Abstract
Streptococcus pyogenes meningitis is a rare invasive disease, accounting for less than 2% of bacterial meningitis. We presented two case reports and conducted a systematic review using PUBMED, covering the database from its inception up to 31 December 2024, of pediatric cases of Streptococcus pyogenes meningitis. Only case reports and case series were included. Differences in clinical and laboratory parameters were compared between uneventful course and complicated admissions. A total of 57 cases were included. The median age at diagnosis was 4 years. A primary infection focus outside the brain was identified in 61.39% of cases. S. pyogenes was identified from cerebrospinal fluid in 66.66% of cases and from blood in 15.79%. Septic shock occurred in 24.56% of cases, and 36.84% had brain anatomical anomalies. All patients received broad-spectrum empiric antibiotics, while protein-synthesis inhibitors were administered in 26.31% of cases. A total of 17% of patients died, and 28.07% experienced sequelae. The identification of S. pyogenes from blood and a Phoenix Sepsis Score ≥ 2 were significantly associated with a complicated clinical course. Our findings may offer useful insights for the clinical management of Streptococcus pyogenes meningitis.
1. Introduction
Group A streptococcus (GAS), also known as Streptococcus pyogenes, is a Gram-positive bacterium and one of the leading human-restricted pathogens affecting children worldwide [1]. This pathogen can cause both non-invasive disease—pharyngitis, scarlet fever, impetigo—as well as life-threatening invasive diseases [1,2,3]. The most common invasive GAS (iGAS) infections are toxic shock syndrome and necrotizing fasciitis [1,2,3]. The main virulence factors of S. pyogenes are the M protein and secreted pyogenic exotoxins (SPE). The M protein is the pivotal virulence factor, functioning as an epithelial adhesion factor and inhibiting bacterial phagocytosis. It is encoded by the emm gene, with more than 180 M protein variants detected to date. In iGAS infections, an association with emm 1, emm 3, SPE A, and SPE B has been described [1,2,3,4]. S. pyogenes meningitis is a rare invasive disease, accounting for less than 2% of iGAS cases [5]. Since 2022, an increase in iGAS infections has been reported in many European countries, with a subsequent rise in GAS meningitis cases [6]. We present two case reports and a systematic review to:
- -
- highlight the key clinical features, common complications, and outcomes of GAS meningitis;
- -
- identify factors associated with a complicated course of disease.
2. Case Reports
Case 1:
A fully immunized 4 years and 8 months old girl presented to our Pediatric Emergency Department (ED) with fever, left earache within the previous 48 h, and incoercible vomiting over the preceding 24 h. Her past medical history was unremarkable. On physical examination, she was alert and reactive but appeared unwell with meningeal signs. Vital parameters showed normal blood pressure 108/83 mmHg, mild tachycardia 105 bpm, and mild tachypnea 40/min. Otoscopy showed left acute otitis media (AOM), while the rest of the examination was normal. Therapy with ceftriaxone and acyclovir was initiated, and blood tests, a head CT, and a lumbar puncture were performed (Table 1). Blood tests revealed neutrophilic leukocytosis and elevated C-reactive protein (CRP). Cerebrospinal fluid (CSF) analysis was compatible with bacterial meningitis (Table 1), and the head CT revealed left mastoiditis with a hypodense fluid collection (maximum thickness: 5 mm), involving the temporal and occipital lobes. Blood and CSF cultures were collected, and a rapid multiplex PCR panel for viruses and bacteria on CSF was negative. Seven hours later, the patient developed focal seizures and worsening neurological status. On examination, she was unresponsive, presenting with anisocoria, right fixed mydriasis, decerebrate posturing, and a positive Babinski sign. The patient was intubated, and an emergent head CT revealed cerebral edema. Dexamethasone and mannitol were administered, and an urgent decompressive craniotomy was performed. Vital parameters showed hypotension (80/50 mmHg), tachycardia 170, and tachypnea 40/min. The CSF culture became positive for Streptococcus pyogenes after 10 h, and therapy was adjusted with the addition of linezolid, while acyclovir was discontinued. Despite those interventions, the patient developed hypotensive shock, and an electroencephalogram (EEG) showed the absence of electrical activity. The patient was declared brain dead 48 h after admission.

Table 1.
Clinical and laboratory features of the two case reports.
Case 2:
A fully immunized 5 year and 9 months old girl presented to a peripheral ED with a 48 h history of fever and lethargy. On examination, she exhibited meningeal signs. Therapy with ceftriaxone and vancomycin was initiated, and the girl was transferred to our pediatric intensive care unit. Upon arrival, she was lethargic with meningeal signs, but no evidence of localized infection was detected. Vital parameters showed normal blood pressure 105/70 mmHg, tachycardia 112 bpm, and mild tachypnea 42/min. Blood tests revealed neutrophilic leukocytosis and elevated CRP. Blood and CSF cultures were collected. CSF analysis was consistent with bacterial meningitis (Table 1). An initial head CT showed a fluid collection (maximum thickness: 4 mm) in the right frontal and parietal regions. BIOFIRE® FILMARRAY® Meningitis/Encephalitis Panel for viruses and bacteria on CSF was negative. After consultation with microbiologists, given the strong suspicion of bacterial meningitis, the Biofire Blood Culture Identification (BCID) Panel designed for blood—which includes a larger number of bacterial targets—was performed on the CSF, yielding a positive result for Streptococcus pyogenes. Therapy with dexamethasone was initiated and therapy with immunoglobulin for 3 days at 400 mg/kg was implemented, while vancomycin was discontinued. After five days, the patient was afebrile but continued to exhibit meningeal signs and developed left hemiparesis; BP was stable 98/75 mmHg, and at lower limit the heart rate was 60 bpm. An EEG revealed asymmetrical brain activity, and a brain MRI showed an enlargement of the fluid collection, now involving the entire right hemisphere with a thickness of 6 mm. Antibiotic treatment was remodulated with addition of linezolid, and an urgent decompressive craniotomy was performed. From that moment, the patient showed a slow but progressive improvement. Physiotherapy was started, and after 10 days, the neurological examination was normal. Audiometry performed prior to discharge was also normal. The patient was discharged home after 21 days in good clinical condition, continuing a tapering of steroid therapy at home. Two years after the event, she remains in good health, with no neurological sequelae.
