Remarks on Life Feasibility on the Red Planet
Abstract
1. Introduction
2. Water on Mars
2.1. Evidence of Liquid Water on Mars
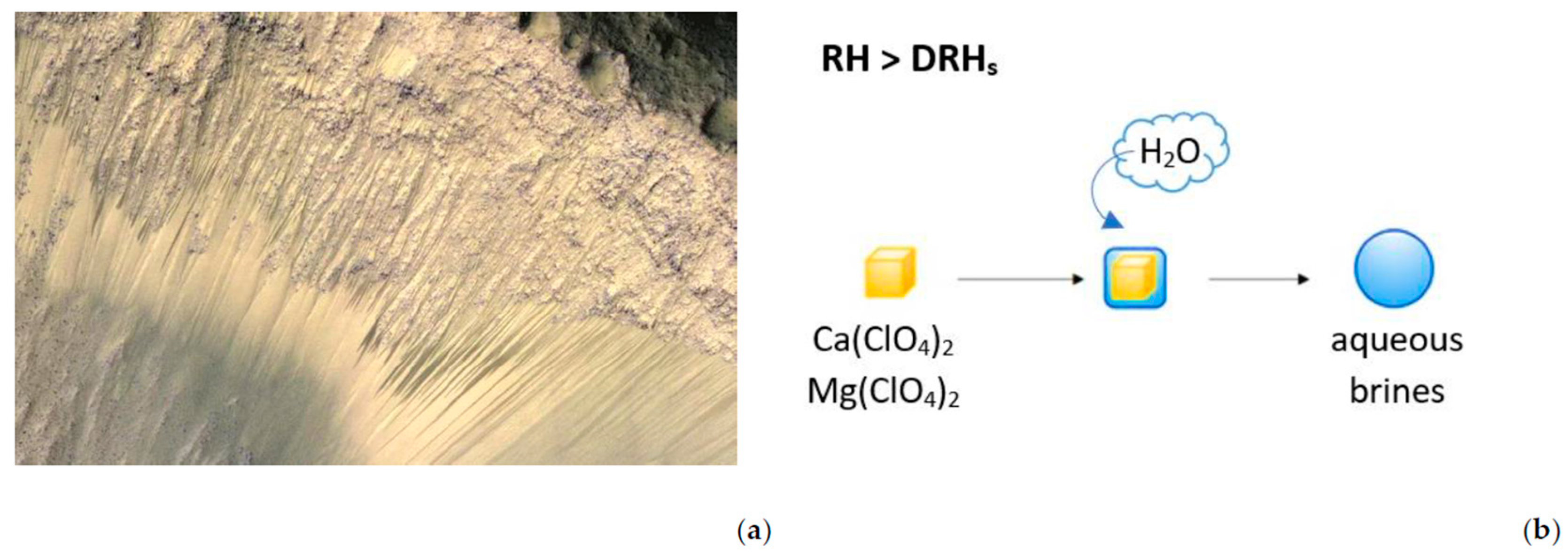
2.2. Martian Brine: Formation and Composition
2.3. Subglacial Liquid Water on Mars
3. Perchlorate Salts
3.1. Detection of Perchlorates on Mars
3.2. Chemical Properties of Perchlorates
4. Perchlorate Salts and Proteins
Effects of Perchlorate Salts on Protein Stability and Protein–Solvent Interactions
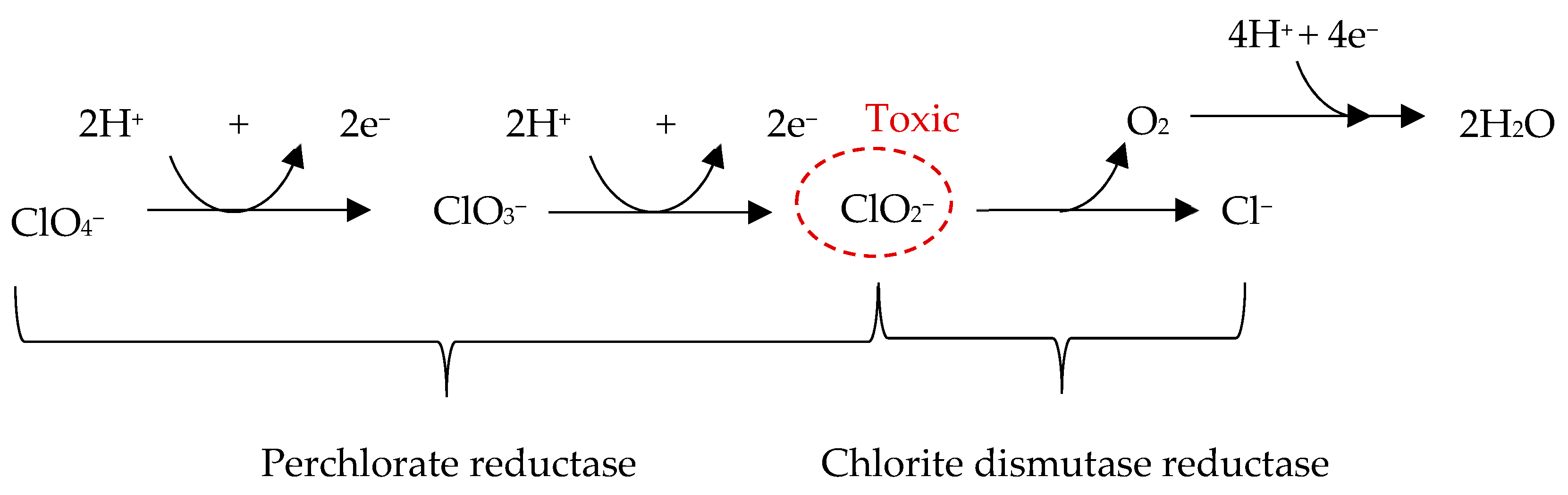
5. Perchlorate Tolerance of Microorganisms
5.1. Evidence and Implications of Perchlorate Resistance of Terrestrial Microorganisms for Putative Life on Mars
5.2. Effects of Perchlorate on Microorganisms Exposed to Low Temperatures
5.3. Kosmotropic Agents Can Protect Microorganisms from Chaotropic Perchlorate Stress
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martínez, G.M.; Newman, C.N.; De Vicente-Retortillo, A.; Fischer, E.; Renno, N.O.; Richardson, M.I.; Fairén, A.G.; Genzer, M.; Guzewich, S.D.; Haberle, R.M.; et al. The Modern Near-Surface Martian Climate: A Review of In-Situ Meteorological Data from Viking to Curiosity. Space Sci. Rev. 2017, 212, 295–338. [Google Scholar] [CrossRef]
- Read, P.L.; Lewis, S.R.; Mulholland, D.P. The Physics of Martian Weather and Climate: A Review. Rep. Prog. Phys. 2015, 78, 125901. [Google Scholar] [CrossRef]
- Antunes, A.; Eder, W.; Fareleira, P.; Santos, H.; Huber, R. Salinisphaera Shabanensis Gen. Nov., Sp. Nov., a Novel, Moderately Halophilic Bacterium from the Brine–Seawater Interface of the Shaban Deep, Red Sea. Extremophiles 2003, 7, 29–34. [Google Scholar] [CrossRef]
- Antunes, A.; França, L.; Rainey, F.A.; Huber, R.; Nobre, M.F.; Edwards, K.J.; Da Costa, M.S. Marinobacter Salsuginis Sp. Nov., Isolated from the Brine–Seawater Interface of the Shaban Deep, Red Sea. Int. J. Syst. Evol. Microbiol. 2007, 57, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Rainey, F.A.; Wanner, G.; Taborda, M.; Pätzold, J.; Nobre, M.F.; da Costa, M.S.; Huber, R. A New Lineage of Halophilic, Wall-Less, Contractile Bacteria from a Brine-Filled Deep of the Red Sea. J. Bacteriol. 2008, 190, 3580. [Google Scholar] [CrossRef]
- Fiala, G.; Woese, C.R.; Langworthy, T.A.; Stetter, K.O. Flexistipes Sinusarabici, a Novel Genus and Species of Eubacteria Occurring in the Atlantis II Deep Brines of the Red Sea. Arch. Microbiol. 1990, 154, 120–126. [Google Scholar] [CrossRef]
