Metabolic Response of Sanghuangporus baumii to Zn2+ Induction and Biosynthesis of a Key Pharmacological Component: Triterpenoid
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Culture and Zn2+ Treatment
2.2. Determination of Mycelial Growth Rate, Biomass, Triterpenoid Content, and Zn2+ Content
2.3. Transcriptome Sequencing of S. baumii
2.4. Quantification of Soluble Sugar Content
2.5. Heterologous Expression of SbHMGS
2.6. UPLC Analysis of Squalene in S. cerevisiae
2.7. Induction Culture of S. cerevisiae
2.8. Data Analysis
3. Results
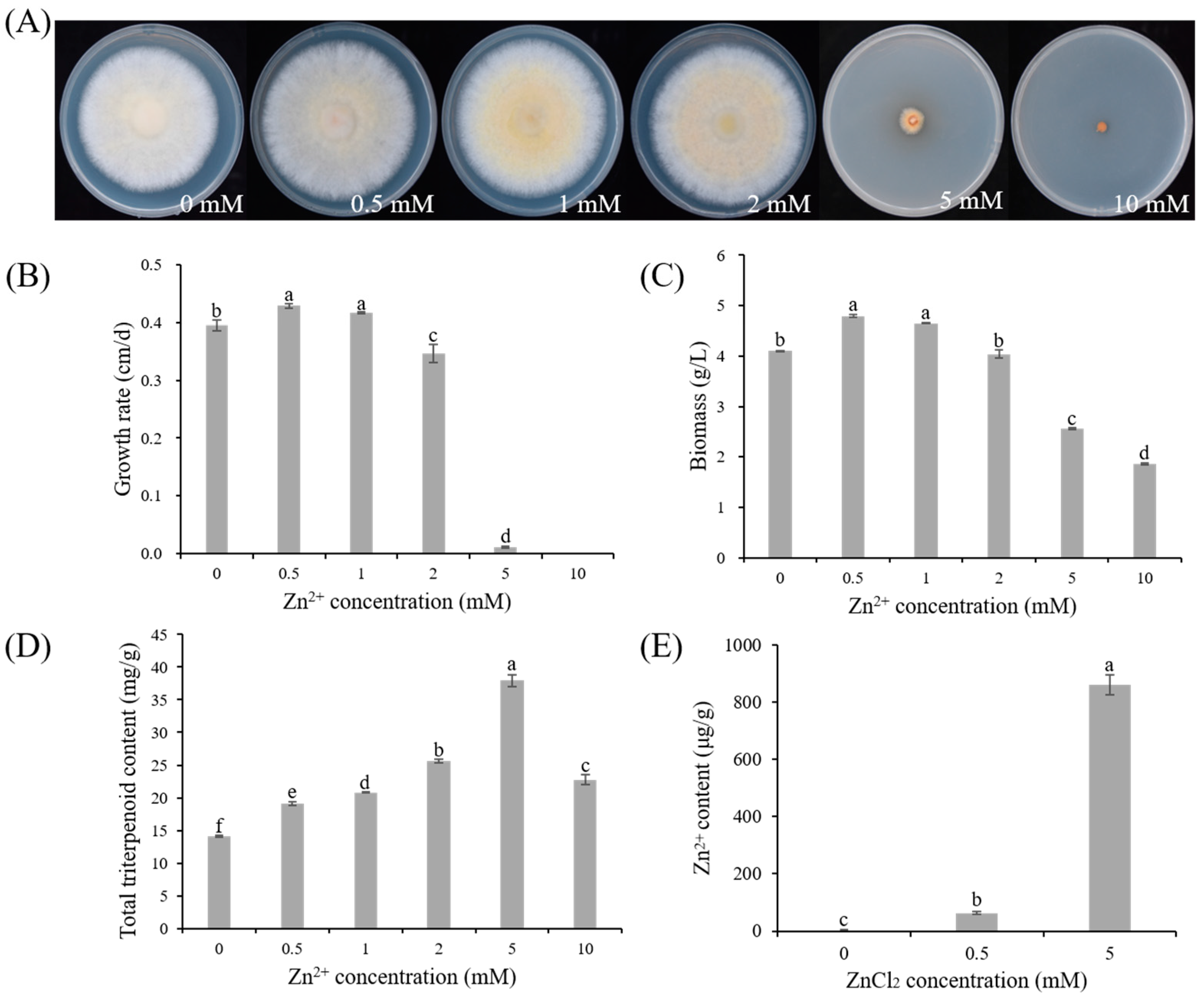
3.1. Effect of Zn2+ on the Growth and Triterpenoid Content of S.baumii
3.1.1. Effect of Zn2+ on the Growth of S. baumii
3.1.2. Effect of Zn2+ on the Triterpenoid Content of S. baumii
3.1.3. Zn2+ Content in S. baumii Mycelia under Zn2+ Treatment
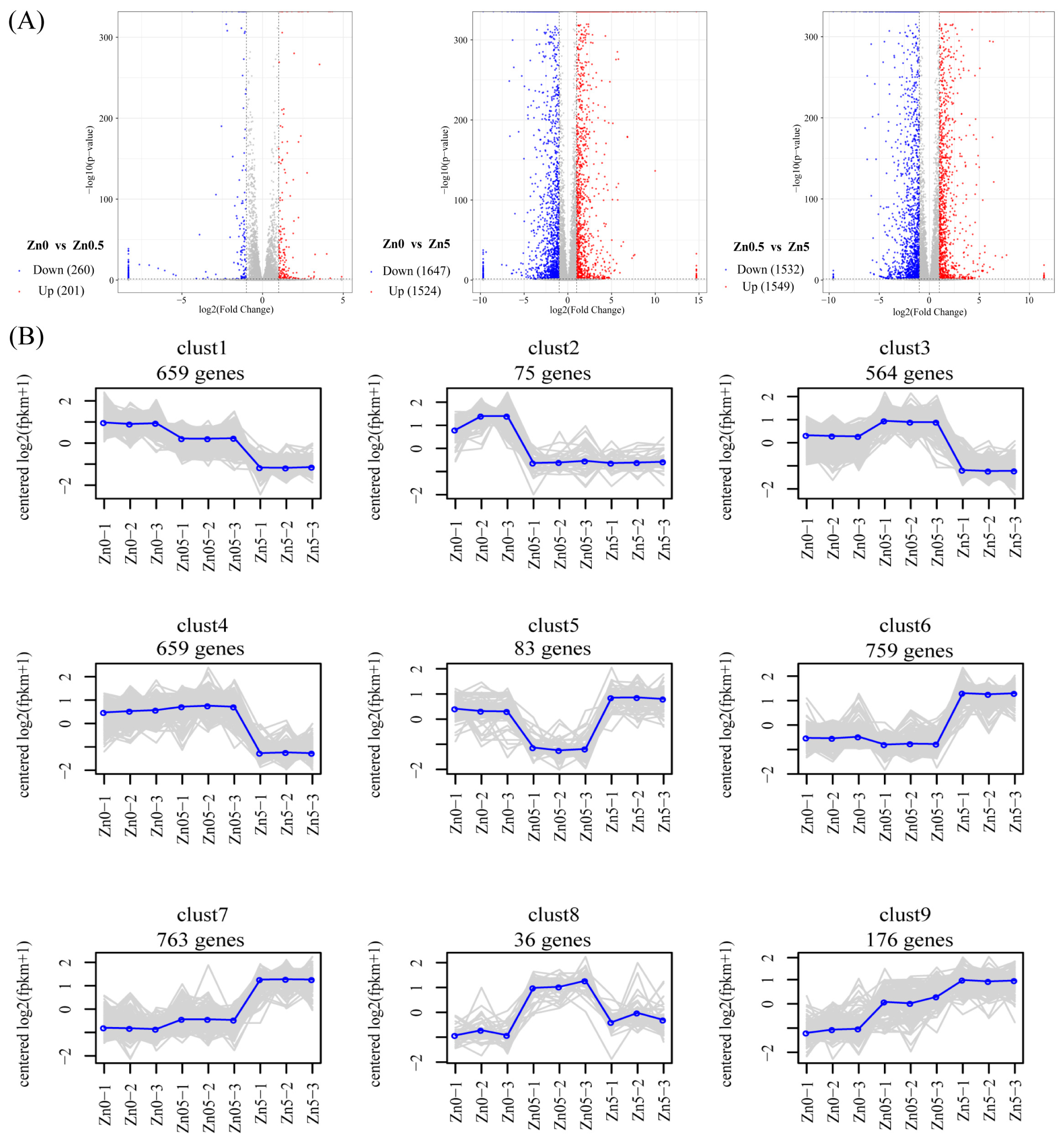
3.2. Transcriptome Data Analysis and qRT-PCR Validation
3.2.1. Overview of RNA-Seq Results
3.2.2. Identification of DEGs and Analysis of Expression Pattern
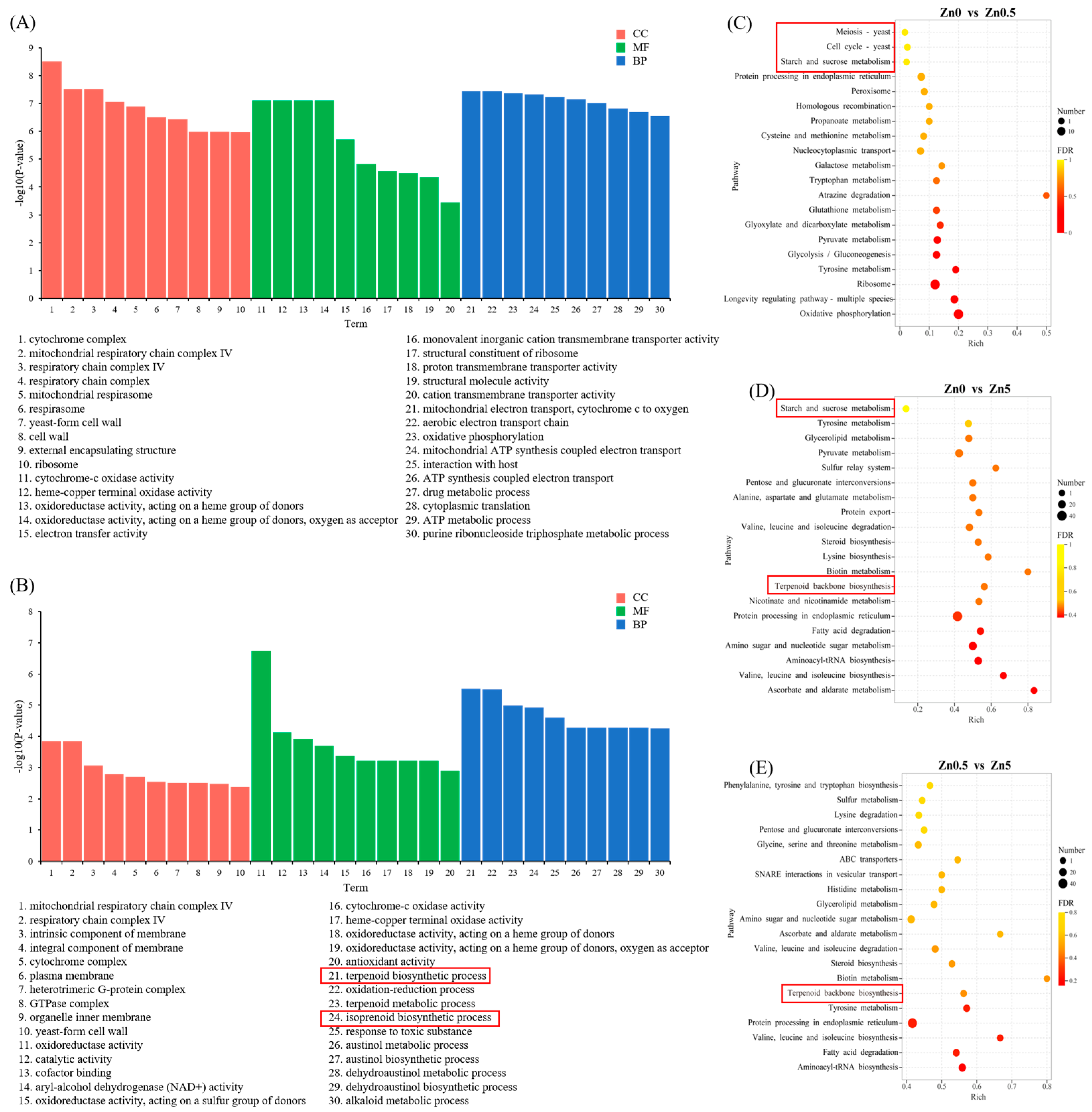
3.2.3. GO and KEGG Pathway Enrichment Analyses
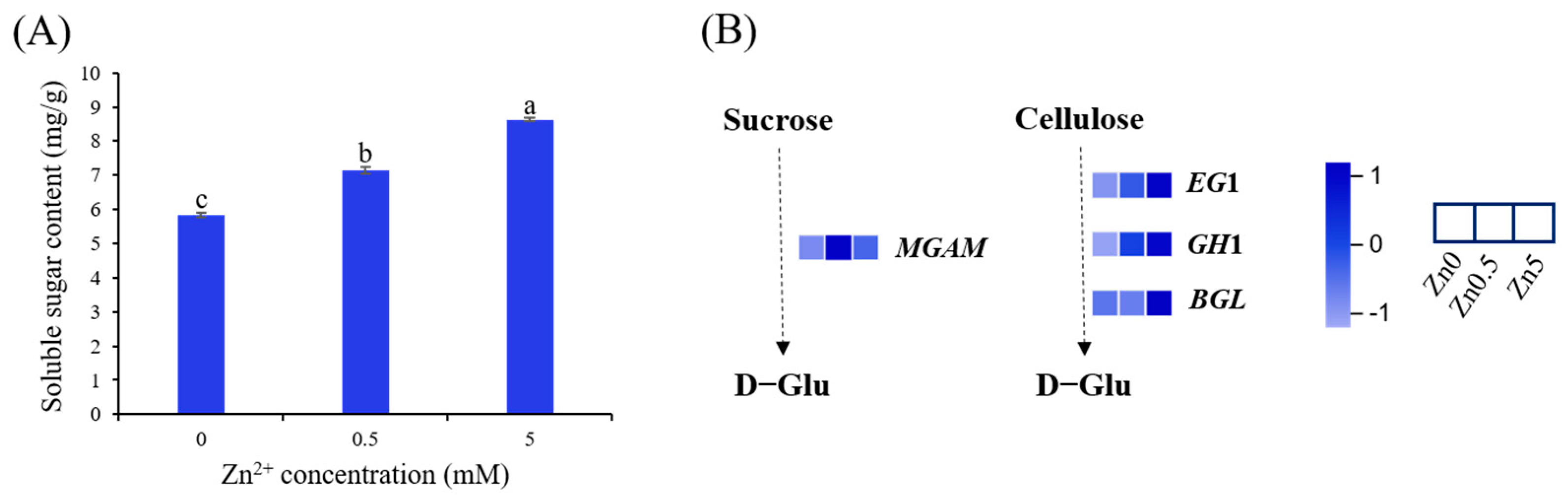
3.2.4. Soluble Sugar Content in S. baumii under Zn2+ Induction and DEGs Involved in Starch and Sucrose Metabolism
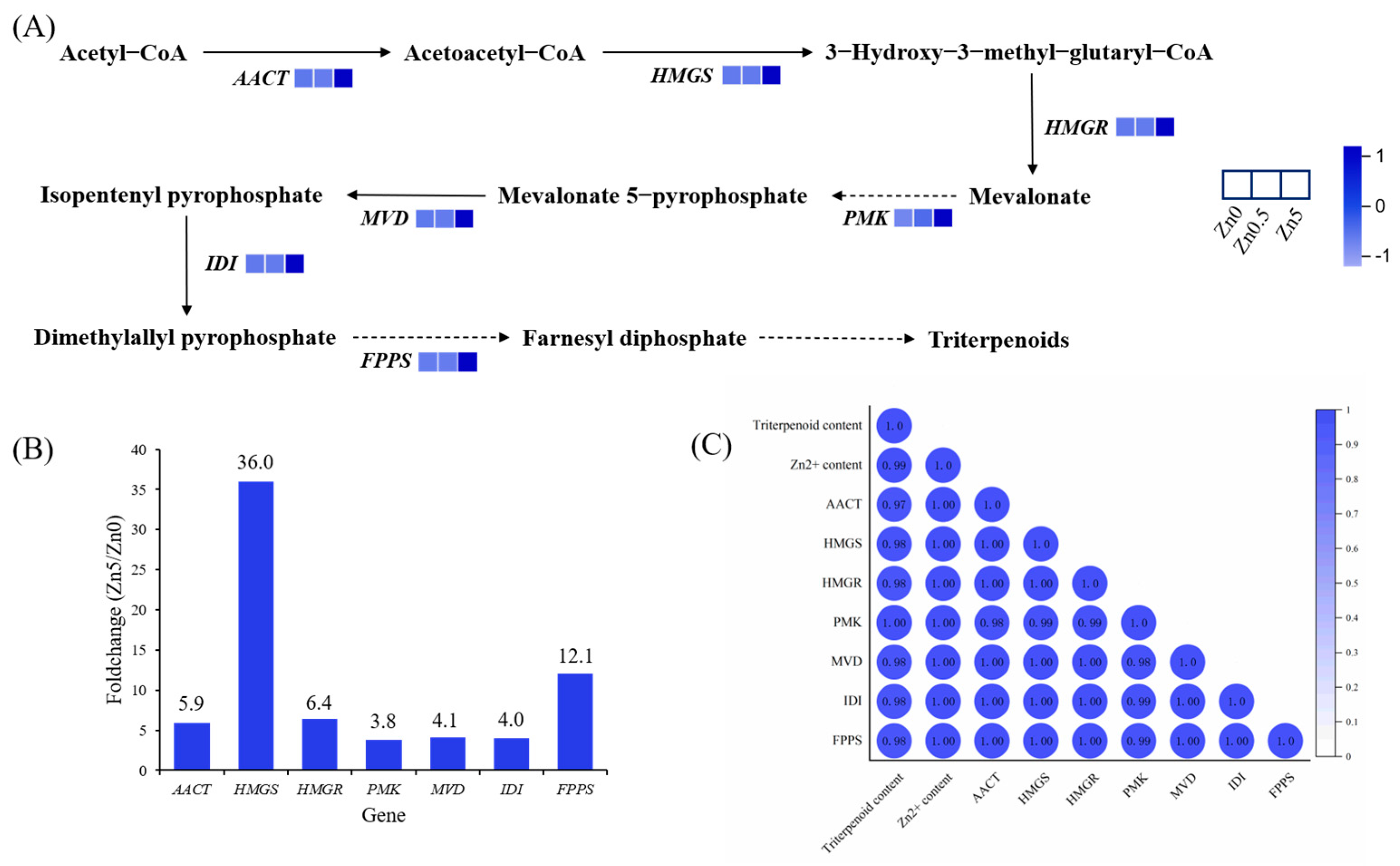
3.2.5. DEGs Involved in Terpenoid Backbone Biosynthesis
3.2.6. qRT-PCR Validation