3. Materials and Methods
A systematic review following the PRISMA 2020 statement was conducted from the inception of the database up to 31 December 2024, using PUBMED. The following keywords were used: Streptococcus pyogenes meningitis, Group A Streptococcus meningitis, and invasive Streptococcus pyogenes disease. The inclusion criteria were as follows: a diagnosis of bacterial meningitis based on CSF features and clinical conditions, along with culture or PCR (from either CSF or blood) positive for S. pyogenes, and patients aged < 18 years. Only case reports and case series were included. Cases from all selected studies were evaluated and analyzed. Study selection followed the PRISMA flow diagram (Figure 1). Two researchers independently assessed the eligibility of the studies. Data extraction from individual studies was performed in duplicate. Any discrepancies in data extraction were discussed and resolved among the authors.
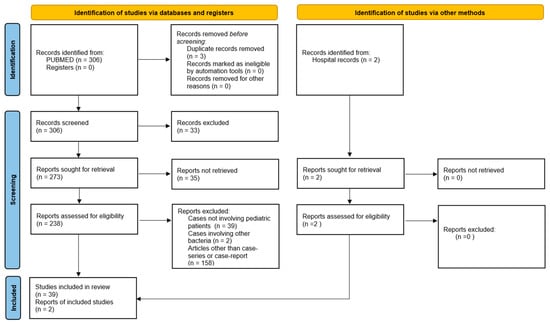
Figure 1.
Prima flowchart.
A total of 306 manuscripts were initially identified. After reviewing titles and abstracts and eliminating duplicates, 238 full-text articles were assessed. Ultimately, 39 papers were selected and included in the review. We analyzed each of the retained studies, as well as our two case reports, to create our own database. The following variables were collected: age, sex, medical history (both past and present), prior S. pyogenes localized infections, laboratory results, CT/MRI findings, treatment regimens, and clinical outcomes. In each study, all categories and variables were analyzed. Data were entered into a dedicated database. Categorical data are presented as frequencies and percentages, while continuous data are expressed as means/medians with ranges, depending on the statistical distribution.
Patients were classified into two groups: those with a uneventful meningitis course, defined as patients who did not experience septic shock, neurological deterioration, or brain involvement on CT/MRI, and those with a complicated course, defined as patients who developed septic shock, neurological instability, brain involvement on CT/MRI, sequelae (including nerve palsy, deafness, neurological impairment), or death. The following variables were compared between the two groups: age, female sex, focus of infection outside the brain, CSF white blood cell (WBC) count, CSF protein levels, CSF glucose levels, WBC blood count, identification of S. pyogenes on blood, Phoenix Sepsis Score ≥ 2 [7], use of antibiotics with activity on protein synthesis inhibition, dexamethasone therapy, and length of hospital stay. A univariate analysis was initially performed to assess the associations between each independent variable and the outcome. The Chi-square (χ2) test was used for nominal variables, with a significance threshold set at 0.05. For independent numerical variables following a normal distribution, comparisons between the two groups were made using a one-tailed t-test, with a significance level of 0.05. Subsequently, a multivariate analysis using logistic regression was conducted, adjusted for age and sex, accounting for potential confounding factors. Odds ratios (OR) were calculated, along with 95% confidence intervals (CI), to assess the strength of the associations. All statistical analyses were performed using Jamovi version 2.6.44.
4. Results
Here, we report the results of a systematic review of the literature and the description of two cases of S. pyogenes meningitis. In total, 39 papers reporting a total of 55 cases were included in the analysis, and we also added 2 cases admitted to our hospital for a total of 57 cases (Figure 1) [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Results are summarized in Table 2 and Figure 2.

Table 2.
Characteristics of the two patients admitted to our hospital and of the patients derived from the systematic review.
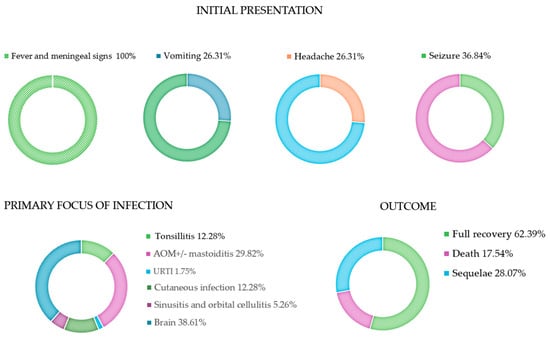
Figure 2.
Initial presentation, primary focus of infection and clinical outcome. AOM: acute otitis media; URTI: upper respiratory tract infections.
The median age at diagnosis was 4 years (range: 1 day–15 years); 54.38% (31/57) were female. Seven percent (4/57) of the cases reported previous predisposing conditions for bacterial meningitis, such as medical devices, HIV infection, and prematurity [10,15,17,28]. A primary focus of infection outside the brain was identified in 61.39% (35/57) of cases, with otitis and skin infections being the most common. Six patients (10.52%) had received a course of S. pyogenes sensitive antibiotics for at least 48 h before admission, including azithromycin, amoxicillin, and cephalexin [14,17,32,37,38,40]. All patients presented with fever and meningeal signs, including altered mental status. Most of them also had vomiting, headache, and seizures. The diagnosis was confirmed by CSF features (turbid CSF, low glucose, high protein, high white blood cells) in all cases. GAS was identified from CSF in 66.66% (38/57) of cases, and in three of those cases, only by PCR. In 15.79% (9/57), S. pyogenes was isolated from blood, while in 17.55% (10/57), S. pyogenes was detected from both CSF and blood. In nine cases, S. pyogenes was detected in other tissues, including ear cultures in patients with otitis, skin vesicles in patients with skin infections, and tonsillar exudates in patients with tonsillitis. In one case, S. pyogenes was detected on the cervix of the newborn’s mother [45].