- Eder, W.; Schmidt, M.; Koch, M.; Garbe-Schönberg, D.; Huber, R. Prokaryotic Phylogenetic Diversity and Corresponding Geochemical Data of the Brine-Seawater Interface of the Shaban Deep, Red Sea. Environ. Microbiol. 2002, 4, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Alam, I.; Bajic, V.B.; Stingl, U. Genome Sequence of Halorhabdus Tiamatea, the First Archaeon Isolated from a Deep-Sea Anoxic Brine Lake. J. Bacteriol. 2011, 193, 4553–4554. [Google Scholar] [CrossRef]
- Antunes, A.; Alam, I.; Simões, M.F.; Daniels, C.; Ferreira, A.J.S.; Siam, R.; El-Dorry, H.; Bajic, V.B. First Insights into the Viral Communities of the Deep-Sea Anoxic Brines of the Red Sea. Genom. Proteom. Bioinform. 2015, 13, 304–309. [Google Scholar] [CrossRef]
- Carr, M.H. Water on Mars; Oxford University Press: Oxford, NY, USA, 1996; ISBN 978-0-19-509938-6. [Google Scholar]
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F.; et al. Global Mineralogical and Aqueous Mars History Derived from OMEGA/Mars Express Data. Science 2006, 312, 400–404. [Google Scholar] [CrossRef]
- Fairén, A.G.; Fernández-Remolar, D.; Dohm, J.M.; Baker, V.R.; Amils, R. Inhibition of Carbonate Synthesis in Acidic Oceans on Early Mars. Nature 2004, 431, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Fairén, A.G.; Davila, A.F.; Gago-Duport, L.; Amils, R.; McKay, C.P. Stability against Freezing of Aqueous Solutions on Early Mars. Nature 2009, 459, 401–404. [Google Scholar] [CrossRef]
- Squyres, S.W.; Grotzinger, J.P.; Arvidson, R.E.; Bell, J.F.; Calvin, W.; Christensen, P.R.; Clark, B.C.; Crisp, J.A.; Farrand, W.H.; Herkenhoff, K.E.; et al. In Situ Evidence for an Ancient Aqueous Environment at Meridiani Planum, Mars. Science 2004, 306, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.-P.; Poulet, F.; Bishop, J.; Dobrea, E.N.; et al. Hydrated Silicate Minerals on Mars Observed by the Mars Reconnaissance Orbiter CRISM Instrument. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Sharabian, M.; Aghababaei, M.; Karakouzian, M.; Karami, M. Water on Mars—A Literature Review. Galaxies 2020, 8, 40. [Google Scholar] [CrossRef]
- Mars Fact Sheet. Available online: https://nssdc.gsfc.nasa.gov/planetary/factsheet/marsfact.html (accessed on 20 October 2024).