3.3. Heterologous Expression of SbHMGS in S. cerevisiae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; He, P.; Li, N. The antitumor potential of extract of the oak bracket medicinal mushroom Inonotus baumii in SMMC-7721 tumor cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 1242784. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Zeng, P.; Li, X.-T.; Shi, L.-G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Ying, Y.; Li, Y.; Cai, M.; Guan, R.; Hu, J.; Sun, P. Cultivated fruit body of Phellinus baumii: A potentially sustainable antidiabetic resource. ACS Omega 2020, 5, 8596–8604. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; Cai, C.S.; Liu, J.J.; Gao, S.; Zhao, G.Z.; He, X.W. In vitro antibacterial and antitumor activity of total triterpenoids from a medicinal mushroom Sanghuangporus sanghuang (Agaricomycetes) in liquid fermentation culture. Int. J. Med. Mushrooms 2021, 23, 27–39. [Google Scholar] [CrossRef]
- Wang, S.; Meng, D.; Feng, M.; Li, C.; Wang, Y. Efficient Plant Triterpenoids Synthesis in Saccharomyces cerevisiae: From Mechanisms to Engineering Strategies. ACS Synth. Biol. 2024, 13, 1059–1076. [Google Scholar] [CrossRef]
- Liu, Z.; Tong, X.; Liu, R.; Zou, L. Metabolome and transcriptome profiling reveal that four terpenoid hormones dominate the growth and development of Sanghuangporus baumii. J. Fungi 2022, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-N.; Zhong, J.-J. Impacts of calcium signal transduction on the fermentation production of antitumor ganoderic acids by medicinal mushroom Ganoderma lucidum. Biotechnol. Adv. 2012, 30, 1301–1308. [Google Scholar] [CrossRef]