Forty-seven percent (27/57) of patients were admitted to the pediatric intensive care unit (PICU). Forty-five percent (26/57) had a Phenix sepsis score > 2. The clinical course was uneventful in 38.59% (22/57). However, 24.56% (14/57) experienced septic shock requiring inotropic support, 33.33% (19/57) entered a coma, 21.05% (12/57) experienced status epilepticus, 8.7% (5/57) had cranial nerve impairments, and 36.84% (24/57) had brain anatomical anomalies detected by cerebral imaging.
A cerebral CT was performed during admission or later in 56.14% (32/57) of cases, with negative results in 25% (8/32) of patients. Brain involvement was detected in 75% (24/32), including 12 cases with an intracranial fluid collection (extra-axial collection n = 8, intra-axial collection n = 4), 7 cases with vascular involvement, 12 cases of brain edema, 5 cases of brain tissue necrosis, 3 cases of ventriculomegaly, 4 cases of cerebritis/ventriculitis, and 1 case of myelitis [9,12,13,14,16,17,18,19,20,23,24,25,26,27,30,32,35,36,37,39,42,46]. All patients received a broad-spectrum empiric antibiotic therapy with third-generation cephalosporins alone in 66.66% (38/57) of cases. Seventeen percent (10/57) received third-generation cephalosporins plus a glycopeptide, mainly vancomycin. The remaining cases received different antibiotic regimens, including meropenem alone or in combination with linezolid, third-generation cephalosporins plus clindamycin, or cephalosporins/penicillin combined with rifampicin or linezolid. In 50.87% (29/57) of cases, therapy was targeted after GAS identification. In 31.67% (18/57) of cases, therapy was de-escalated to intravenous penicillin based on susceptibility testing. Other regimens included the addition of clindamycin, rifampicin, or linezolid to cephalosporins/penicillin or the substitution of the previous therapy with meropenem. Overall, protein-synthesis inhibitors (clindamycin, linezolid, or rifampicin) were used in 26.31% (15/57) of cases. The median length of therapy was 18 days (range: 10–42 days, n = 36). Dexamethasone with or without mannitol was used in 33.33% (19/57) of cases [9,12,17,23,25,27,30,31,32,33,35,36,39]. Other treatments included anticonvulsant therapy for patients with seizures, heparin for those with thrombosis, and supportive care for patients in critical conditions. Immunoglobulins were used in three patients (3.50%) [30,36].
Surgical procedures were performed in 13 patients (22.80%). In particular, three patients had a liquor derivation, two underwent myringotomy/tympanostomy, two had mastoidectomy, two required abscess drainage, two had craniectomy, one had both mastectomy and abscess drainage, and one patient had a combination of mastoidectomy, myringotomy, and craniectomy [9,11,12,16,17,27,32,35,37,42,46].
Seventeen percent (10/57) of patients died, all within the first 48 h of hospitalization, while 28.07% (16/57) had sequelae, including hearing loss, nerve palsy, and varying degrees of neurological impairment defined according to Pediatric Cerebral Performance Category [9,10,11,12,13,14,16,17,19,20,27,28,33,34,35,36,37,39,41,42,43,46]. Sixty-two percent (31/57) had a complete recovery. Table 3 shows the univariate analysis of the comparisons between patients with an uneventful course versus those with a complicated course. Age, sex, presence of predisposing conditions, the primary non-invasive S. pyogenes focus, use of dexamethasone, and antibiotics that interfere with protein synthesis were not statistically significantly different between the comparison groups in the statistical analysis. The presence of S. pyogenes on blood and a Phoenix Sepsis Score ≥ 2 were significantly associated with a complicated course. Regarding laboratory exams, patients with a complicated course had significantly higher levels of WBC and proteins in the CSF. CSF glucose and blood WBC were not statistically different between the groups. Table 4 reports the logistic regression analysis conducted to identify factors associated with a complicated course adjusted for age and sex: the presence of S. pyogenes on blood was significantly associated with a complicated course (OR: 6.101, p = 0.01), indicating a more than 6-fold increase in the likelihood of a complicated course in patients with GAS bacteremia. Similarly, a Phoenix Sepsis Score ≥ 2 was strongly associated with a complicated course (OR: 68.570, p < 0.001), suggesting that this subset of patients have an higher risk of sequelae and/or death.

Table 3.
Comparison between patients with uneventful and complicated course of disease.

Table 4.
Logistic regression between patients with uneventful and complicated course of disease adjusted for age and sex.
5. Discussion
Bacterial meningitis is a life-threatening condition that causes high morbidity and mortality in children. The most common pathogens involved are Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B [47]. GAS is responsible for fewer than 2% of bacterial meningitis [5].
Since 2022, the WHO has reported an increase in iGAS infections in at least five European countries, particularly in the age group from 0 to 5 years old [6,48,49,50,51,52,53]. In our tertiary pediatric hospital, between April 2023 and July 2024, we recorded 2 cases of GAS meningitis out of 34 cases of iGAS infections.
S. pyogenes meningitis exhibits peculiar features. According to our study and previous reports, in half of the patients, the primary focus of infection was not the brain, with otitis and skin infections being the most common sources of bacterial spread. Meningitis may occur through brain invasion via the hematogenous route or by direct spread through continuity. The hematogenous route could be responsible for dissemination from the skin and the nasopharyngeal mucosa, while direct spread seems to be involved in GAS infections of the ear and mastoid bone [5,9,16,23,54].
The main S. pyogenes virulence factors involved in adhesion and bacterial dissemination are the M protein, Streptolysin O, streptococcal DNases, and the IL-8 protease SpyCEP. So far, studies have failed to demonstrate differential expression of these factors in patients with GAS meningitis compared with those with otitis or oropharyngeal colonization. Therefore, host characteristics may represent the key discriminant in this process [55].
To better define the pathogenesis and risk factors for local infection spreading, more studies are advocated [54,55].
Based on our findings and those of previous studies, we recommend some specific diagnostic and therapeutic steps in cases of S. pyogenes meningitis.
