Survival of Filamentous Cyanobacteria Through Martian ISRU: Combined Effects of Desiccation and UV-B Radiation
Abstract
1. Introduction
2. Materials and Methods
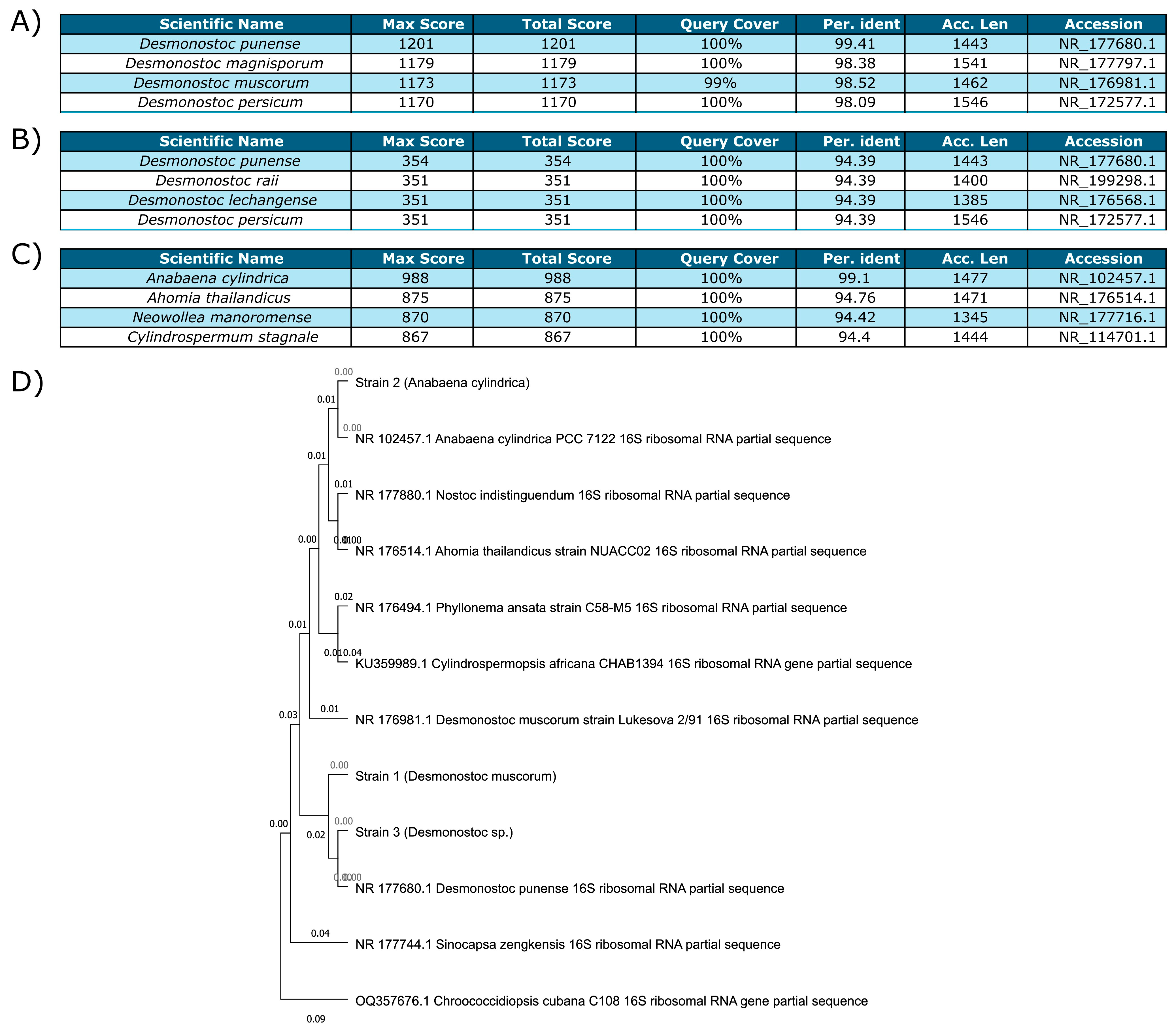
2.1. Cyanobacteria Culturing and Identification
2.2. Measurement of Cyanobacterial Fluorescence
2.3. Martian Regolith Simulants
2.4. Oxidative Capacity of the Regoliths
2.5. Preparation of the Soluble Fraction of Martian Regolith Simulants
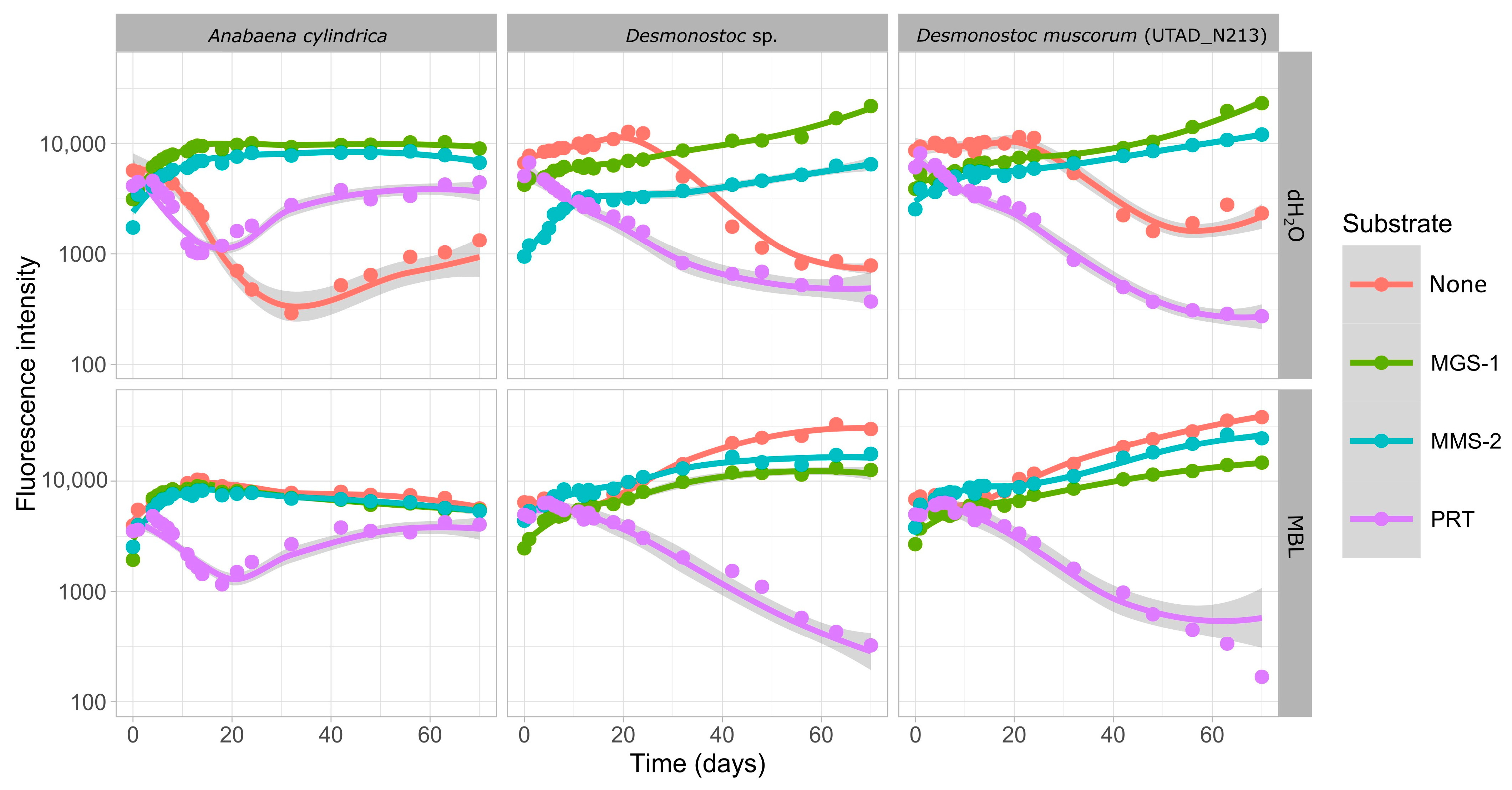
2.6. Cyanobacteria Growth Over Martian Regolith Simulants and in Their Soluble Fraction
2.6.1. Growth in the Soluble Fraction of 3 Martian Regolith Simulants
2.6.2. Growth over Three Regolith Simulants
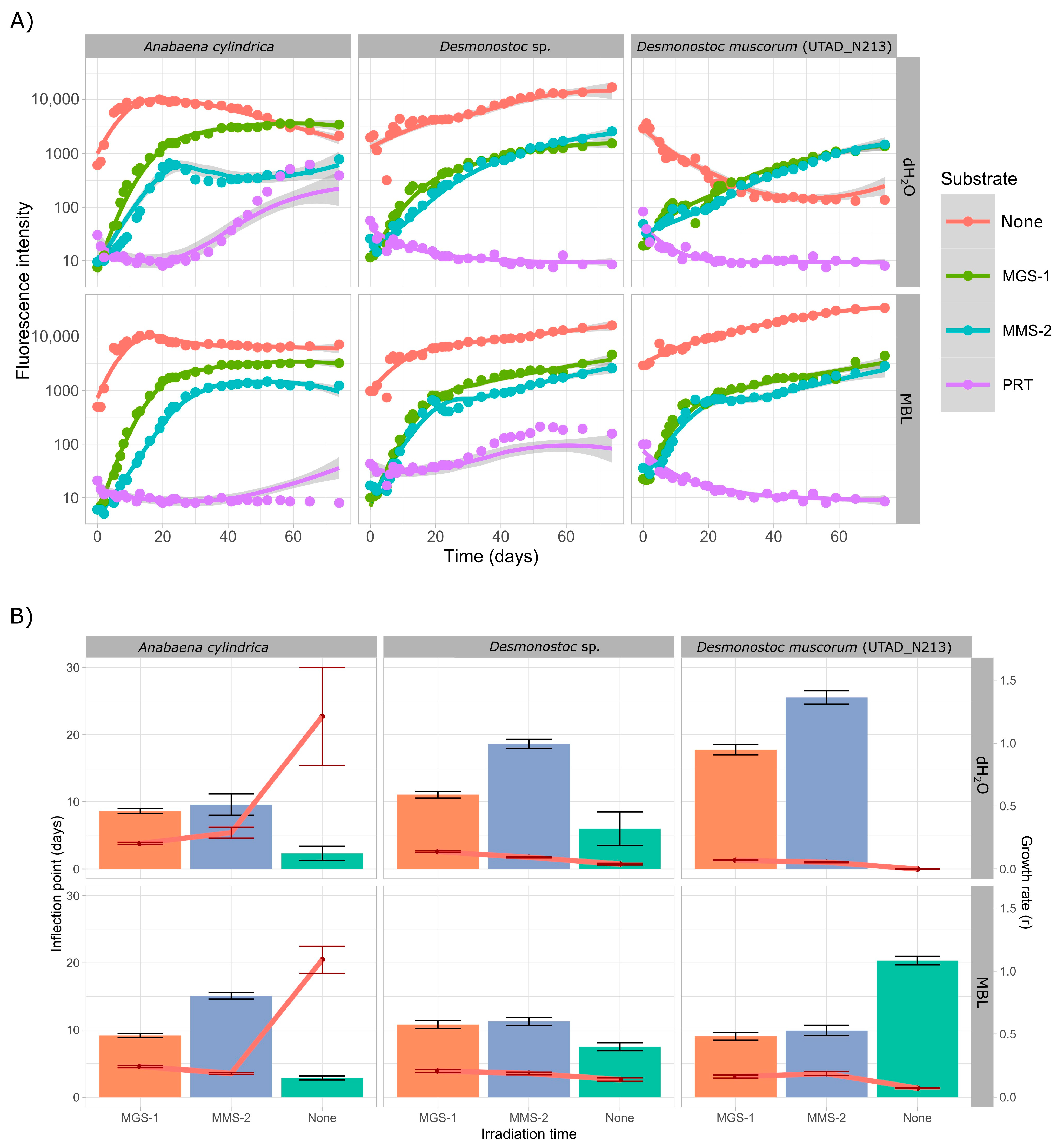
2.7. Cyanobacteria Growth in Regolith Under Stress
2.7.1. Drying and UV Exposure
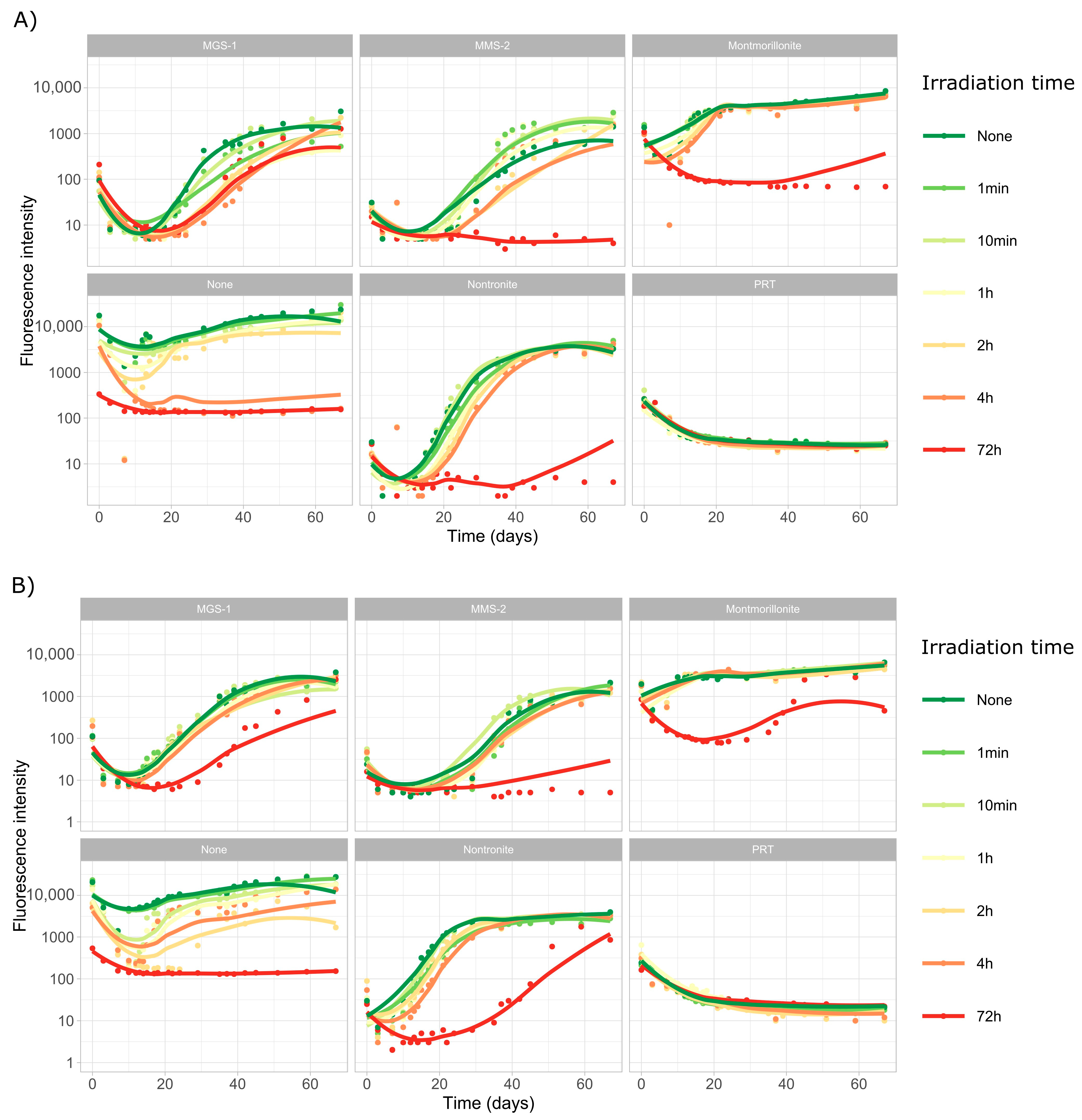
2.7.2. UV Exposure in Regolith Simulants in the Presence of Water
2.8. Curve Modeling Using Growthcurver
3. Results
3.1. Determination of the Oxidative Pressure Exerted by the Regolith Simulants
3.2. Cyanobacterial Growth in Martian Regolith Simulants
3.2.1. Identification of the Cyanobacterial Strains
3.2.2. Growth in the Soluble Fraction of Martian Regolith Simulants
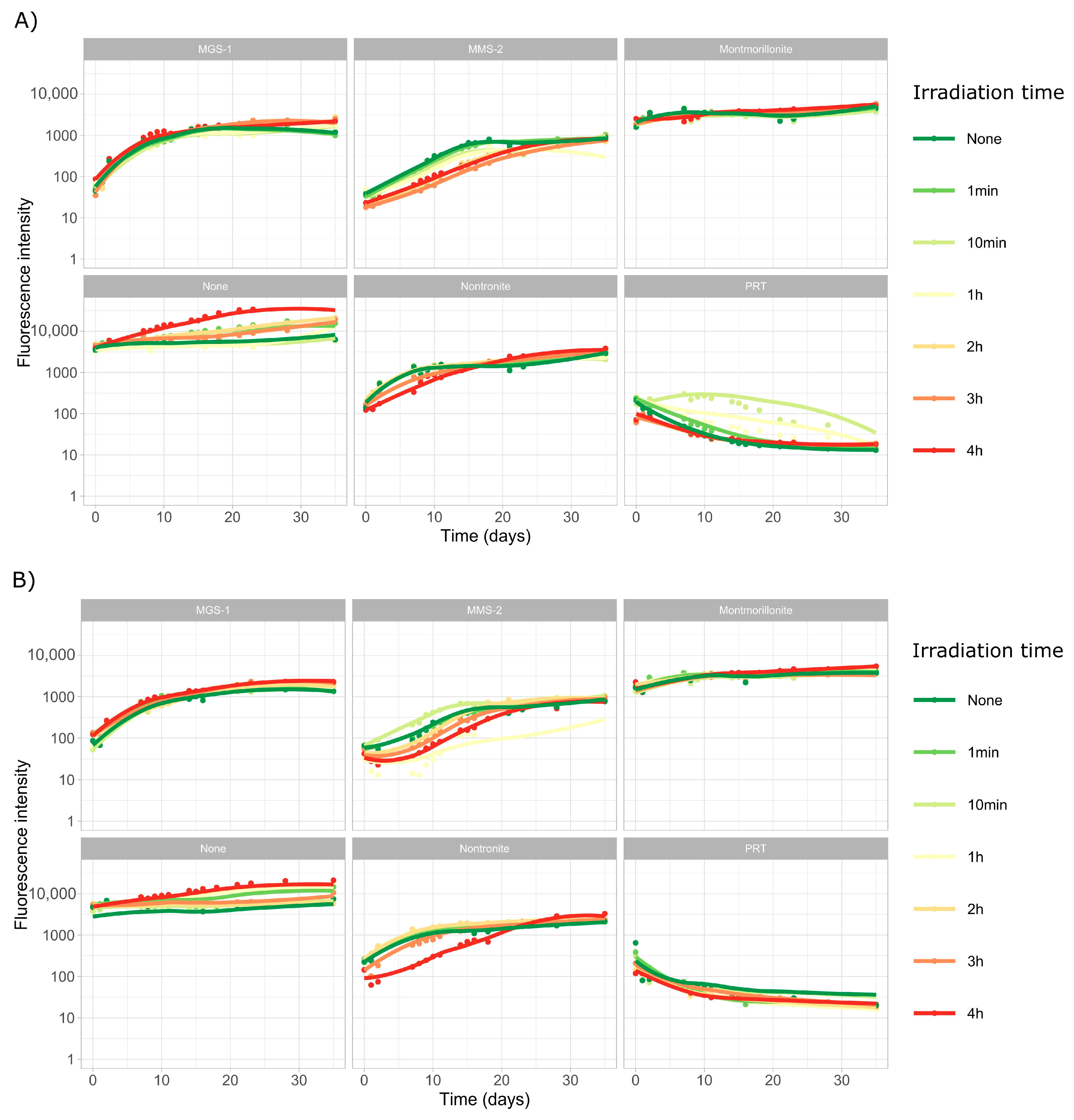
3.2.3. Growth over Martian Regolith Simulants
3.3. Cyanobacterial Survival Under Deleterious Martian Surface Conditions
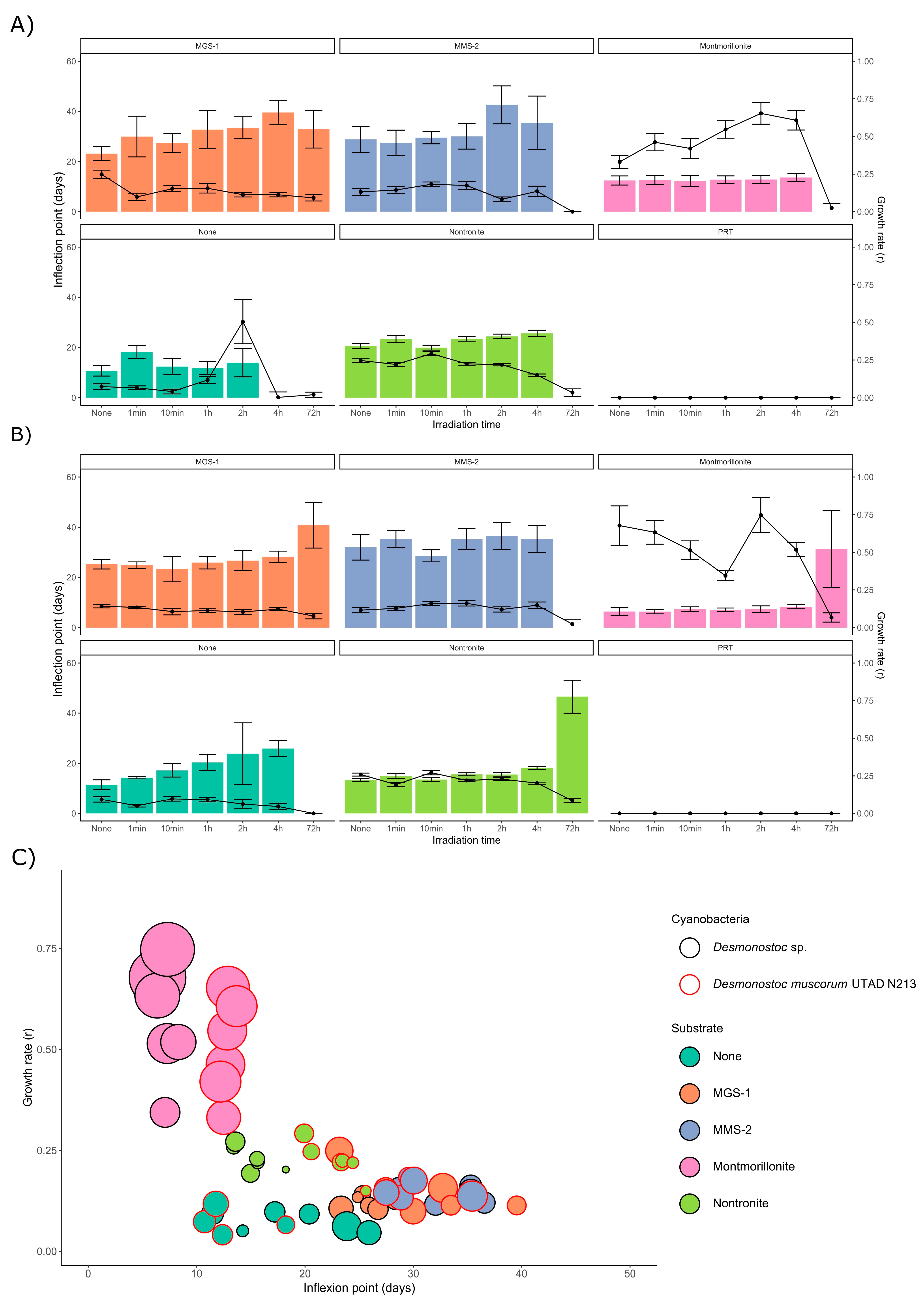
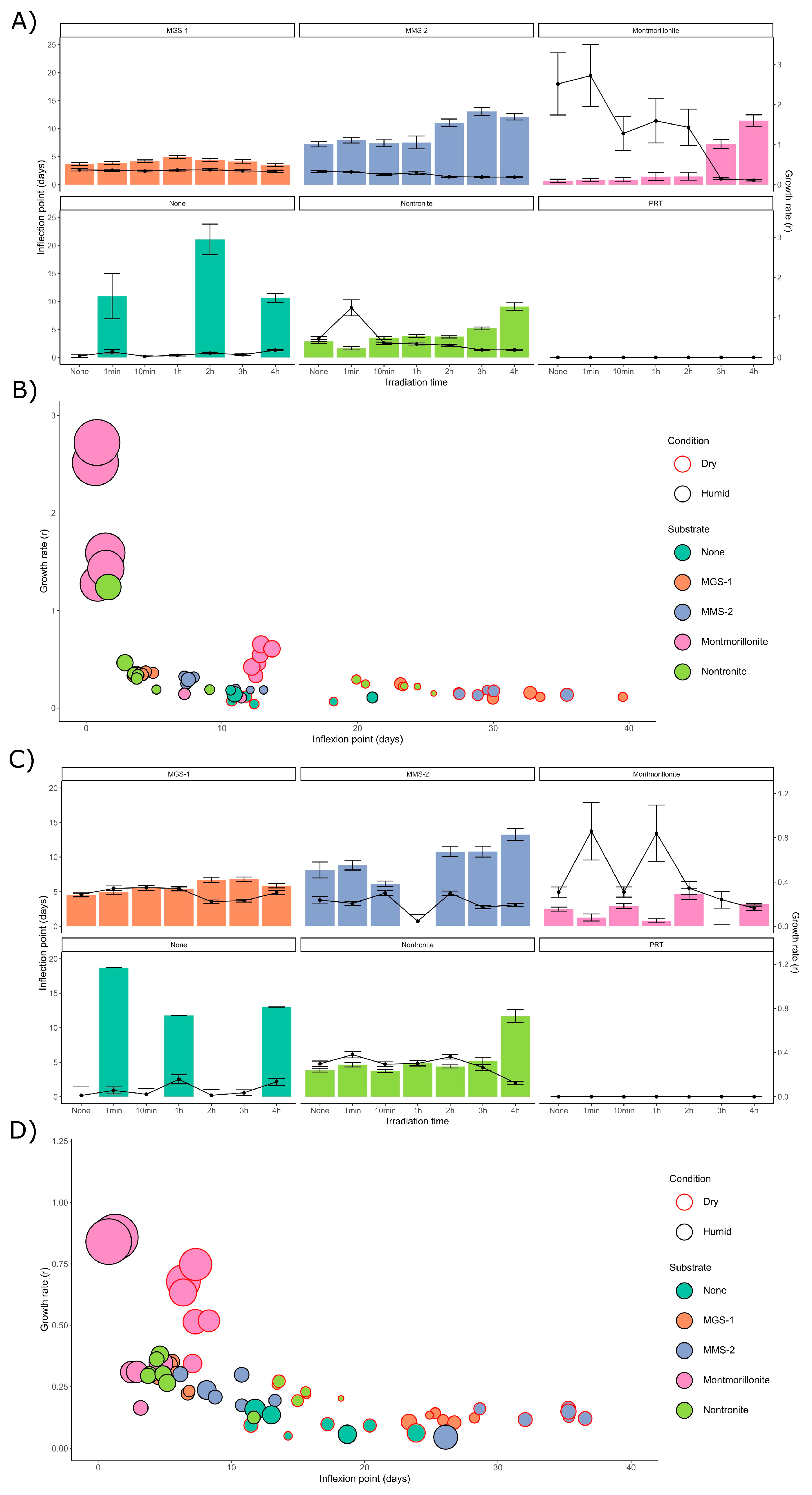
3.3.1. Survival and Growth After UV-B Irradiation and Desiccation
3.3.2. Survival and Growth After UV-B Irradiation in a Humid Environment
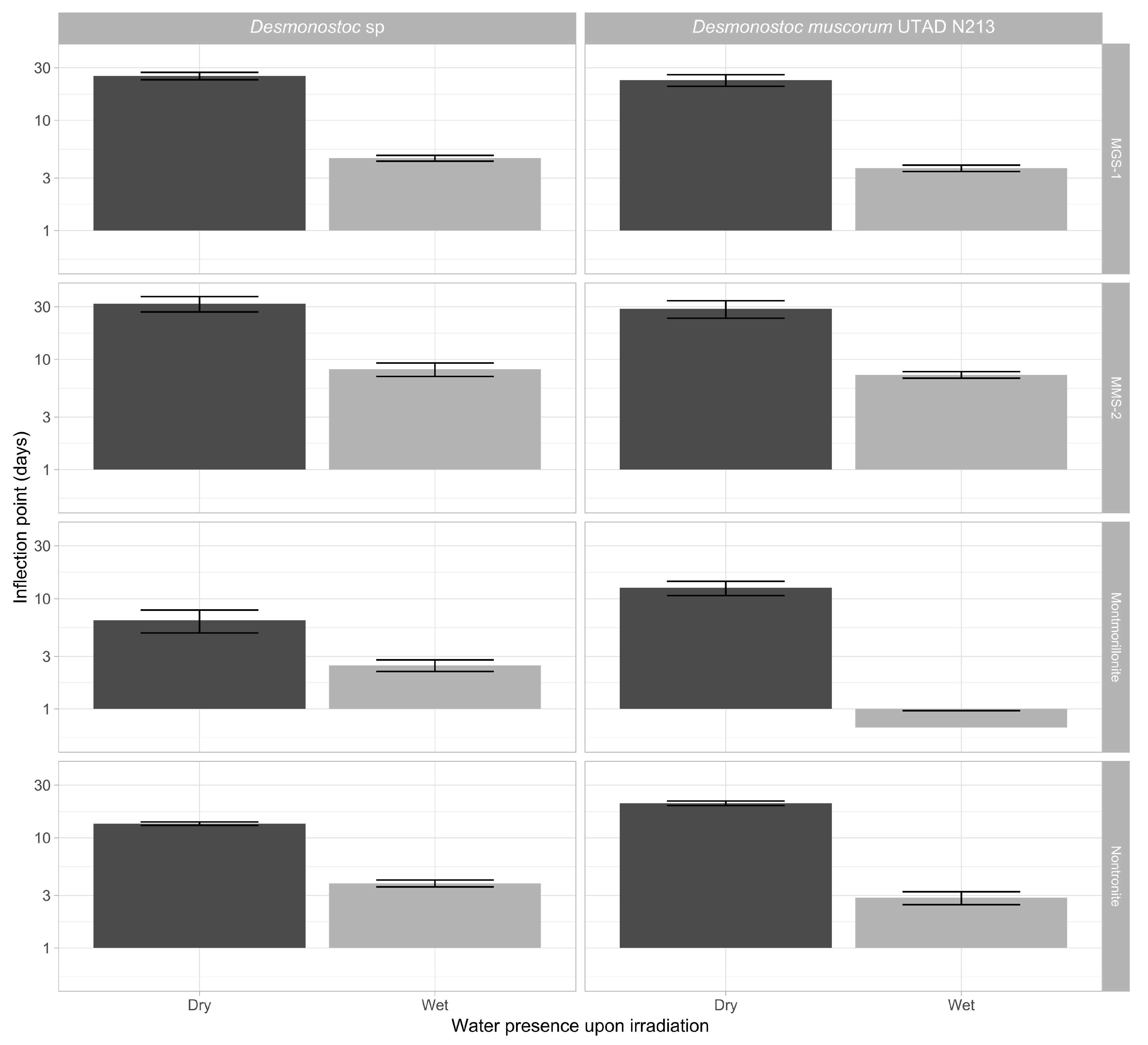
3.3.3. Effects of Desiccation on Cyanobacterial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, B.C.; Kolb, V.M.; Steele, A.; House, C.H.; Lanza, N.L.; Gasda, P.J.; VanBommel, S.J.; Newsom, H.E.; Martínez-Frías, J. Origin of life on mars: Suitability and opportunities. Life 2021, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Kite, E.S.; Conway, S. Geological evidence for multiple climate transitions on Early Mars. Nat. Geosci. 2024, 17, 10–19. [Google Scholar] [CrossRef]