- Mischna, M.A.; Piqueux, S. The Role of Atmospheric Pressure on Mars Surface Properties and Early Mars Climate Modeling. Icarus 2020, 342, 113496. [Google Scholar] [CrossRef]
- Bell, J. (Ed.) The Martian Surface: Composition, Mineralogy and Physical Properties; Cambridge Planetary Science; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-86698-9. [Google Scholar]
- Bibring, J.-P.; Langevin, Y.; Poulet, F.; Gendrin, A.; Gondet, B.; Berthé, M.; Soufflot, A.; Drossart, P.; Combes, M.; Bellucci, G.; et al. Perennial Water Ice Identified in the South Polar Cap of Mars. Nature 2004, 428, 627–630. [Google Scholar] [CrossRef]
- Kieffer, H.H. Mars South Polar Spring and Summer Temperatures: A Residual CO2 Frost. J. Geophys. Res. Solid. Earth 1979, 84, 8263–8288. [Google Scholar] [CrossRef]
- Titus, T.N.; Kieffer, H.H.; Christensen, P.R. Exposed Water Ice Discovered near the South Pole of Mars. Science 2003, 299, 1048–1051. [Google Scholar] [CrossRef]
- Kieffer, H.H.; Titus, T.N. TES Mapping of Mars’ North Seasonal Cap. Icarus 2001, 154, 162–180. [Google Scholar] [CrossRef]
- Fedorova, A.A.; Baklanova, I.V.; Rodin, A.V. Mars Atmospheric Water Vapor in the Southern Hemisphere: MAWD Observations Revisited. Adv. Space Res. 2004, 34, 1677–1682. [Google Scholar] [CrossRef]
- Fouchet, T.; Lellouch, E.; Ignatiev, N.I.; Forget, F.; Titov, D.V.; Tschimmel, M.; Montmessin, F.; Formisano, V.; Giuranna, M.; Maturilli, A.; et al. Martian Water Vapor: Mars Express PFS/LW Observations. Icarus 2007, 190, 32–49. [Google Scholar] [CrossRef]
- Smith, M.D. The Annual Cycle of Water Vapor on Mars as Observed by the Thermal Emission Spectrometer. J. Geophys. Res. Planets 2002, 107, 25-1–25-19. [Google Scholar] [CrossRef]
- McEwen, A.S.; Ojha, L.; Dundas, C.M.; Mattson, S.S.; Byrne, S.; Wray, J.J.; Cull, S.C.; Murchie, S.L.; Thomas, N.; Gulick, V.C. Seasonal Flows on Warm Martian Slopes. Science 2011, 333, 740–743. [Google Scholar] [CrossRef]
- McEwen, A.S.; Dundas, C.M.; Mattson, S.S.; Toigo, A.D.; Ojha, L.; Wray, J.J.; Chojnacki, M.; Byrne, S.; Murchie, S.L.; Thomas, N. Recurring Slope Lineae in Equatorial Regions of Mars. Nat. Geosci. 2014, 7, 53–58. [Google Scholar] [CrossRef]
- Heinz, J.; Schulze-Makuch, D.; Kounaves, S.P. Deliquescence-Induced Wetting and RSL-like Darkening of a Mars Analogue Soil Containing Various Perchlorate and Chloride Salts. Geophys. Res. Lett. 2016, 43, 4880–4884. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Christensen, P.R. Recurring Slope Lineae and Chlorides on the Surface of Mars. J. Geophys. Res. Planets 2016, 121, 1411–1428. [Google Scholar] [CrossRef]
- Zorzano, M.-P.; Mateo-Martí, E.; Prieto-Ballesteros, O.; Osuna, S.; Renno, N. Stability of Liquid Saline Water on Present Day Mars. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Munaretto, G.; Pajola, M.; Cremonese, G.; Re, C.; Lucchetti, A.; Simioni, E.; McEwen, A.S.; Pommerol, A.; Becerra, P.; Conway, S.J.; et al. Implications for the Origin and Evolution of Martian Recurring Slope Lineae at Hale Crater from CaSSIS Observations. Planet. Space Sci. 2020, 187, 104947. [Google Scholar] [CrossRef]
- McEwen, A.S.; Schaefer, E.I.; Dundas, C.M.; Sutton, S.S.; Tamppari, L.K.; Chojnacki, M. Mars: Abundant Recurring Slope Lineae (RSL) Following the Planet-Encircling Dust Event (PEDE) of 2018. J. Geophys. Res. Planets 2021, 126, e2020JE006575. [Google Scholar] [CrossRef]
- Clark, B.C.; Kounaves, S.P. Evidence for the Distribution of Perchlorates on Mars. Int. J. Astrobiol. 2016, 15, 311–318. [Google Scholar] [CrossRef]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Kounaves, S.P.; Carrier, B.L.; O’Neil, G.D.; Stroble, S.T.; Claire, M.W. Evidence of Martian Perchlorate, Chlorate, and Nitrate in Mars Meteorite EETA79001: Implications for Oxidants and Organics. Icarus 2014, 229, 206–213. [Google Scholar] [CrossRef]
- Sutter, B.; McAdam, A.C.; Mahaffy, P.R.; Ming, D.W.; Edgett, K.S.; Rampe, E.B.; Eigenbrode, J.L.; Franz, H.B.; Freissinet, C.; Grotzinger, J.P.; et al. Evolved Gas Analyses of Sedimentary Rocks and Eolian Sediment in Gale Crater, Mars: Results of the Curiosity Rover’s Sample Analysis at Mars Instrument from Yellowknife Bay to the Namib Dune. J. Geophys. Res. Planets 2017, 122, 2574–2609. [Google Scholar] [CrossRef]
- Cull, S.C.; Arvidson, R.E.; Catalano, J.G.; Ming, D.W.; Morris, R.V.; Mellon, M.T.; Lemmon, M. Concentrated Perchlorate at the Mars Phoenix Landing Site: Evidence for Thin Film Liquid Water on Mars. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D., Jr.; Atreya, S.K.; Brinckerhoff, W.B.; et al. Evidence for Perchlorates and the Origin of Chlorinated Hydrocarbons Detected by SAM at the Rocknest Aeolian Deposit in Gale Crater. J. Geophys. Res. Planets 2013, 118, 1955–1973. [Google Scholar] [CrossRef]