- Li, H.-J.; Zhang, D.-H.; Han, L.-L.; Yu, X.Y.; Zhao, P.; Li, T.; Zhong, J.-J.; Xu, J.-W. Further improvement in ganoderic acid production in static liquid culture of Ganoderma lucidum by integrating nitrogen limitation and calcium ion addition. Bioprocess Biosyst. Eng. 2016, 39, 75–80. [Google Scholar] [CrossRef]
- Sun, W.; Qin, L.; Xue, H.; Yu, Y.; Ma, Y.; Wang, Y.; Li, C. Novel trends for producing plant triterpenoids in yeast. Crit. Rev. Biotechnol. 2019, 39, 618–632. [Google Scholar] [CrossRef]
- Jin, K.; Shi, X.; Liu, J.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Combinatorial metabolic engineering enables the efficient production of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. Bioresour. Technol. 2023, 374, 128819. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, C.; Wang, Q.; Fang, Y.; Wang, J.; Wang, M.; Xiao, H. Biosynthesis of mushroom-derived type II ganoderic acids by engineered yeast. Nat. Commun. 2022, 13, 7740. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ji, W.; Zhao, Y.Q.; Zhang, J.; Wei, D.; Wang, F.-Q. De novo biosynthesis of betulinic acid in engineered Saccharomyces cerevisiae. Bioorg. Chem. 2024, 152, 107737. [Google Scholar] [CrossRef]
- Bröker, J.N.; Müller, B.; van Deenen, N.; Prüfer, D.; Schulze Gronover, C. Upregulating the mevalonate pathway and repressing sterol synthesis in Saccharomyces cerevisiae enhances the production of triterpenes. Appl. Microbiol. Biotechnol. 2018, 102, 6923–6934. [Google Scholar] [CrossRef]