- Guzman, M.; McKay, C.P.; Quinn, R.C.; Szopa, C.; Davila, A.F.; Navarro-González, R.; Freissinet, C. Identification of Chlorobenzene in the Viking Gas Chromatograph-Mass Spectrometer Data Sets: Reanalysis of Viking Mission Data Consistent With Aromatic Organic Compounds on Mars. J. Geophys. Res. Planets 2018, 123, 1674–1683. [Google Scholar] [CrossRef]
- Finkel, P.L.; Carrizo, D.; Parro, V.; Sánchez-García, L. An Overview of Lipid Biomarkers in Terrestrial Extreme Environments with Relevance for Mars Exploration. Astrobiology 2023, 23, 563–604. [Google Scholar] [CrossRef]
- Moreno-Paz, M.; Severino, R.S.d.S.; Sánchez-García, L.; Manchado, J.M.; García-Villadangos, M.; Aguirre, J.; Fernández-Martínez, M.A.; Carrizo, D.; Kobayashi, L.; Dave, A.; et al. Life Detection and Microbial Biomarker Profiling with Signs of Life Detector-Life Detector Chip During a Mars Drilling Simulation Campaign in the Hyperarid Core of the Atacama Desert. Astrobiology 2023, 23, 1259–1283. [Google Scholar] [CrossRef] [PubMed]
- Kate, I.L.T.; Garry, J.R.C.; Peeters, Z.; Quinn, R.; Foing, B.; Ehrenfreund, P. Amino acid photostability on the Martian surface. Meteorit. Planet. Sci. 2005, 40, 1185–1193. [Google Scholar] [CrossRef]
- Megevand, V.; Viennet, J.; Balan, E.; Gauthier, M.; Rosier, P.; Morand, M.; Garino, Y.; Guillaumet, M.; Pont, S.; Beyssac, O.; et al. Impact of UV Radiation on the Raman Signal of Cystine: Implications for the Detection of S-rich Organics on Mars. Astrobiology 2021, 21, 566–574. [Google Scholar] [CrossRef]
- Liu, D.; Kounaves, S.P. Degradation of Amino Acids on Mars by UV Irradiation in the Presence of Chloride and Oxychlorine Salts. Astrobiology 2021, 21, 793–801. [Google Scholar] [CrossRef]
- Pavlov, A.A.; McLain, H.L.; Glavin, D.P.; Roussel, A.; Dworkin, J.P.; Elsila, J.E.; Yocum, K.M. Rapid Radiolytic Degradation of Amino Acids in the Martian Shallow Subsurface: Implications for the Search for Extinct Life. Astrobiology 2022, 22, 1099–1115. [Google Scholar] [CrossRef]