The initial approach is the same for all bacterial meningitis. In case of suspicion of meningitis, a thorough examination is mandatory, especially to recognize meningeal signs and any source of peripheral infection [47,56,57,58]. It is crucial to collect blood samples and cultures, as well as perform lumbar puncture for the CSF analysis and culture. Broad-spectrum antibiotics should be initiated as soon as cultures are collected; however, the execution of head CT/MRI, blood tests, and lumbar puncture should not delay the administration of antibiotics [47,56,57,58]. If possible, we recommend using molecular techniques, especially in patients who received antibiotics before blood and CSF collection [59]. The most common rapid multiplex PCR panel does not include GAS, but in cases of high suspicion—such as concomitant tonsillitis, otitis, or skin impetigo—a more comprehensive panel should be considered [16]. At our center, when rapid multiplex PCR results on CSF are negative and bacterial meningitis is highly suspected, a more comprehensive panel designed for blood testing on cerebrospinal fluid is performed. This approach has also been suggested in other studies [60,61].
Empiric therapy typically involves ceftriaxone plus vancomycin, depending on local resistance patterns [47,56,57,58]. Once S. pyogenes is isolated, the approach should be personalized for each case and involve a multidisciplinary team, including pediatricians, intensivists, neurosurgeons, and infectious disease specialists. The antibiotic regimen should be targeted based on the antibiogram. Penicillin G is usually the antibiotic of choice [56]. S. pyogenes meningitis has been associated with specific M types and exotoxin production, particularly SPEA, SPEB, and SPEC. Based on these virulence features, there is a growing trend of using protein-synthesis inhibitors (such as linezolid, rifampicin, and clindamycin) in combination with penicillin or cephalosporins [4,16,20,35,62,63]. In both our patients, we decided to add linezolid, an oxazolidinone that inhibits bacterial protein synthesis by binding to the bacterial 23S ribosomial RNA of the 50S subunit, based on its good cerebral penetration, more favorable pharmacokinetic properties compared to vancomycin, and a theoretical activity against the production of pyrogenic exotoxins A and B [64]. In our study, 26% of patients were treated with this approach. Clindamycin has been reported to have poor CSF penetration, and therefore its role in the management of CNS infections is debated [65,66]. Nowadays, no comparative studies have been conducted comparing beta-lactam monotherapy with beta-lactam plus protein-synthesis inhibitors in S. pyogenes meningitis, and further research is needed to define the most effective strategy [67,68].
Dexamethasone may play a positive role in reducing brain edema and the inflammatory cascade. The results of its use in Streptococcus pneumoniae meningitis encourage its administration in S. pyogenes meningitis as well. In a 2015 Cochrane meta-analysis and systematic review regarding corticosteroid use for acute bacterial meningitis, the use of steroids in Streptococcus pneumoniae meningitis was proven to be protective against death but it had no significant beneficial effect on hearing loss sequelae [69]. In our analysis, steroid use was not statistically different between patients with an uneventful course and those with a complicated course [35,70,71,72]. Of note, our results cannot define the efficacy of steroids in this setting, primary because of the small sample size and secondly because in many cases, steroids were introduced only after the evidence of brain edema at the MRI, rather than immediately after the detection of the bacteria. Additionally, the efficacy demonstrated in Streptococcus pneumoniae is not enough to advise its use in S. pyogenes meningitis. Even if they belong to the same genus (Streptococcus), they are distinct species with different pathogenic profiles. Based on the current literature, GAS meningitis exhibits a higher rate of brain anomalies on brain CT/MRI (17% vs. 36.84%), death (13% vs. 17.54%), and sequelae (23% vs. 28.07%) compared to S. pneumoniae meningitis [41,42]. Our results align with previous studies [5,9,29,35,38,72,73,74].
We recommend continuous monitoring of the patient during the first 48–72 h, particularly for those with elevated WBC or protein levels in the CSF, as well as those with GAS bacteremia or signs of sepsis, as these factors have been associated with a complicated course, sequelae, or death in our study. Fatal complications occurred in 17% of our cohort, all of them within the first 48 h; thus an intensive setting should be considered at the time of admission. In the event of worsening of the clinical condition or lack of improvement, we recommend performing additional tests based on the clinical presentation, including imaging, as cerebral parenchyma involvement or vascular alterations are common in S. pyogenes meningitis [4,16,20,35,45,73,74]. Surgical intervention should be considered, especially in cases of brain anomalies (e.g., hydrocephalus or fluid collections) or when otitis or mastoiditis is the source of infection. In cases of thrombosis, the use of heparin and high doses of steroids has been proposed [12,37].
Looking to the future, some considerations need to be done in terms of possible concerns in the management of S. pyogenes meningitis. In the last three years, studies in the Netherlands have reported a concerning increase not only in iGAS infections but also specifically in GAS meningitis. Moreover, while molecular studies before 2022 showed that approximately 35% of isolated strains belonged to the emm1 subtype, after 2022, nearly 90% of cases were associated with the emm1 subtype, and of these, 80% were identified as the toxigenic M1uk variant, known to be associated with more severe clinical courses [75].
Interestingly, the current concern is not limited to the emergence of S. pyogenes strains with additional virulence factors but also includes the rise in antibiotic resistance. Penicillin remains the antibiotic of choice; however, over the last 20 years, there has been a troubling trend of reduced penicillin susceptibility, not yet a case of resistance [76]. Several mechanisms have been proposed for this phenomenon: (1) intracellular persistence of S. pyogenes within tonsillar tissues, where poor antibiotic penetration reduces treatment efficacy, and (2) beta-lactamase-mediated inactivation, where co-infecting bacteria produce extracellular beta-lactamase that degrades penicillin in the absence of an inhibitor. One of the latest discoveries as mechanisms of resistance involves mutations in the pbp2x gene, which encodes a key enzyme in peptidoglycan synthesis [76,77]. These mutations have been linked to reduced susceptibility to penicillins and cephalosporins and are associated with multiple emm types exhibiting high mortality rates. Even more concerning is the growing resistance to macrolides. Over the past decade, the incidence of macrolide resistance has increased dramatically, reaching rates as high as 20–40%. The mechanisms behind this resistance typically involves the methylation of the 23S rRNA target by erm (erythromycin ribosomal methylase) genes (ermA and ermB) [76,77,78].