- Ming, D.W.; Archer, P.D.; Glavin, D.P.; Eigenbrode, J.L.; Franz, H.B.; Sutter, B.; Brunner, A.E.; Stern, J.C.; Freissinet, C.; McAdam, A.C.; et al. Volatile and Organic Compositions of Sedimentary Rocks in Yellowknife Bay, Gale Crater, Mars. Science 2014, 343, 1245267. [Google Scholar] [CrossRef]
- Sutter, B.; Quinn, R.C.; Archer, P.D.; Glavin, D.P.; Glotch, T.D.; Kounaves, S.P.; Osterloo, M.M.; Rampe, E.B.; Ming, D.W. Measurements of Oxychlorine Species on Mars. Int. J. Astrobiol. 2017, 16, 203–217. [Google Scholar] [CrossRef]
- Meslin, P.-Y.; Forni, O.; Beck, P.; Cousin, A.; Beyssac, O.; Lopez-Reyes, G.; Benzerara, K.; Ollila, A.; Mandon, L.; Wiens, R.C.; et al. Evidence for Perchlorate and Sulfate Salts in Jezero Crater, Mars, from Supercam Observations. In Proceedings of the Lunar and Planetary Science Conference, The Woodlands, TX, USA, 7–11 March 2022; Volume 53, p. 2694. [Google Scholar]
- Corpolongo, A.; Jakubek, R.S.; Burton, A.S.; Brown, A.J.; Yanchilina, A.; Czaja, A.D.; Steele, A.; Wogsland, B.V.; Lee, C.; Flannery, D.; et al. SHERLOC Raman Mineral Class Detections of the Mars 2020 Crater Floor Campaign. J. Geophys. Res. Planets 2023, 128, e2022JE007455. [Google Scholar] [CrossRef]
- Tice, M.M.; Hurowitz, J.A.; Allwood, A.C.; Jones, M.W.M.; Orenstein, B.J.; Davidoff, S.; Wright, A.P.; Pedersen, D.A.K.; Henneke, J.; Tosca, N.J.; et al. Alteration History of Séítah Formation Rocks Inferred by PIXL X-Ray Fluorescence, x-Ray Diffraction, and Multispectral Imaging on Mars. Sci. Adv. 2022, 8, eabp9084. [Google Scholar] [CrossRef]
- Simon, J.I.; Hickman-Lewis, K.; Cohen, B.A.; Mayhew, L.E.; Shuster, D.L.; Debaille, V.; Hausrath, E.M.; Weiss, B.P.; Bosak, T.; Zorzano, M.-P.; et al. Samples Collected From the Floor of Jezero Crater With the Mars 2020 Perseverance Rover. J. Geophys. Res. Planets 2023, 128, e2022JE007474. [Google Scholar] [CrossRef]
- Clark, B.C.; Baird, A.K. Is the Martian Lithosphere Sulfur Rich? J. Geophys. Res. Solid. Earth 1979, 84, 8395–8403. [Google Scholar] [CrossRef]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian Layered Terrains: The OMEGA/Mars Express View. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Massé, M.; Le Mouélic, S.; Bourgeois, O.; Combe, J.-P.; Le Deit, L.; Sotin, C.; Bibring, J.-P.; Gondet, B.; Langevin, Y. Mineralogical Composition, Structure, Morphology, and Geological History of Aram Chaos Crater Fill on Mars Derived from OMEGA Mars Express Data. J. Geophys. Res. Planets 2008, 113. [Google Scholar] [CrossRef]
- Wray, J.J.; Murchie, S.L.; Squyres, S.W.; Seelos, F.P.; Tornabene, L.L. Diverse Aqueous Environments on Ancient Mars Revealed in the Southern Highlands. Geology 2009, 37, 1043–1046. [Google Scholar] [CrossRef]
- Osterloo, M.M.; Hamilton, V.E.; Bandfield, J.L.; Glotch, T.D.; Baldridge, A.M.; Christensen, P.R.; Tornabene, L.L.; Anderson, F.S. Chloride-Bearing Materials in the Southern Highlands of Mars. Science 2008, 319, 1651–1654. [Google Scholar] [CrossRef]
- Ojha, L.; Wilhelm, M.B.; Murchie, S.L.; McEwen, A.S.; Wray, J.J.; Hanley, J.; Massé, M.; Chojnacki, M. Spectral Evidence for Hydrated Salts in Recurring Slope Lineae on Mars. Nat. Geosci. 2015, 8, 829–832. [Google Scholar] [CrossRef]
- Chevrier, V.F.; Hanley, J.; Altheide, T.S. Stability of Perchlorate Hydrates and Their Liquid Solutions at the Phoenix Landing Site, Mars. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Davila, A.F.; Duport, L.G.; Melchiorri, R.; Jänchen, J.; Valea, S.; de los Rios, A.; Fairén, A.G.; Möhlmann, D.; McKay, C.P.; Ascaso, C.; et al. Hygroscopic Salts and the Potential for Life on Mars. Astrobiology 2010, 10, 617–628. [Google Scholar] [CrossRef]
- Connon, S.A.; Lester, E.D.; Shafaat, H.S.; Obenhuber, D.C.; Ponce, A. Bacterial Diversity in Hyperarid Atacama Desert Soils. J. Geophys. Res. Biogeosciences 2007, 112. [Google Scholar] [CrossRef]
- Davila, A.F.; Gómez-Silva, B.; de los Rios, A.; Ascaso, C.; Olivares, H.; McKay, C.P.; Wierzchos, J. Facilitation of Endolithic Microbial Survival in the Hyperarid Core of the Atacama Desert by Mineral Deliquescence. J. Geophys. Res. Biogeosciences 2008, 113. [Google Scholar] [CrossRef]