- Baqué, M.; Verseux, C.; Böttger, U.; Rabbow, E.; de Vera, J.-P.P.; Billi, D. Preservation of Biomarkers from Cyanobacteria Mixed with Mars Like Regolith Under Simulated Martian Atmosphere and UV Flux. Orig. Life Evol. Biosph. 2016, 46, 289–310. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B.; Hippmann, N.; Kaczmarek, Ł. Screening the Survival of Cyanobacteria Under Perchlorate Stress. Potential Implications for Mars In Situ Resource Utilization. Astrobiology 2022, 22, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S. Trajectories of Martian Habitability. Astrobiology 2014, 14, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Bosak, T.; Shuster, D.L.; Scheller, E.L.; Siljeström, S.; Zawaski, M.J.; Mandon, L.; Simon, J.I.; Weiss, B.P.; Stack, K.M.; Mansbach, E.N.; et al. Astrobiological Potential of Rocks Acquired by the Perseverance Rover at a Sedimentary Fan Front in Jezero Crater, Mars. AGU Adv. 2024, 5, e2024AV001241. [Google Scholar] [CrossRef]
- Mapstone, L.J.; Leite, M.N.; Purton, S.; Crawford, I.A.; Dartnell, L. Cyanobacteria and microalgae in supporting human habitation on Mars. Biotechnol. Adv. 2022, 59, 107946. [Google Scholar] [CrossRef]
- Koehle, A.P.; Brumwell, S.L.; Seto, E.P.; Lynch, A.M.; Urbaniak, C. Microbial applications for sustainable space exploration beyond low Earth orbit. NPJ Microgravity 2023, 9, 1–27. [Google Scholar] [CrossRef]
- Ramalho, T.P.; Chopin, G.; Salman, L.; Baumgartner, V.; Heinicke, C.; Verseux, C. On the growth dynamics of the cyanobacterium Anabaena sp. PCC 7938 in Martian regolith. NPJ Microgravity 2022, 8, 1–8. [Google Scholar]
- Verseux C, Heinicke C, Ramalho TP, Determann J, Duckhorn M, Smagin M; et al. A Low-Pressure, N2/CO2 Atmosphere Is Suitable for Cyanobacterium-Based Life-Support Systems on Mars. Front Microbiol. 2021, 12, 611798. [Google Scholar]
- Kaur, J.; Nigam, A. Extremophiles in Space Exploration. Indian J. Microbiol. Springer 2024, 64, 418–428. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Veloso, T.; Frankenbach, S.; Serôdio, J.; Passos, H.; Sousa, C.; Gonçalves, F.J.M.; Ventura, S.P.M.; Pereira, J.L. Cyanobacteria as Candidates to Support Mars Colonization: Growth and Biofertilization Potential Using Mars Regolith as a Resource. Front. Microbiol. 2022, 13, 840098. [Google Scholar] [CrossRef]