- Chimienti, F.; Aouffen, M.; Favier, A.; Seve, M. Zinc homeostasis-regulating proteins: New drug targets for triggering cell fate. Curr. Drug Targets 2003, 4, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Liang, C.-H.; Ou, C.-H.; Liang, Z.-C. Zinc ion addition to grain media enhanced hispidin production during solid-state fermentation of Phellinus linteus. J. Taiwan Inst. Chem. Eng. 2021, 121, 101–107. [Google Scholar] [CrossRef]
- Cui, Y.H.; Zhang, K.C. Effect of metal ions on the growth and metabolites production of Ganoderma lucidum in submerged culture. Afr. J. Biotechnol. 2011, 10, 11983–11989. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, T.; Wang, S.; Zou, L. Cloning, molecular properties and differential expression analysis of the isopentenyl diphosphate isomerase gene in Sanghuangporus baumii. Biotechnol. Biotechnol. Equip. 2020, 34, 623–630. [Google Scholar] [CrossRef]
- Ghuniem, M.M. Determination of some element’s migrants in aqueous simulant from plastic food contact products by inductively coupled plasma mass spectrometer. Food Anal. Methods 2024, 17, 1497–1510. [Google Scholar] [CrossRef]
- Sedbare, R.; Raudone, L.; Zvikas, V.; Viskelis, J.; Liaudanskas, M.; Janulis, V. Development and validation of the UPLC-DAD methodology for the detection of triterpenoids and phytosterols in fruit samples of Vaccinium macrocarpon aiton and Vaccinium oxycoccos L. Molecules 2022, 27, 4403. [Google Scholar] [CrossRef]
