3 | 99.6 | 99.6 | 99.3 | 99.3 | 100 | 100 | 100 | 99.9 | |
| 20. PP993176 FAdV D isolate Gz BSU18 | 96.7 | 95.2 | 99.6 | 99.6 | 99.6 | 99.6 | 99.6 | 99.6 | 98.5 | 99.6 | 99.3 | 99.3 | 99.6 | 99.6 | 99.3 | 99.6 | 99.6 | 100 | 99.6 | 99.9 | |
| 21. PP993177 FAdV D isolate Gz BSU19 | 97.1 | 95.7 | 99.2 | 99.3 | 99.3 | 99.2 | 100 | 100 | 99.2 | 99.3 | 99.7 | 99.7 | 99.2 | 99.2 | 98.9 | 99.3 | 99.6 | 99.6 | 99.3 | 99.3 | |
| Amino acid identity % | |||||||||||||||||||||
| Region | Amino Acids Position | Majority | Strains in this Study (No. 19) | Other Egyptian Strains (No. 91) |
|---|---|---|---|---|
| HVR1 (17–81) | 58 | T | -- | I (1/33), -- (N = 58) |
| 59 | R | -- | H (1/34), -- (N = 57) | |
| 60 | N | -- | Y (1/34), -- (N = 57) | |
| 61 | V | V (3/19), -- (N = 16) | I (1/34), L (1/34), -- (N = 57) | |
| 62 | T | T (5/19), S (1/19), -- (N = 13) | A (1 /34), -- (N = 57) | |
| 63 | T | T (11/19), -- (N = 8) | A (1/34), -- (N = 57) | |
| 75 | P | P (16/19), -- (N = 3) | A (3/34), -- (N = 57) | |
| 78 | T | T (17/19), -- (N = 2) | -- (N = 57), H (1/34), N (1/34) | |
| 79 | D | D (17/19), -- (N = 2) | G (1 /34), -- (N = 57) | |
| HVR2 (82–97) | 84 | S | S (18/19), G (1/19) | S (33), -- (N = 58) |
| 92 | N | N (19/19) | D (1/34), H (1/34), -- (N = 57) | |
| HVR3 (113–143) | 113 | D | D (19/19) | G (1/75), -- (N = 16) |
| 114 | R | R (19/19) | G (1/75), S (1/75), -- (N = 16) | |
| 115 | G | G (19/19) | R (1/75), -- (N = 16) | |
| 116 | P | P (19/19) | S (1/75), -- (N = 16) | |
| 117 | S | S (19/19) | F (2/75), -- (N = 16) | |
| 118 | F | F (19/19) | S (1/76), P (1/76), N (1/76), -- (N = 15) | |
| 119 | K | K (19/19) | T (4/78), N (2/78), Q (1/87), -- (N = 13) | |
| 120 | P | P (19/19) | K (1/79), A (1/79), L (1/79), -- (N = 12) | |
| 121 | Y | Y (19/19) | S (1/83), -- (N = 8) | |
| 122 | G | G (19/19) | V (1/83), A (1/83), -- (N = 8) | |
| 123 | G | G (19/19) | E (1/84), -- (N = 7) | |
| 124 | T | T (19/19) | A (1/84), G (1/84), -- (N = 7) | |
| 125 | A | A (19/19) | T (1/90), V (1/90), D (1/90), G (1/90), -- (N = 1) | |
| 126 | Y | Y (19/19) | K (1/90), H (1/90), -- (N = 1) | |
| 135 | F | F (19/19) | L (1/91) | |
| 140 | I | I (19/19) | V (10/91), L (3/91) | |
| 141 | D | D (19/19) | G (1/91), Q (1/91), E (1/91) | |
| 142 | T | T (19/19) | S (2/91), A (1/91) | |
| 143 | G | G (19/19) | E (11/91), D (2/91) | |
| HVR4 (162–167) | 162 | S | S (19/19) | G (7/91), A (2/91) T (1/91) |
| 163 | A | A (19/19) | N (3/91) | |
| 164 | K | K (19/19) | N (2/91), Q (1/91) | |
| 165 | D | D (19/19) | T (3/91) | |
| 166 | K | K (18/19), T (1/19) | N (3/91), E (1/91), T (1/91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellatif, D.M.; El-Sawah, A.A.; Elkady, M.F.; Ali, A.; Abdelaziz, K.; Shany, S.A.S. Widespread Detection of Fowl Adenovirus Serotype 2/11 Species D Among Cases of Inclusion Body Hepatitis–Hydropericardium Syndrome in Chickens in Egypt. Microorganisms 2025, 13, 1107. https://doi.org/10.3390/microorganisms13051107
Abdellatif DM, El-Sawah AA, Elkady MF, Ali A, Abdelaziz K, Shany SAS. Widespread Detection of Fowl Adenovirus Serotype 2/11 Species D Among Cases of Inclusion Body Hepatitis–Hydropericardium Syndrome in Chickens in Egypt. Microorganisms. 2025; 13(5):1107. https://doi.org/10.3390/microorganisms13051107
Chicago/Turabian StyleAbdellatif, Doaa M., Azza A. El-Sawah, Magdy F. Elkady, Ahmed Ali, Khaled Abdelaziz, and Salama A. S. Shany. 2025. "Widespread Detection of Fowl Adenovirus Serotype 2/11 Species D Among Cases of Inclusion Body Hepatitis–Hydropericardium Syndrome in Chickens in Egypt" Microorganisms 13, no. 5: 1107. https://doi.org/10.3390/microorganisms13051107
APA StyleAbdellatif, D. M., El-Sawah, A. A., Elkady, M. F., Ali, A., Abdelaziz, K., & Shany, S. A. S. (2025). Widespread Detection of Fowl Adenovirus Serotype 2/11 Species D Among Cases of Inclusion Body Hepatitis–Hydropericardium Syndrome in Chickens in Egypt. Microorganisms, 13(5), 1107. https://doi.org/10.3390/microorganisms13051107