- Gaysina LA, Saraf A, Singh, P. Chapter 1—Cyanobacteria in Diverse Habitats. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Jung, P.; Harion, F.; Wu, S.; Nürnberg, D.J.; Bellamoli, F.; Guillen, A.; Leira, M.; Lakatos, M. Dark blue-green: Cave-inhabiting cyanobacteria as a model for astrobiology. Front. Astron. Space Sci. 2023, 10, 1107371. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Zinser, E.R.; Coe, A.; McNulty, N.P.; Woodward, E.M.S.; Chisholm, S.W. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 2006, 311, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Bezalel-Hazony, N.; Zer, H.; Nathanson, S.; Shevtsov-Tal, S.; Ostersetzer-Biran, O.; Keren, N. Functional flexibility of cyanobacterial light harvesting phycobilisomes enable acclimation to the complex light regime of mixing marine water columns. FEBS J. 2022, 290, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeon, J.; Kwak, M.S.; Kim, G.H.; Koh, I.; Rho, M. Photosynthetic functions of Synechococcus in the ocean microbiomes of diverse salinity and seasons. PLoS ONE 2018, 13, e0190266. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Johnson HE, King SR, Banack SA, Webster C, Callanaupa WJ, Cox PA. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J. Ethnopharmacol. 2008, 118, 159–165. [Google Scholar] [CrossRef]
- Mutale-Joan, C.; Sbabou, L.; Hicham, E.A. Microalgae and Cyanobacteria: How Exploiting These Microbial Resources Can Address the Underlying Challenges Related to Food Sources and Sustainable Agriculture: A Review. J. Plant Growth Regul. 2023, 42, 1–20. [Google Scholar] [CrossRef]
- Farrokh, P.; Sheikhpour, M.; Kasaeian, A.; Asadi, H.; Bavandi, R. Cyanobacteria as an eco-friendly resource for biofuel production: A critical review. Biotechnol. Prog. 2019, 35, e2835. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.; Kalaji, H.M. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 2017, 55, 510–521. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Alwaleed, E.A.; Ibrahim, A.; Saber, H. Detrimental effect of UV-B radiation on growth, photosynthetic pigments, metabolites and ultrastructure of some cyanobacteria and freshwater chlorophyta. Int. J. Radiat. Biol. 2020, 97, 265–275. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Nakamoto, H.; Incharoensakdi, A. Resilience and self-regulation processes of microalgae under UV radiation stress. J. Photochem. Photobiol. C Photochem. Rev. 2020, 43, 100322. [Google Scholar] [CrossRef]