- Gajewska, J.; Floryszak-Wieczorek, J.; Sobieszczuk-Nowicka, E.; Mattoo, A.; Arasimowicz-Jelonek, M. Fungal and oomycete pathogens and heavy metals: An inglorious couple in the environment. IMA Fungus 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Hu, P.-F.; Huang, J.; Chen, L.; Ding, Z.; Liu, L.; Molnár, I.; Zhang, B.-B. Oxidative stress induction is a rational strategy to enhance the productivity of Antrodia cinnamomea fermentations for the antioxidant secondary metabolite Antrodin C. J. Agric. Food Chem. 2020, 68, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ding, P.; Yang, G.X.; He, G.Y. Advances on the plant terpenoid isoprenoid biosynthetic pathway and its key enzymes. Biotechnol. Bull. 2006, 1, 22–30. [Google Scholar]
- Afroz, S.; Warsi, Z.I.; Khatoon, K.; Sangwan, N.S.; Khan, F.; Rahman, L.U. Molecular cloning and characterization of triterpenoid biosynthetic pathway gene HMGS in Centella asiatica (Linn.). Mol. Biol. Rep. 2022, 49, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhao, Y.-J.; Zhang, M.; Su, P.; Wang, X.-J.; Zhang, X.-N.; Gao, W.; Huang, L.-Q. Cloning and characterisation of the gene encoding 3-hydroxy-3-methylglutaryl-CoA synthase in Tripterygium wilfordii. Molecules 2014, 19, 19696–19707. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Wu, K.; Wang, F.; Yang, Q.; Han, R.; Liang, Z.; Jia, Q. Cloning and characterization of the gene encoding HMGS synthase in Polygonatum sibiricum. BioMed. Res. Int. 2022, 47, 7441296. [Google Scholar] [CrossRef]