Finally, despite global advances, a reliable vaccination is still not yet available, but a number of vaccine candidates are in early human trials [79].
The limitations of our study include, primarily, the small sample size, mainly due to the rarity of the disease. Another significant limitation is that the data were derived from retrospective studies, each focusing on different aspects of the disease. As a result, some data may have been omitted by the original authors based on the focus they chose for their paper. Additionally, it is important to consider that severe cases may be overrepresented, as they might have been more likely to be published compared to milder cases, introducing a significant bias. Furthermore, the length of follow-up varied across case reports which could have influenced the reporting of sequelae.
6. Conclusions
Our study reviewed the management of S. pyogenes meningitis. No guidelines or unique approaches to this invasive infection are available yet. Our study may help clinicians quickly identify high-risk patients with S. pyogenes meningitis who require more intensive monitoring during hospitalization to reduce morbidity and mortality associated with this infection. Further studies are needed to enhance knowledge for the management of iGAS infections.
Author Contributions
Conceptualization M.D.L., L.C. and M.P.; methodology M.D.L., L.R., L.C., L.L., R.B. and P.B.; formal analysis L.D.M. and S.M.; investigation A.M.M. and L.L.; resources, A.M.M., M.P., V.C. and G.V.; data curation, M.P. and S.M.; writing—original draft preparation, L.D.M., M.D.L. and L.R.; writing—review and editing, M.D.L., L.C., P.B., L.L. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health with Current Research funds.
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Bambino Gesù Pediatric Hospital code Prot. n. 201; Pratica n° 3596/2025 and date of approval 8 May 2025).” for studies involving humans.
Informed Consent Statement
Informed consent statement was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author. The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
All the authors acknowledge ChatGPT version 4o-mini (OpenAI) for helping improve the clarity and accuracy of the English language in this article. The AI was not used for content generation or any other purpose.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GAS | Group A streptococcus |
| iGAS | Invasive GAS |
| SPE | Secreted pyogenic exotoxins |
| ED | Emergency Department |
| AOM | Acute Otitis Media |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| WBC | White blood cell |
| OR | Odds Ratio |
| PICU | Pediatric Intensive Care Unit |
References
- Brouwer, S.; Rivera-Hernandez, T.; Curren, B.F.; Harbison-Price, N.; De Oliveira, D.M.P.; Jespersen, M.G.; Davies, M.R.; Walker, M.J. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023, 21, 431–447. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group a streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Waddington, C.S.; Snelling, T.L.; Carapetis, J.R. Management of invasive group A streptococcal infections. J. Infect. 2014, 69 (Suppl. S1), S63–S69. [Google Scholar] [CrossRef] [PubMed]
- Link-Gelles, R.; Toews, K.-A.; Schaffner, W.; Edwards, K.M.; Wright, C.; Beall, B.; Barnes, B.; Jewell, B.; Harrison, L.H.; Kirley, P.D.; et al. Characteristics of Intracranial Group A Streptococcal Infections in US Children, 1997–2014. J. Pediatr. Infect. Dis. Soc. 2020, 9, 30–35. [Google Scholar] [CrossRef]
- World Health Organization Disease Outbreak News; Increased Incidence of Scarlet Fever and Invasive Group A Streptococcus Infection—Multi-Country. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429 (accessed on 15 December 2022).
- Schlapbach, L.J.; Watson, R.S.; Sorce, L.R.; Argent, A.C.; Menon, K.; Hall, M.W.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665–674. [Google Scholar] [CrossRef]
- Abuhammour, W.; Hasan, R.A.; Unuvar, E. Group A beta-hemolytic streptococcal bacteremia. Indian J. Pediatr. 2004, 71, 915–919. [Google Scholar] [CrossRef]
- Arnoni, M.V.; Berezin, E.N.; Sáfadi, M.A.; Almeida, F.J.; Lopes, C.R. Streptococcus pyogenes meningitis in children: Report of two cases and literature review. Braz. J. Infect. Dis. 2007, 11, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Givner, L.B. Invasive disease due to group A beta-hemolytic streptococci: Continued occurrence in children in North Carolina. South. Med. J. 1998, 91, 333–337. [Google Scholar] [CrossRef]
- Harnden, A.; Lennon, D. Serious suppurative group A streptococcal infections in previously well children. Pediatr. Infect. Dis. J. 1988, 7, 714–718. [Google Scholar] [CrossRef]
- Hummel, B.A.; Blackburn, J.; Pham-Huy, A.; Muir, K. High-dose steroid and heparin: A novel therapy for cerebral vasculitis associated with presumed group A Streptococcus meningitis. BMJ Case Rep. 2021, 14, e239618. [Google Scholar] [CrossRef]