- Warren-Rhodes, K.A.; Rhodes, K.L.; Pointing, S.B.; Ewing, S.A.; Lacap, D.C.; Gómez-Silva, B.; Amundson, R.; Friedmann, E.I.; McKay, C.P. Hypolithic Cyanobacteria, Dry Limit of Photosynthesis, and Microbial Ecology in the Hyperarid Atacama Desert. Microb. Ecol. 2006, 52, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wierzchos, J.; Ascaso, C.; McKay, C.P. Endolithic Cyanobacteria in Halite Rocks from the Hyperarid Core of the Atacama Desert. Astrobiology 2006, 6, 415–422. [Google Scholar] [CrossRef]
- Orosei, R.; Lauro, S.E.; Pettinelli, E.; Cicchetti, A.; Coradini, M.; Cosciotti, B.; Di Paolo, F.; Flamini, E.; Mattei, E.; Pajola, M.; et al. Radar Evidence of Subglacial Liquid Water on Mars. Science 2018, 361, 490–493. [Google Scholar] [CrossRef]
- Sori, M.M.; Bramson, A.M. Water on Mars, With a Grain of Salt: Local Heat Anomalies Are Required for Basal Melting of Ice at the South Pole Today. Geophys. Res. Lett. 2019, 46, 1222–1231. [Google Scholar] [CrossRef]
- Fisher, D.A.; Hecht, M.H.; Kounaves, S.P.; Catling, D.C. A Perchlorate Brine Lubricated Deformable Bed Facilitating Flow of the North Polar Cap of Mars: Possible Mechanism for Water Table Recharging. J. Geophys. Res. 2010, 115, 2009JE003405. [Google Scholar] [CrossRef]
- Lauro, S.E.; Pettinelli, E.; Caprarelli, G.; Guallini, L.; Rossi, A.P.; Mattei, E.; Cosciotti, B.; Cicchetti, A.; Soldovieri, F.; Cartacci, M.; et al. Multiple Subglacial Water Bodies below the South Pole of Mars Unveiled by New MARSIS Data. Nat. Astron. 2021, 5, 63–70. [Google Scholar] [CrossRef]
- Bowling, J.S.; Livingstone, S.J.; Sole, A.J.; Chu, W. Distribution and Dynamics of Greenland Subglacial Lakes. Nat. Commun. 2019, 10, 2810. [Google Scholar] [CrossRef]
- Fox, D. The Hunt for Life below Antarctic Ice. Nature 2018, 564, 180–182. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Hecht, M.H.; Kapit, J.; Gospodinova, K.; DeFlores, L.; Quinn, R.C.; Boynton, W.V.; Clark, B.C.; Catling, D.C.; Hredzak, P.; et al. Wet Chemistry Experiments on the 2007 Phoenix Mars Scout Lander Mission: Data Analysis and Results. J. Geophys. Res. Planets 2010, 115. [Google Scholar] [CrossRef]
- Scheller, E.L.; Razzell Hollis, J.; Cardarelli, E.L.; Steele, A.; Beegle, L.W.; Bhartia, R.; Conrad, P.; Uckert, K.; Sharma, S.; Ehlmann, B.L.; et al. Aqueous Alteration Processes in Jezero Crater, Mars—Implications for Organic Geochemistry. Science 2022, 378, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, E.A.; Royle, S.H.; Claire, M.W.; Kounaves, S.P.; Sephton, M.A. Indigenous Organic-Oxidized Fluid Interactions in the Tissint Mars Meteorite. Geophys. Res. Lett. 2019, 46, 3090–3098. [Google Scholar] [CrossRef]
- Rzymski, P.; Losiak, A.; Heinz, J.; Szukalska, M.; Florek, E.; Poniedziałek, B.; Kaczmarek, Ł.; Schulze-Makuch, D. Perchlorates on Mars: Occurrence and Implications for Putative Life on the Red Planet. Icarus 2024, 421, 116246. [Google Scholar] [CrossRef]
- Catling, D.C.; Claire, M.W.; Zahnle, K.J.; Quinn, R.C.; Clark, B.C.; Hecht, M.H.; Kounaves, S. Atmospheric Origins of Perchlorate on Mars and in the Atacama. J. Geophys. Res. Planets 2010, 115. [Google Scholar] [CrossRef]
- Steele, A.; Benning, L.G.; Wirth, R.; Siljeström, S.; Fries, M.D.; Hauri, E.; Conrad, P.G.; Rogers, K.; Eigenbrode, J.; Schreiber, A.; et al. Organic Synthesis on Mars by Electrochemical Reduction of CO2. Sci. Adv. 2018, 4, eaat5118. [Google Scholar] [CrossRef]
- Wilson, E.H.; Atreya, S.K.; Kaiser, R.I.; Mahaffy, P.R. Perchlorate Formation on Mars through Surface Radiolysis-Initiated Atmospheric Chemistry: A Potential Mechanism. J. Geophys. Res. Planets 2016, 121, 1472–1487. [Google Scholar] [CrossRef]
- Brown, G.M.; Gu, B. The Chemistry of Perchlorate in the Environment. In Perchlorate: Environmental Occurrence, Interactions and Treatment; Gu, B., Coates, J.D., Eds.; Springer: Boston, MA, USA, 2006; pp. 17–47. ISBN 978-0-387-31113-5. [Google Scholar]
- Marcus, Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- Jungwirth, P.; Cremer, P.S. Beyond Hofmeister. Nat. Chem. 2014, 6, 261–263. [Google Scholar] [CrossRef]
- Ball, P.; Hallsworth, J.E. Water Structure and Chaotropicity: Their Uses, Abuses and Biological Implications. Phys. Chem. Chem. Phys. 2015, 17, 8297–8305. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Asadzadeh, B. Effect of 1-Carboxymethyl-3-Methylimidazolium Chloride, [HOOCMMIM][Cl], Ionic Liquid on Volumetric, Acoustic and Transport Behavior of Aqueous Solutions of l-Serine and l-Threonine at T = 298.15 K. J. Mol. Liq. 2015, 202, 79–85. [Google Scholar] [CrossRef]