- Pradipta, P.; Manali, D.; Pritam, K.; Amit, G. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Deng, L.; Xu, P. Genetic and bioprocess engineering to improve squalene production in Yarrowia lipolytica. Bioresour. Technol. 2020, 317, 123991. [Google Scholar] [CrossRef]
- Luvitaa, K.S.; Wambui, M.A.; Fredrick, M.; Otieno, O.D. Zinc bioaccessibility in finger millet porridge blended with zinc-dense mushroom. Heliyon 2023, 9, e18901. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Campos-Ortiz, A.; Sánchez-Chávez, E.; González, A.; Ruiz-Espinoza, F.; Ojeda-Barrios, D.; Hernandez-Montiel, L.G. Zinc biofortification improves yield, nutraceutical quality and antioxidant capacity in lettuce. Trop. Subtrop. Agroecosyst. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X.; Lv, D.; Wang, J.; Shen, Y.; Peng, S.; Yang, S.; Huang, J.; Sun, X. Synthesis of chitin nanocrystals supported Zn2+ with high activity against tobacco mosaic virus. Int. J. Biol. Macromol. 2023, 250, 126168. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bai, G.; Niu, C.; Feng, Y.; Wei, Z.; Lei, Z.; Chen, K.; Guo, X. Construction of charge-switchable and pH-sensitive carboxymethyl chitosan/Zn2+/heterocycle nanocomposite for the treatment of phytopathogens in agriculture. Chem. Eng. J. 2023, 473, 145308. [Google Scholar] [CrossRef]