- Lee, J.; Blackburn, J.; Pham-Huy, A. Uncommon clinical presentation of a common bug: Group A Streptococcus meningitis. Paediatr. Child Health 2020, 26, e129–e131. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.N.; Griffith, J.A.; Carvajal, H.F. Localized meningoencephalitis and group A streptococcal bacteremia. Clin. Pediatr. 1992, 31, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S.; Patel, C.C.; Buck, G. Meningitis caused by toxigenic group a beta-hemolytic streptococcus in a pediatric patient with acquired immunodeficiency syndrome. Pediatr. Infect. Dis. J. 1991, 10, 339–340. [Google Scholar] [CrossRef]
- Paul, S.P.; Jerwood, S. Group A streptococcal septicemia, meningitis and cerebral abscess: Case report and literature review. Turk. J. Pediatr. 2012, 54, 180–183. [Google Scholar] [PubMed]
- Pettersen, G.; Ovetchkine, P.; Tapiero, B. Group a streptococcal meningitis in a pediatric patient following cochlear implantation: Report of the first case and review of the literature. J. Clin. Microbiol. 2005, 43, 5816–5818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruvinsky, R.O.; Schindler, Y.; Urman, G.; Lopera, L.C.; Carrano, J.; Paolillo, A.L.; Grosman, A. Meningitis por Streptococcus pyogenes: Informe de un caso pediátrico. [Streptococcus pyogenes meningitis: A pediatric case report]. Arch. Argent. Pediatr. 2020, 118, e309–e312. [Google Scholar] [CrossRef]
- Rezvani, M.; Yager, J.Y.; Hartfield, D.S. Group A streptococcal meningitis as a complication of an infected capillary haemangioma. Eur. J. Pediatr. 2004, 163, 19–21. [Google Scholar] [CrossRef]
- Shetty, A.K.; Frankel, L.R.; Maldonado, Y.; Falco, D.A.; Lewis, D.B. Group A streptococcal meningitis: Report of a case and review of literature since 1976. Pediatr. Emerg. Care 2001, 17, 430–434. [Google Scholar] [CrossRef]
- van Zitteren, L.M.; Arents, N.L.; Halbertsma, F. Group-A-streptococcal meningitis in a 7-year-old child—A rare pathogen in a non-immune compromised patient. BMJ Case Rep. 2011, 2011, bcr1020114896corr1. [Google Scholar] [CrossRef]
- Walsh, M.; Chodock, R.; Quinn, C.; Peglow, S. Group A beta-hemolytic streptococcal meningitis associated with uncomplicated varicella. Am. J. Emerg. Med. 1994, 12, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.; Herdeg, S.; Gordjani, N.; Brandis, M. Streptococcus pyogenes meningitis: Report of a case and review of the literature. Eur. J. Pediatr. 2000, 159, 527–529. [Google Scholar] [CrossRef]
- González, E.J.; Gutiérrez, P.B.; Villán, E.A.; Aguado, I.C. Meningitis y Streptococcus pyogenes: Un cruce de caminos poco frecuente [Meningitis and Streptococcus pyogenes: A rare cross-roads]. An. Pediatr. 2013, 80, 65–66. [Google Scholar] [CrossRef]
- Brandt, C.M.; Kitz, R.; Lütticken, R.; Brade, V. Streptococcus pyogenes meningitis complicating varicella in a 3-month-old child. Scand. J. Infect. Dis. 2003, 35, 876–878. [Google Scholar] [CrossRef]
- Núñez Ramiro, A.G.; Adell Sales, A.; Calderón Fernández, R.J.; Frasquet, J.; Pérez Tamarit, A. Meningitis bacteriana aguda por Streptococcus pyogenes [Acute bacterial meningitis caused by Streptococcus pyogenes]. An. Pediatr. 2013, 78, 135. [Google Scholar] [CrossRef]
- Steppberger, K.; Adams, I.; Deutscher, J.; Müller, H.; Kiess, W. Meningitis in a girl with recurrent otitis media caused by Streptococcus pyogenes—Otitis media has to be treated appropriately. Infection 2001, 29, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Jouhadi, Z.; Sadiki, H.; Lehlimi, M.; Honsali, Z.; Najib, J.; Zerouali, K.; Belabess, H.; Mdaghri, N. Meningites à streptocoque du groupe A. Med. Mal. Infect. 2012, 42, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Perera, N.; Abulhoul, L.; Green, M.; Swann, R. Group A streptococcal meningitis: Case report and review of the literature. J. Infect. 2005, 51, E1–E4. [Google Scholar] [CrossRef]
- Moses, A.; Beeri, M.; Engelhard, D. Group A streptococcal meningitis: Report of two cases. J. Infect. 1998, 36, 116–118. [Google Scholar] [CrossRef]
- Busetti, M.; Marchetti, F.; Croci, E.; L’Erario, I.; Creti, R.; D’Agaro, P. Group A streptococcal meningitis: A case report. New Microbiol. 2013, 36, 419–422. [Google Scholar]
- Fanella, S.; Embree, J. Group A Streptococcal Meningitis in a Pediatric Patient. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.C.; Pickering, L.K.; Baker, C.J. Group A streptococcal meningitis without predisposing factors. South. Med. J. 1981, 74, 1029–1030. [Google Scholar] [CrossRef]
- Jevon, G.P.; Dunne, W.M.; Hawkins, H.K.; Armstrong, D.L.; Musser, J.M. Fatal group a streptococcal meningitis and toxic shock-like syndrome: Case report. Clin. Infect. Dis. 1994, 18, 91–93. [Google Scholar] [CrossRef]
- Bruun, T.; Kittang, B.R.; Mylvaganam, H.; Lund-Johansen, M.; Skrede, S. Clinical, microbiological and molecular characteristics of six cases of group A streptococcal meningitis in western Norway. Scand. J. Infect. Dis. 2010, 42, 665–671. [Google Scholar] [CrossRef]
- González, L.A.; Peñaranda, N.A.D.; Fernández, J.M.R.; Alonso, J.M.C. Post-infective transverse myelitis following Streptococcus pyogenes meningitis. An. Pediatr. 2024, 101, 224–225. [Google Scholar] [CrossRef]
- Hutton, D.; Kameda-Smith, M.; Afshari, F.T.; Elawadly, A.; Hogg, F.; Mehta, S.; Samarasekara, J.; Aquilina, K.; Jeelani, N.U.O.; Tahir, M.Z.; et al. Intracranial invasive group A Streptococcus: A neurosurgical emergency in children. J. Neurosurg. Pediatr. 2023, 32, 478–487. [Google Scholar] [CrossRef]