- Dwivedi, S.; Ahmad, I.Z. Evaluation of the effect of UV-B Radiation on Growth, Photosynthetic Pigment, and Antioxidant Enzymes of some Cyanobacteria. Environ. Res. 2023, 218, 114943. [Google Scholar] [CrossRef] [PubMed]
- Billi, D.; Verseux, C.; Fagliarone, C.; Napoli, A.; Baqué, M.; de Vera, J.-P. A Desert Cyanobacterium under Simulated Mars-like Conditions in Low Earth Orbit: Implications for the Habitability of Mars. Astrobiology 2019, 19, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Fagliarone, C.; Napoli, A.; Chiavarini, S.; Baqué, M.; de Vera, J.-P.; Billi, D. Biomarker Preservation and Survivability Under Extreme Dryness and Mars-Like UV Flux of a Desert Cyanobacterium Capable of Trehalose and Sucrose Accumulation. Front. Astron. Space Sci. 2020, 7, 546498. [Google Scholar] [CrossRef]
- Lin, W.; Shen, J.; Chen, Y.; Sun, Y.; Liu, L.; Pan, Y. Detection of biosignatures in Terrestrial analogs of Martian regions: Strategical and technical assessments. Earth Planet. Phys. 2022, 6, 431–450. [Google Scholar]
- Azua-Bustos, A.; Zúñiga, J.; Arenas-Fajardo, C.; Orellana, M.; Salas, L.; Rafael, V. Gloeocapsopsis AAB1, an extremely desiccation-tolerant cyanobacterium isolated from the Atacama Desert. Extremophiles 2014, 18, 61–74. [Google Scholar] [CrossRef]
- Amils, R.; Escudero, C.; Huang, T.; Fernádez-Remolar, D. Geomicrobiology of Río Tinto (Iberian Pyrite Belt): A Geological and Mineralogical Mars Analogue. In Geomicrobiol: Nat Anthropog Settings; Springer: Berlin/Heidelberg, Germany, 2024; pp. 123–150. Available online: https://link.springer.com/chapter/10.1007/978-3-031-54306-7_7 (accessed on 24 October 2024).
- Cannon, K.M.; Britt, D.T.; Smith, T.M.; Fritsche, R.F.; Batcheldor, D. Mars global simulant MGS-1: A Rocknest-based open standard for basaltic martian regolith simulants. Icarus 2019, 317, 470–478. [Google Scholar] [CrossRef]