- Quinn, R.C.; Martucci, H.F.H.; Miller, S.R.; Bryson, C.E.; Grunthaner, F.J.; Grunthaner, P.J. Perchlorate Radiolysis on Mars and the Origin of Martian Soil Reactivity. Astrobiology 2013, 13, 515–520. [Google Scholar] [CrossRef]
- Góbi, S.; Abplanalp, M.J.; Kaiser, R.I. Effect of perchlorates on electron radiolysis of glycine with application to mars. ApJ 2016, 822, 8. [Google Scholar] [CrossRef]
- Misra, G.; Smith, W.; Garner, M.; Loureiro, R. Potential Biological Remediation Strategies for Removing Perchlorate from Martian Regolith. New Space 2021, 9, 4. [Google Scholar] [CrossRef]
- Coates, J.D.; Michaelidou, U.; Bruce, R.A.; O’Connor, S.M.; Crespi, J.N.; Achenbach, L.A. Ubiquity and Diversity of Dissimilatory (Per)Chlorate-Reducing Bacteria. Appl. Environ. Microbiol. 1999, 65, 5234–5241. [Google Scholar] [CrossRef]
- Vijaya Nadaraja, A.; Gangadharan Puthiya Veetil, P.; Bhaskaran, K. Perchlorate Reduction by an Isolated Serratia Marcescens Strain under High Salt and Extreme pH. FEMS Microbiol. Lett. 2013, 339, 117–121. [Google Scholar] [CrossRef]
- Coates, J.D.; Achenbach, L.A. Microbial Perchlorate Reduction: Rocket-Fueled Metabolism. Nat. Rev. Microbiol. 2004, 2, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Zhang, H.; Mulvaney, P.; Milner, M.G.; Head, I.M.; Unz, R.F. Kinetics of Perchlorate- and Chlorate-Respiring Bacteria. Appl. Environ. Microbiol. 2001, 67, 2499–2506. [Google Scholar] [CrossRef]
- Carlström, C.I.; Wang, O.; Melnyk, R.A.; Bauer, S.; Lee, J.; Engelbrektson, A.; Coates, J.D. Physiological and Genetic Description of Dissimilatory Perchlorate Reduction by the Novel Marine Bacterium Arcobacter Sp. Strain CAB. mBio 2013, 4, 10–1128. [Google Scholar] [CrossRef]
- Carlström, C.I.; Loutey, D.E.; Wang, O.; Engelbrektson, A.; Clark, I.; Lucas, L.N.; Somasekhar, P.Y.; Coates, J.D. Phenotypic and Genotypic Description of Sedimenticola Selenatireducens Strain CUZ, a Marine (per)Chlorate-Respiring Gammaproteobacterium, and Its Close Relative the Chlorate-Respiring Sedimenticola Strain NSS. Appl. Environ. Microbiol. 2015, 81, 2717–2726. [Google Scholar] [CrossRef]
- Graziano, G. On the Molecular Origin of Cold Denaturation of Globular Proteins. Phys. Chem. Chem. Phys. 2010, 12, 14245–14252. [Google Scholar] [CrossRef]
- Graziano, G. On the Mechanism of Cold Denaturation. Phys. Chem. Chem. Phys. 2014, 16, 21755–21767. [Google Scholar] [CrossRef] [PubMed]
- Pica, A.; Graziano, G. Shedding Light on the Extra Thermal Stability of Thermophilic Proteins. Biopolymers 2016, 105, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Graziano, G. Water and Cold Denaturation of Small Globular Proteins. J. Mol. Liq. 2018, 264, 579–584. [Google Scholar] [CrossRef]
- Lee, B.; Richards, F.M. The Interpretation of Protein Structures: Estimation of Static Accessibility. J. Mol. Biol. 1971, 55, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Spassov, V.Z.; Karshikoff, A.D.; Ladenstein, R. The Optimization of Protein-Solvent Interactions: Thermostability and the Role of Hydrophobic and Electrostatic Interactions. Protein Sci. 1995, 4, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Royer, C.A. Revisiting Volume Changes in Pressure-Induced Protein Unfolding. Biochim. Biophys. Acta 2002, 1595, 201–209. [Google Scholar] [CrossRef]
- Chalikian, T.V. Volumetric Properties of Proteins. Annu. Rev. Biophys. 2003, 32, 207–235. [Google Scholar] [CrossRef]
- Graziano, G. The Gibbs Energy Cost of Cavity Creation Depends on Geometry. J. Mol. Liq. 2015, 211, 1047–1051. [Google Scholar] [CrossRef]
- Wallqvist, A.; Berne, B.J. Molecular Dynamics Study of the Dependence of Water Solvation Free Energy on Solute Curvature and Surface Area. J. Phys. Chem. 1995, 99, 2885–2892. [Google Scholar] [CrossRef]
- Patel, A.J.; Varilly, P.; Chandler, D.; Garde, S. Quantifying Density Fluctuations in Volumes of All Shapes and Sizes Using Indirect Umbrella Sampling. J. Stat. Phys. 2011, 145, 265–275. [Google Scholar] [CrossRef]
- Sosso, G.C.; Caravati, S.; Rotskoff, G.; Vaikuntanathan, S.; Hassanali, A. On the Role of Nonspherical Cavities in Short Length-Scale Density Fluctuations in Water. J. Phys. Chem. A 2017, 121, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Reiss, H. Scaled Particle Methods in the Statistical Thermodynamics of Fluids. In Advances in Chemical Physics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1–84. ISBN 978-0-470-14355-1. [Google Scholar]
- Kell, G.S. Density, Thermal Expansivity, and Compressibility of Liquid Water from 0.Deg. to 150.Deg. Correlations and Tables for Atmospheric Pressure and Saturation Reviewed and Expressed on 1968 Temperature Scale. J. Chem. Eng. Data 1975, 20, 97–105. [Google Scholar] [CrossRef]