- Torres, L.; Rodrigues, A.M.; Francisco, C.; Santos, S.; Carvalho, P. Streptococcus pyogenes Meningitis in a Pediatric Patient: Case Report. Acta Medica Port. 2024, 37, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Krebs, V.L.J.; Chieffi, L.N.; Ceccon, M.E.J.R.; Diniz, E.M.D.A.; Feferbaum, R.; Takeuchi, C.A.; Marques-Dias, M.J.; Carneiro, J.D.A.; Vaz, F.A.C. Meningite neonatal por Streptococcus pyogenes e trombose de seio sagital: Relato de caso. [Neonatal Streptococcus pyogenes meningitis and sagittal sinus thrombosis: Case report]. Arq. Neuro Psiquiatr. 1998, 56, 829–832. [Google Scholar] [CrossRef]
- Bacalhau, S.; Zarcos, M.M.; Rezende, T. Meningite bacteriana. Uma etiologia pouco frequente [Bacterial meningitis. A rare cause]. Acta Med. Port. 2011, 24 (Suppl. S3), 627–630. [Google Scholar]
- Gupta, V.; Jain, S. Meningitis with bilateral acute suppurative otitis media caused by Group A Streptococcus. Indian Pediatr. 2005, 42, 79–80. [Google Scholar]
- Hmami, F.; Oulmaati, A.; Mahmoud, M.; Boubou, M.; Tizniti, S.; Bouharrou, A. Méningite néonatale à streptocoque A et thrombose porte: Une association fortuite ? [Neonatal group A streptococcal meningitis and portal vein thrombosis: A casual association? Arch. Pediatr. 2014, 21, 1020–1023. [Google Scholar] [CrossRef]
- Gertner, M.; Rodriguez, L.; Barnett, S.H.; Shah, K. Group A beta-hemolytic Streptococcus and Waterhouse-Friderichsen syndrome. Pediatr. Infect. Dis. J. 1992, 11, 595–596. [Google Scholar] [CrossRef]
- Nutman, J.; Henig, E.; Wilunsky, E.; Reisner, S.H. Acute necrotising fasciitis due to streptococcal infection in a newborn infant. Arch. Dis. Child. 1979, 54, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Yagupsky, P.; Giladi, Y. Group A beta-hemolytic streptococcal septicemia complicating infected hemangioma in children. Pediatr. Dermatol. 1987, 4, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.-Z.; Li, W.; Hu, H.-L.; Guo, X.; Hu, B.; Chen, T.-M.; Chen, H.-Y.; Guo, L.-Y.; Liu, G. Group A Streptococcal meningitis in children: A short case series and systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Acute bacterial meningitis in infants and children. Lancet Infect. Dis. 2010, 10, 32–42. [Google Scholar] [CrossRef]
- de Gier, B.; Marchal, N.; de Beer-Schuurman, I.; Wierik, M.T.; Hooiveld, M.; ISIS-AR Study Group; GAS Study group; de Melker, H.E.; van Sorge, N.M. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in The Netherlands, 2022. Euro. Surveill. 2023, 28, 2200941. [Google Scholar] [CrossRef]
- Guy, R.; Henderson, K.L.; Coelho, J.; Hughes, H.; Mason, E.L.; Gerver, S.M.; Demirjian, A.; Watson, C.; Sharp, A.; Brown, C.S.; et al. Increase in invasive group A streptococcal infection notifications, England, 2022. Euro. Surveill. 2023, 28, 2200942. [Google Scholar] [CrossRef]
- Lassoued, Y.; Assad, Z.; Ouldali, N.; Caseris, M.; Mariani, P.; Birgy, A.; Bonacorsi, S.; Bidet, P.; Faye, A. Unexpected Increase in Invasive Group A Streptococcal Infections in Children After Respiratory Viruses Outbreak in France: A 15-Year Time-Series Analysis. Open Forum. Infect. Dis. 2023, 10, ofad188. [Google Scholar] [CrossRef]
- Cobo-Vázquez, E.; Aguilera-Alonso, D.; Carrasco-Colom, J.; Calvo, C.; Saavedra-Lozano, J.; Mellado, I.; Grandioso, D.; Rincón, E.; Jové, A.; Cercenado, E.; et al. Increasing incidence and severity of invasive Group A streptococcal disease in Spanish children in 2019–2022. Lancet Reg. Health Eur. 2023, 27, 100597. [Google Scholar] [CrossRef]
- van Kempen, E.B.; Bruijning-Verhagen, P.C.J.; Borensztajn, D.M.; Vermont, C.L.; Quaak, M.S.W.; Janson, J.-A.; Maat, I.; Stol, K.; Vlaminckx, B.J.M.; Wieringa, J.W.; et al. Increase in Invasive Group a Streptococcal Infections in Children in the Netherlands, A Survey Among 7 Hospitals in 2022. Pediatr. Infect. Dis. J. 2023, 42, e122–e124. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Ficari, A.; Romani, L.; De Luca, M.; Tripiciano, C.; Chiurchiù, S.; Carducci, F.I.C.; Cursi, L.; Di Giuseppe, M.; Krzysztofiak, A.; et al. The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy. Children 2024, 11, 383. [Google Scholar] [CrossRef]
- Aznar, S.L.; Martinez, C.V.; Fonz, R.B.; Lobera, I.B.; Alonso, M.B. Mastoiditis aguda con complicación intracraneal. Reporte de un caso pediátrico. Arch. Argent. Pediatr. 2020, 118, e166–e169. [Google Scholar] [CrossRef]
- Marquardt, L.; Andreoni, F.; Boumasmoud, M.; Schweizer, T.A.; Heuberger, D.M.; Parietti, E.; Hertegonne, S.; Epprecht, J.; Mattle, D.; Raez, A.K.; et al. Group A Streptococcus strains causing meningitis without distinct invasive phenotype. Microbiologyopen 2024, 13, e1394. [Google Scholar] [CrossRef]