- Caporale, A.G.; Vingiani, S.; Palladino, M.; El-Nakhel, C.; Duri, L.G.; Pannico, A.; Rouphael, Y.; De Pascale, S.; Adamo, P. Geo-mineralogical characterisation of Mars simulant MMS-1 and appraisal of substrate physico-chemical properties and crop performance obtained with variable green compost amendment rates. Sci. Total. Environ. 2020, 720, 137543. [Google Scholar] [CrossRef]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Carrier, B.L.; O’neil, G.D.; Stroble, S.T.; Claire, M.W. Evidence of martian perchlorate, chlorate, and nitrate in Mars meteorite EETA79001: Implications for oxidants and organics. Icarus 2014, 229, 206–213. [Google Scholar] [CrossRef]
- McAdam, A.C.; Sutter, B.; Archer, P.D.; Franz, H.B.; Wong, G.M.; Lewis, J.M.T.; Clark, J.V.; Millan, M.; Williams, A.J.; Eigenbrode, J.L.; et al. Evolved Gas Analyses of Sedimentary Rocks From the Glen Torridon Clay-Bearing Unit, Gale Crater, Mars: Results From the Mars Science Laboratory Sample Analysis at Mars Instrument Suite. J. Geophys. Res. Planets 2022, 127, e2022JE007179. [Google Scholar] [CrossRef]
- Clark, B.C.; Kounaves, S.P. Evidence for the distribution of perchlorates on Mars. Int. J. Astrobiol. 2016, 15, 311–318. [Google Scholar] [CrossRef]
- Rzymski, P.; Losiak, A.; Heinz, J.; Szukalska, M.; Florek, E.; Poniedziałek, B.; Kaczmarek, Ł.; Schulze-Makuch, D. Perchlorates on Mars: Occurrence and implications for putative life on the Red Planet. Icarus 2024, 421, 1162476. [Google Scholar] [CrossRef]
- Stone, D.; Bress, W. Addressing public health risks for cyanobacteria in recreational freshwaters: The oregon and vermont framework. Integr. Environ. Assess. Manag. 2007, 3, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Galhano, V.; de Figueiredo, D.R.; Alves, A.; Correia, A.; Pereira, M.J.; Gomes-Laranjo, J.; Peixoto, F. Morphological, biochemical and molecular characterization of Anabaena, Aphanizomenon and Nostoc strains (Cyanobacteria, Nostocales) isolated from Portuguese freshwater habitats. Hydrobiologia 2011, 663, 187–203. [Google Scholar] [CrossRef]
- Patel, H.M.; Roszak, A.W.; Madamwar, D.; Cogdell, R.J. Crystal structure of phycocyanin from heterocyst-forming filamentous cyanobacterium Nostoc sp. WR13. Int. J. Biol. Macromol. 2019, 135, 62–68. [Google Scholar] [CrossRef]