- Novotny, P.; Sohnel, O. Densities of Binary Aqueous Solutions of 306 Inorganic Substances. J. Chem. Eng. Data 1988, 33, 49–55. [Google Scholar] [CrossRef]
- Lenton, S.; Rhys, N.H.; Towey, J.J.; Soper, A.K.; Dougan, L. Highly Compressed Water Structure Observed in a Perchlorate Aqueous Solution. Nat. Commun. 2017, 8, 919. [Google Scholar] [CrossRef]
- Kaulgud, M.V.; Pokale, W.K. Measurement of the Temperature of Maximum Density of Aqueous Solutions of Some Salts and Acids. J. Chem. Soc. Faraday Trans. 1995, 91, 999–1004. [Google Scholar] [CrossRef]
- Troncoso, J.; González-Salgado, D. The Temperature of Maximum Density for Aqueous Solutions. J. Chem. Phys. 2024, 160, 100902. [Google Scholar] [CrossRef]
- Graziano, G. Water: Cavity Size Distribution and Hydrogen Bonds. Chem. Phys. Lett. 2004, 396, 226–231. [Google Scholar] [CrossRef]
- Sorenson, J.M.; Hura, G.; Glaeser, R.M.; Head-Gordon, T. What Can X-Ray Scattering Tell Us about the Radial Distribution Functions of Water? J. Chem. Phys. 2000, 113, 9149–9161. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic Radii in Aqueous Solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Cozzolino, S.; Oliva, R.; Graziano, G.; Vecchio, P.D. Counteraction of Denaturant-Induced Protein Unfolding Is a General Property of Stabilizing Agents. Phys. Chem. Chem. Phys. 2018, 20, 29389–29398. [Google Scholar] [CrossRef]
- Gault, S.; Cockell, C.S. Perchlorate Salts Exert a Dominant, Deleterious Effect on the Structure, Stability, and Activity of α-Chymotrypsin. Astrobiology 2021, 21, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gault, S.; Jaworek, M.W.; Winter, R.; Cockell, C.S. Perchlorate Salts Confer Psychrophilic Characteristics in α-Chymotrypsin. Sci. Rep. 2021, 11, 16523. [Google Scholar] [CrossRef]
- Pica, A.; Graziano, G. On the Effect of Sodium Salts on the Coil-to-Globule Transition of Poly(N-Isopropylacrylamide). Phys. Chem. Chem. Phys. 2015, 17, 27750–27757. [Google Scholar] [CrossRef]
- Pica, A.; Graziano, G. Effect of Sodium Thiocyanate and Sodium Perchlorate on Poly(N-Isopropylacrylamide) Collapse. Phys. Chem. Chem. Phys. 2020, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Paladino, A.; Balasco, N.; Vitagliano, L.; Graziano, G. A Structure-Based Mechanism for the Denaturing Action of Urea, Guanidinium Ion and Thiocyanate Ion. Biology 2022, 11, 1764. [Google Scholar] [CrossRef]
- Gregory, K.P.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Page, A.J. The Electrostatic Origins of Specific Ion Effects: Quantifying the Hofmeister Series for Anions. Chem. Sci. 2021, 12, 15007–15015. [Google Scholar] [CrossRef]
- Gregory, K.P.; Elliott, G.R.; Robertson, H.; Kumar, A.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Andersson, G.G.; Page, A.J. Understanding Specific Ion Effects and the Hofmeister Series. Phys. Chem. Chem. Phys. 2022, 24, 12682–12718. [Google Scholar] [CrossRef]
- Paladino, A.; Balasco, N.; Graziano, G.; Vitagliano, L. A Protein Data Bank Survey of Multimodal Binding of Thiocyanate to Proteins: Evidence for Thiocyanate Promiscuity. Int. J. Biol. Macromol. 2022, 208, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mykytczuk, N.C.S.; Wilhelm, R.C.; Whyte, L.G. Planococcus Halocryophilus Sp. Nov., an Extreme Sub-Zero Species from High Arctic Permafrost. Int. J. Syst. Evol. Microbiol. 2012, 62, 1937–1944. [Google Scholar] [CrossRef]
- Heinz, J.; Waajen, A.C.; Airo, A.; Alibrandi, A.; Schirmack, J.; Schulze-Makuch, D. Bacterial Growth in Chloride and Perchlorate Brines: Halotolerances and Salt Stress Responses of Planococcus Halocryophilus. Astrobiology 2019, 19, 1377–1387. [Google Scholar] [CrossRef]
- Gries, A.; Heinz, J.; Schulze-Makuch, D. Perchlorate Stress Responses of Haloferax Volcanii and Implications on the Habitability of Mars. In Proceedings of the Europlanet Science Congress 2022, Granada, Spain, 18–23 September 2022. [Google Scholar]
- Laye, V.J.; DasSarma, S. An Antarctic Extreme Halophile and Its Polyextremophilic Enzyme: Effects of Perchlorate Salts. Astrobiology 2018, 18, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Heinz, J.; Krahn, T.; Schulze-Makuch, D. A New Record for Microbial Perchlorate Tolerance: Fungal Growth in NaClO4 Brines and Its Implications for Putative Life on Mars. Life 2020, 10, 53. [Google Scholar] [CrossRef]