- Xiang, S.; Chen, M.; Luo, X.; Zhang, S.; Shen, Y.; Chen, X.; Zhang, X.; Wang, J.; Tang, H.; Huang, J.; et al. Harnessing CNC-Carrier nanomaterials for enhanced Zn2+-mediated inhibition of oomycete asexual reproduction. J. Agric. Food Chem. 2025, 73, 8214–8224. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Yu, Y.; Huang, J.; Xu, Y.; Wang, A.; Liu, Z.; Zou, L. Metabolic Response of Sanghuangporus baumii to Zn2+ Induction and Biosynthesis of a Key Pharmacological Component: Triterpenoid. Microorganisms 2025, 13, 1067. https://doi.org/10.3390/microorganisms13051067
Tong X, Yu Y, Huang J, Xu Y, Wang A, Liu Z, Zou L. Metabolic Response of Sanghuangporus baumii to Zn2+ Induction and Biosynthesis of a Key Pharmacological Component: Triterpenoid. Microorganisms. 2025; 13(5):1067. https://doi.org/10.3390/microorganisms13051067
Chicago/Turabian StyleTong, Xinyu, Ying Yu, Jin Huang, Ying Xu, Anxin Wang, Zengcai Liu, and Li Zou. 2025. "Metabolic Response of Sanghuangporus baumii to Zn2+ Induction and Biosynthesis of a Key Pharmacological Component: Triterpenoid" Microorganisms 13, no. 5: 1067. https://doi.org/10.3390/microorganisms13051067
APA StyleTong, X., Yu, Y., Huang, J., Xu, Y., Wang, A., Liu, Z., & Zou, L. (2025). Metabolic Response of Sanghuangporus baumii to Zn2+ Induction and Biosynthesis of a Key Pharmacological Component: Triterpenoid. Microorganisms, 13(5), 1067. https://doi.org/10.3390/microorganisms13051067