- Amils, R.; Fernández-Remolar, D.; The IPBSL Team. Río Tinto: A geochemical and mineralogical terrestrial analogue of Mars. Life 2014, 4, 511–534. [Google Scholar] [CrossRef]
- Cockell, C.S.; Catling, D.C.; Davis, W.L.; Snook, K.; Kepner, R.L.; Lee, P.; McKay, C.P. The Ultraviolet Environment of Mars: Biological Implications Past, Present, and Future. Icarus 2000, 146, 343–359. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, H.-J.; Kim, M.H.; Kim, J.-S.; Kang, N.; Lee, J.-Y.; Kim, K.-T.; Lee, J.I.; Kim, D.-D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Duri, L.G.; Caporale, A.G.; Rouphael, Y.; Vingiani, S.; Palladino, M.; De Pascale, S.; Adamo, P. The Potential for Lunar and Martian Regolith Simulants to Sustain Plant Growth: A Multidisciplinary Overview. Front. Astron. Space Sci. 2022, 8, 747821. [Google Scholar] [CrossRef]
- Eichler, A.; Hadland, N.; Pickett, D.; Masaitis, D.; Handy, D.; Perez, A.; Batcheldor, D.; Wheeler, B.; Palmer, A. Challenging the agricultural viability of martian regolith simulants. Icarus 2021, 354, 114022. [Google Scholar] [CrossRef]
- Gómez, F.; Mateo-Martí, E.; Prieto-Ballesteros, O.; Martín-Gago, J.; Amils, R. Protection of chemolithoautotrophic bacteria exposed to simulated Mars environmental conditions. Icarus 2010, 209, 482–487. [Google Scholar] [CrossRef]
- Morris, J.J.; Rose, A.L.; Lu, Z. Reactive oxygen species in the world ocean and their impacts on marine ecosystems. Redox Biol. 2022, 52, 102285. [Google Scholar] [CrossRef]
- Rasul, F.; You, D.; Jiang, Y.; Liu, X.; Daroch, M. Thermophilic cyanobacteria—Exciting, yet challenging biotechnological chassis. Appl. Microbiol. Biotechnol. 2024, 108, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fagliarone, C.; Mosca, C.; Di Stefano, G.; Leuko, S.; Moeller, R.; Rabbow, E.; Rettberg, P.; Billi, D. Enabling deep-space experimentations on cyanobacteria by monitoring cell division resumption in dried Chroococcidiopsis sp. 029 with accumulated DNA damage. Front. Microbiol. 2023, 14, 1150224. [Google Scholar] [CrossRef]