- Heinz, J.; Rambags, V.; Schulze-Makuch, D. Physicochemical Parameters Limiting Growth of Debaryomyces Hansenii in Solutions of Hygroscopic Compounds and Their Effects on the Habitability of Martian Brines. Life 2021, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Breuer, U.; Harms, H. Debaryomyces Hansenii—An Extremophilic Yeast with Biotechnological Potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Heim, S.; Timmis, K.N. Chaotropic Solutes Cause Water Stress in Pseudomonas Putida. Environ. Microbiol. 2003, 5, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Heinz, J.; Doellinger, J.; Maus, D.; Schneider, A.; Lasch, P.; Grossart, H.-P.; Schulze-Makuch, D. Perchlorate-Specific Proteomic Stress Responses of Debaryomyces Hansenii Could Enable Microbial Survival in Martian Brines. Environ. Microbiol. 2022, 24, 5051–5065. [Google Scholar] [CrossRef]
- Zancan, P.; Sola-Penna, M. Trehalose and Glycerol Stabilize and Renature Yeast Inorganic Pyrophosphatase Inactivated by Very High Temperatures. Arch. Biochem. Biophys. 2005, 444, 52–60. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Prior, B.A.; Nomura, Y.; Iwahara, M.; Timmis, K.N. Compatible Solutes Protect against Chaotrope(Ethanol)-Induced, Nonosmotic WaterStress. Appl. Environ. Microbiol. 2003, 69, 7032–7034. [Google Scholar] [CrossRef]
- Chin, J.P.; Megaw, J.; Magill, C.L.; Nowotarski, K.; Williams, J.P.; Bhaganna, P.; Linton, M.; Patterson, M.F.; Underwood, G.J.C.; Mswaka, A.Y.; et al. Solutes Determine the Temperature Windows for Microbial Survival and Growth. Proc. Natl. Acad. Sci. USA 2010, 107, 7835. [Google Scholar] [CrossRef]
- Gilichinsky, D.; Rivkina, E.; Shcherbakova, V.; Laurinavichuis, K.; Tiedje, J. Supercooled Water Brines within Permafrost-an Unknown Ecological Niche for Microorganisms: A Model for Astrobiology. Astrobiology 2003, 3, 331–341. [Google Scholar] [CrossRef]
- Debenedetti, P.G. Metastable Liquids: Concepts and Principles; Princeton University Press: Princeton, NJ, USA, 1996; Volume 1, ISBN 978-0-691-08595-1. [Google Scholar]
- Clarke, A.; Morris, G.J.; Fonseca, F.; Murray, B.J.; Acton, E.; Price, H.C. A Low Temperature Limit for Life on Earth. PLoS ONE 2013, 8, e66207. [Google Scholar] [CrossRef] [PubMed]
- Gault, S.; Fonseca, F.; Cockell, C.S. Preservation of Bacillus Subtilis’ Cellular Liquid State at Deep Sub-Zero Temperatures in Perchlorate Brines. Commun. Biol. 2024, 7, 588. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.H.; Cockell, C.S. A Systematic Study of the Limits of Life in Mixed Ion Solutions: Physicochemical Parameters Do Not Predict Habitability. Front. Microbiol. 2020, 11, 1478. [Google Scholar] [CrossRef]
- Heinz, J.; Schirmack, J.; Airo, A.; Kounaves, S.P.; Schulze-Makuch, D. Enhanced Microbial Survivability in Subzero Brines. Astrobiology 2018, 18, 1171. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; La Cono, V.; Spada, G.L.; Bortoluzzi, G.; Messina, E.; Smedile, F.; Arcadi, E.; Borghini, M.; Ferrer, M.; Schmitt-Kopplin, P.; et al. Microbial Community of the Deep-Sea Brine Lake Kryos Seawater-Brine Interface Is Active below the Chaotropicity Limit of Life as Revealed by Recovery of mRNA. Environ. Microbiol. 2015, 17, 364–382. [Google Scholar] [CrossRef]
- Carré, L.; Gonzalez, D.; Girard, É.; Franzetti, B. Effects of Chaotropic Salts on Global Proteome Stability in Halophilic Archaea: Implications for Life Signatures on Mars. Environ. Microbiol. 2023, 25, 2216–2230. [Google Scholar] [CrossRef]
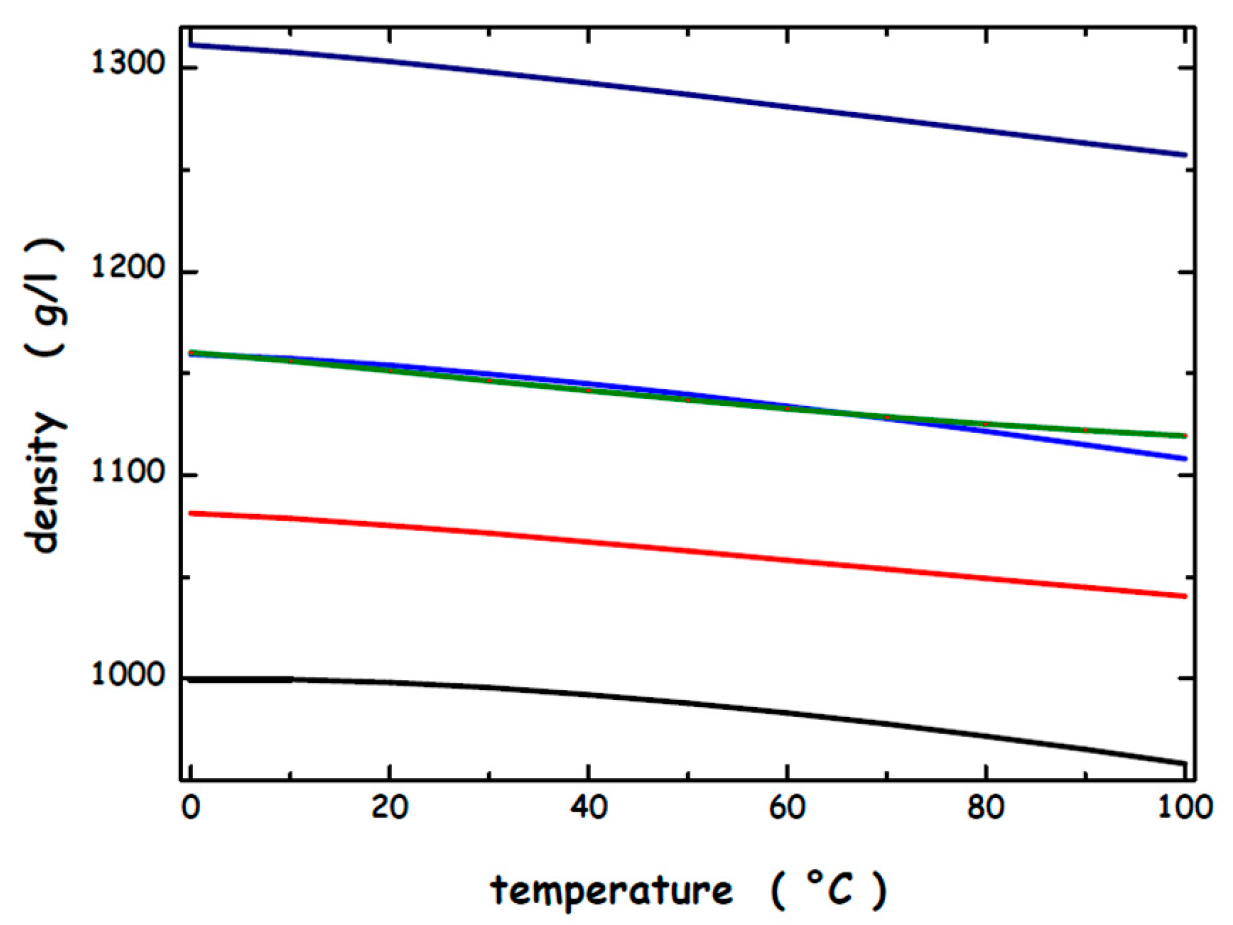

| [H2O] M | ξ3 | ΔΔGc kJ mol−1 | ΔGd kJ mol−1 | T·ΔSconf kJ mol−1 | ΔEa kJ mol−1 | |
|---|---|---|---|---|---|---|
| H2O | 55.4 | 0.383 | 792.2 | 23.6 | 768.6 | 0 |
| 1 M NaClO4 | 52.9 | 0.404 | 844.5 | 10 | 768.6 | −65.9 |
| 2 M NaClO4 | 50.3 | 0.423 | 900.0 | 5 | 768.6 | −126.4 |
| 1 M Mg(ClO4)2 | 51.7 | 0.428 | 924.3 | −10 | 768.6 | −165.7 |
| 2 M Mg(ClO4)2 | 47.6 | 0.471 | 1075.2 | −30 | 768.6 | −276.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, F.; Graziano, G. Remarks on Life Feasibility on the Red Planet. Microorganisms 2025, 13, 1105. https://doi.org/10.3390/microorganisms13051105
Mancini F, Graziano G. Remarks on Life Feasibility on the Red Planet. Microorganisms. 2025; 13(5):1105. https://doi.org/10.3390/microorganisms13051105
Chicago/Turabian StyleMancini, Fiorella, and Giuseppe Graziano. 2025. "Remarks on Life Feasibility on the Red Planet" Microorganisms 13, no. 5: 1105. https://doi.org/10.3390/microorganisms13051105
APA StyleMancini, F., & Graziano, G. (2025). Remarks on Life Feasibility on the Red Planet. Microorganisms, 13(5), 1105. https://doi.org/10.3390/microorganisms13051105