- de Almeida Torres, R.S.; Fedalto, L.E.; Steer, A.C.; Smeesters, P.R. Group A Streptococcus meningitis in Children. Pediatr. Infect. Dis. J. 2013, 32, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; McGill, F.; Solomon, T. Management of acute meningitis. Clin. Med. 2018, 18, 164–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alamarat, Z.; Hasbun, R. Management of Acute Bacterial Meningitis in Children. Infect. Drug Resist. 2020, 13, 4077–4089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trujillo-Gómez, J.; Tsokani, S.; Arango-Ferreira, C.; Atehortúa-Muñoz, S.; Jimenez-Villegas, M.J.; Serrano-Tabares, C.; Veroniki, A.-A.; Florez, I.D. Biofire FilmArray Meningitis/Encephalitis panel for the aetiological diagnosis of central nervous system infections: A systematic review and diagnostic test accuracy meta-analysis. eClinicalMedicine 2022, 44, 101275. [Google Scholar] [CrossRef]
- López-Amor, L.; García-Prieto, E.; Fernández-Suárez, J.; Escudero, D.; Vázquez, F.; Fernández, J. Evaluation of a commercial multiplex PCR for diagnosis of central nervous system (CNS) nosocomial infections. J. Microbiol. Methods 2020, 171, 105865. [Google Scholar] [CrossRef]
- Leitner, E.; Hoenigl, M.; Wagner, B.; Krause, R.; Feierl, G.; Grisold, A.J. Performance of the FilmArray Blood culture identification panel in positive blood culture bottles and cerebrospinal fluid for the diagnosis of sepsis and meningitis. GMS Infect. Dis. 2016, 4, Doc06. [Google Scholar] [CrossRef]
- Rezahosseini, O.; Roed, C.; Holler, J.G.; Frimodt-Møller, N.; Harboe, Z.B. Adjunctive antibiotic therapy with clindamycin or linezolid in patients with group A streptococcus (GAS) meningitis. Infect. Dis. 2023, 55, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, H.; Medina, L.M.P.; Morgan, M.; Moll, K.; Skutlaberg, D.H.; Skrede, S.; Wajima, T.; Svensson, M.; Norrby-Teglund, A. Adjunctive Rifampicin Increases Antibiotic Efficacy in Group A Streptococcal Tissue Infection Models. Antimicrob. Agents Chemother. 2021, 65, e0065821. [Google Scholar] [CrossRef]
- Cascone, A.; De Luca, M.; Simeoli, R.; Goffredo, B.M.; Cursi, L.; Tripiciano, C.; Romani, L.; Mercadante, S.; Di Giuseppe, M.; Carducci, F.I.C.; et al. Therapeutic Drug Monitoring-Guided Linezolid Therapy for the Treatment of Multiple Staphylococcal Brain Abscesses in a 3-Month-Old Infant. Pathogens 2024, 14, 4. [Google Scholar] [CrossRef]
- Meesters, K.; Alemayehu, T.; Benou, S.; Buonsenso, D.; Decloedt, E.H.; Lorente, V.P.-F.; Downes, K.J.; Allegaert, K. Pharmacokinetics of Antimicrobials in Children with Emphasis on Challenges Faced by Low and Middle Income Countries, a Clinical Review. Antibiotics 2022, 12, 17. [Google Scholar] [CrossRef]
- Sullins, A.K.; Abdel-Rahman, S.M. Pharmacokinetics of Antibacterial Agents in the CSF of Children and Adolescents. Pediatr. Drugs 2013, 15, 93–117. [Google Scholar] [CrossRef]
- Coyle, E.A. Targeting Bacterial Virulence: The role of protein synthesis inhibitors in severe infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, F.; Zürcher, C.; Tarnutzer, A.; Schilcher, K.; Neff, A.; Keller, N.; Maggio, E.M.; Poyart, C.; Schuepbach, R.A.; Zinkernagel, A.S. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J. Infect. Dis. 2016, 215, 269–277. [Google Scholar] [CrossRef][Green Version]
- Brouwer, M.C.; McIntyre, P.; Prasad, K.; van de Beek, D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 2015, 2018, CD004405. [Google Scholar] [CrossRef]
- Schaad, U.; Wedgwood, J.; Lips, U.; Gnehm, H.; Heinzer, I.; Blumberg, A. Dexamethasone therapy for bacterial meningitis in children. Lancet 1993, 342, 457–461. [Google Scholar] [CrossRef]
- Girgis, N.I.; Farid, Z.; Mikhail, I.A.; Farrag, I.; Sultan, Y.; Kilpatrick, M.E. Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr. Infect. Dis. J. 1989, 8, 848–851. [Google Scholar] [CrossRef]
- McIntyre, P.B.; MacIntyre, C.R.; Gilmour, R.; Wang, H. A population based study of the impact of corticosteroid therapy and delayed diagnosis on the outcome of childhood pneumococcal meningitis. Arch. Dis. Child. 2005, 90, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, P.; Fusco, U.; Attanasio, V.; Rossi, M.; Pantosti, A.; Conte, M.; Faella, F.S. Pneumococcal meningitis in childhood: A longitudinal prospective study. FEMS Immunol. Med. Microbiol. 2007, 51, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Pelton, S.I.; Yogev, R. Improving the outcome of pneumococcal meningitis. Arch. Dis. Child. 2005, 90, 333–334. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, B.C.L.; Vlaminckx, B.J.M.; de Gier, B.; Graaf, W.F.-D.; van Sorge, N.M. Group A Streptococcal Meningitis With the M1UK Variant in the Netherlands. JAMA 2023, 329, 1791–1792. [Google Scholar] [CrossRef]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Rubio-López, V.; Valdezate, S.; Álvarez, D.; Villalón, P.; Medina, M.J.; Salcedo, C.; Sáez-Nieto, J.-A. Molecular epidemiology, antimicrobial susceptibilities and resistance mechanisms of Streptococcus pyogenes isolates resistant to erythromycin and tetracycline in Spain (1994–2006). BMC Microbiol. 2012, 12, 215. [Google Scholar] [CrossRef]
- Berbel, D.; González-Díaz, A.; de Egea, G.L.; Càmara, J.; Ardanuy, C. An Overview of Macrolide Resistance in Streptococci: Prevalence, Mobile Elements and Dynamics. Microorganisms 2022, 10, 2316. [Google Scholar] [CrossRef]
- Castro, S.A.; Dorfmueller, H.C. Update on the development of Group A Streptococcus vaccines. npj Vaccines 2023, 8, 135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).