- Raanan, H.; Oren, N.; Treves, H.; Keren, N.; Ohad, I.; Berkowicz, S.M.; Hagemann, M.; Koch, M.; Shotland, Y.; Kaplan, A. Towards clarifying what distinguishes cyanobacteria able to resurrect after desiccation from those that cannot: The photosynthetic aspect. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef]
- Legrand, B.; Miras, Y.; Beauger, A.; Dussauze, M.; Latour, D. Akinetes and ancient DNA reveal toxic cyanobacterial recurrences and their potential for resurrection in a 6700-year-old core from a eutrophic lake. Sci. Total Environ. 2019, 687, 1369–1380. [Google Scholar] [CrossRef]
- Wood, S.M.; Kremp, A.; Savela, H.; Akter, S.; Vartti, V.-P.; Saarni, S.; Suikkanen, S. Cyanobacterial Akinete Distribution, Viability, and Cyanotoxin Records in Sediment Archives From the Northern Baltic Sea. Front. Microbiol. 2021, 12, 681881. [Google Scholar] [CrossRef]
| Absorbance | SD | Consumption (pmol DTT/g) | |
|---|---|---|---|
| Control+ | 0.906 | 0.091 | 0 |
| MGS-1 | 0.412 | 0.043 | 545 |
| MMS-2 | 0.284 | 0.032 | 686 |
| PRT | 0.232 | 0.034 | 744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arribas Tiemblo, M.; Macário, I.P.E.; Tornero, A.; Yáñez, A.; Andrejkovičová, S.; Gómez, F. Survival of Filamentous Cyanobacteria Through Martian ISRU: Combined Effects of Desiccation and UV-B Radiation. Microorganisms 2025, 13, 1083. https://doi.org/10.3390/microorganisms13051083
Arribas Tiemblo M, Macário IPE, Tornero A, Yáñez A, Andrejkovičová S, Gómez F. Survival of Filamentous Cyanobacteria Through Martian ISRU: Combined Effects of Desiccation and UV-B Radiation. Microorganisms. 2025; 13(5):1083. https://doi.org/10.3390/microorganisms13051083
Chicago/Turabian StyleArribas Tiemblo, Miguel, Inês P. E. Macário, Antonio Tornero, Ana Yáñez, Slavka Andrejkovičová, and Felipe Gómez. 2025. "Survival of Filamentous Cyanobacteria Through Martian ISRU: Combined Effects of Desiccation and UV-B Radiation" Microorganisms 13, no. 5: 1083. https://doi.org/10.3390/microorganisms13051083
APA StyleArribas Tiemblo, M., Macário, I. P. E., Tornero, A., Yáñez, A., Andrejkovičová, S., & Gómez, F. (2025). Survival of Filamentous Cyanobacteria Through Martian ISRU: Combined Effects of Desiccation and UV-B Radiation. Microorganisms, 13(5), 1083. https://doi.org/10.3390/microorganisms13051083








