Synergistic Recruitment of Symbiotic Fungi by Potting and Scleroderma bovista Inoculation Suppresses Pathogens in Hazel Rhizosphere Microbiomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Measurement and Analysis of Root Parameters of Hazel Seedlings
2.3. Extraction and Sequencing of Soil DNA
2.4. Sequencing Data Analysis
2.5. Statistical Analysis
3. Results
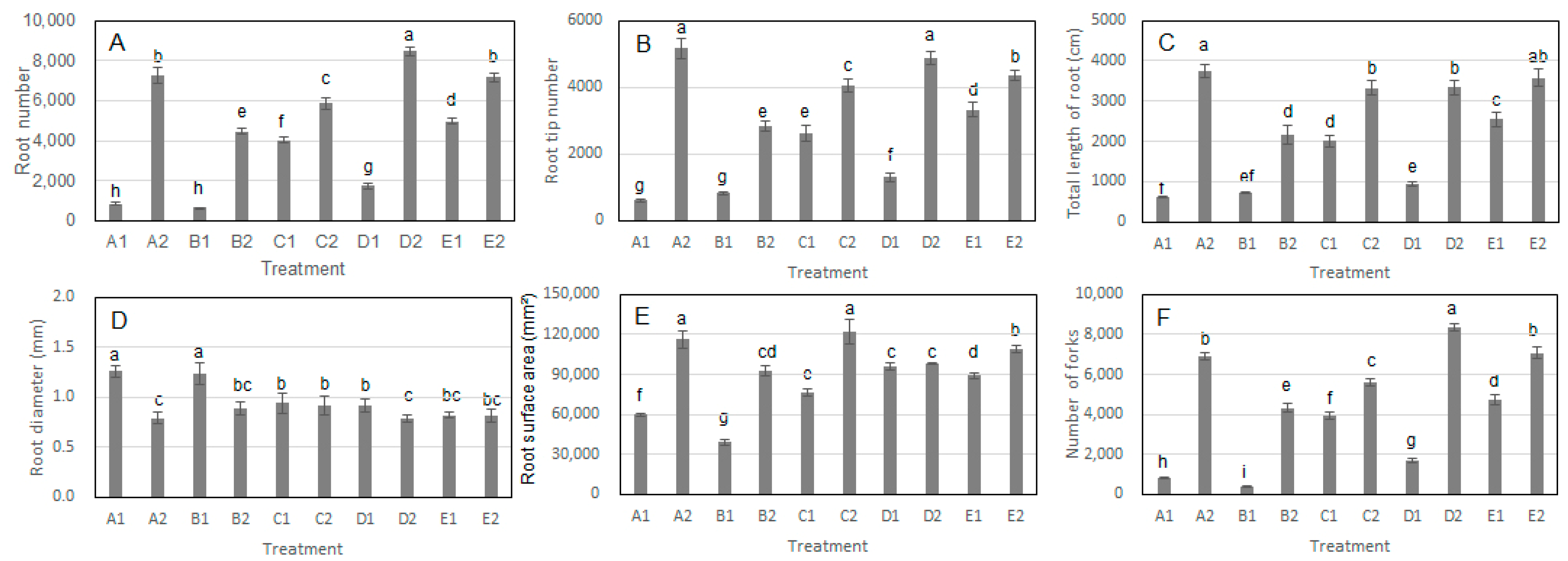
3.1. Effects of S. bovista Inoculation on Root Index in Split-Root Cultivation Experiment
3.2. Raw Data Quality Control and OTU/ASV Analysis
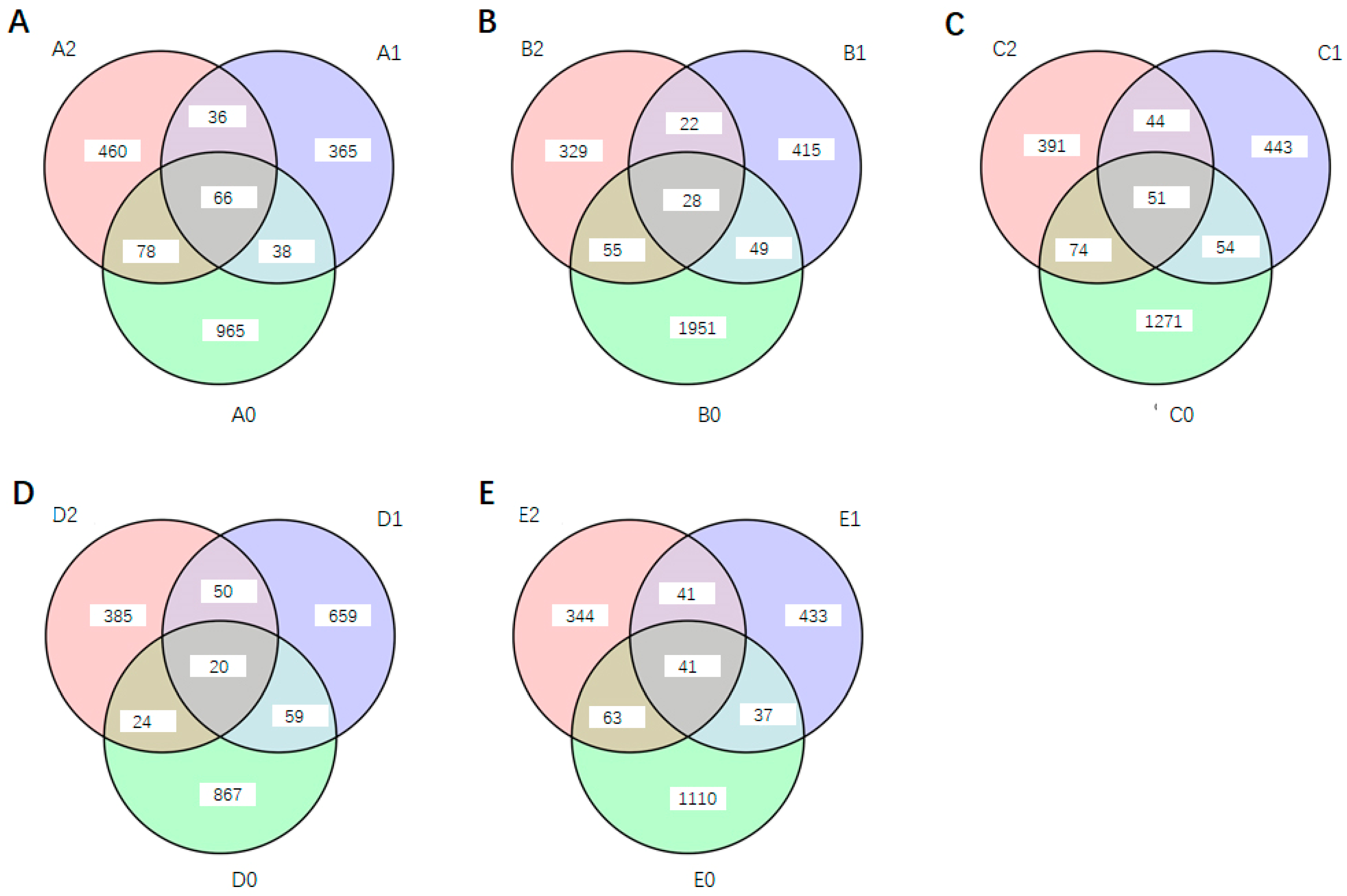
3.3. Impact of Potting and S. bovista Inoculation on Soil Feature Number
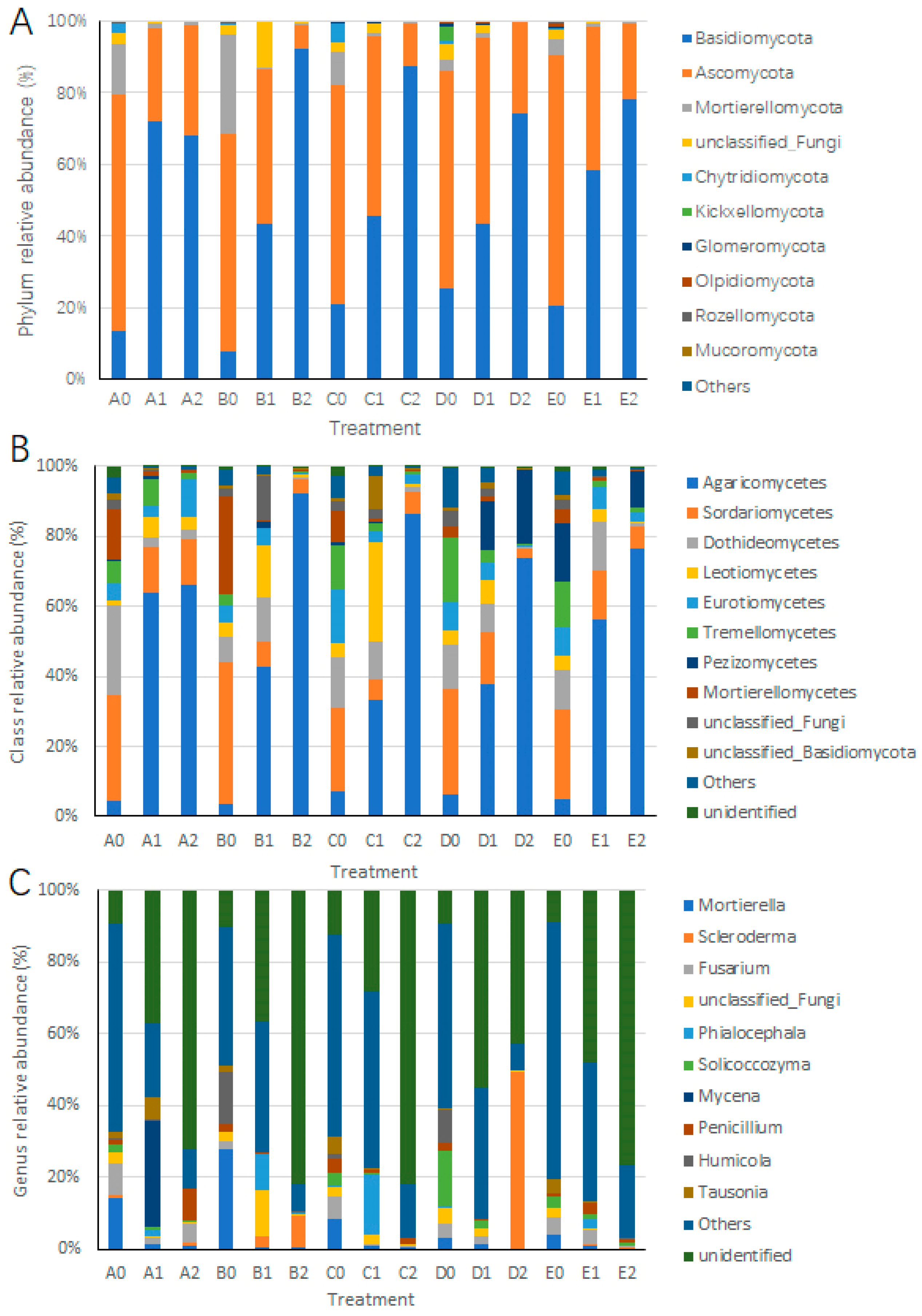
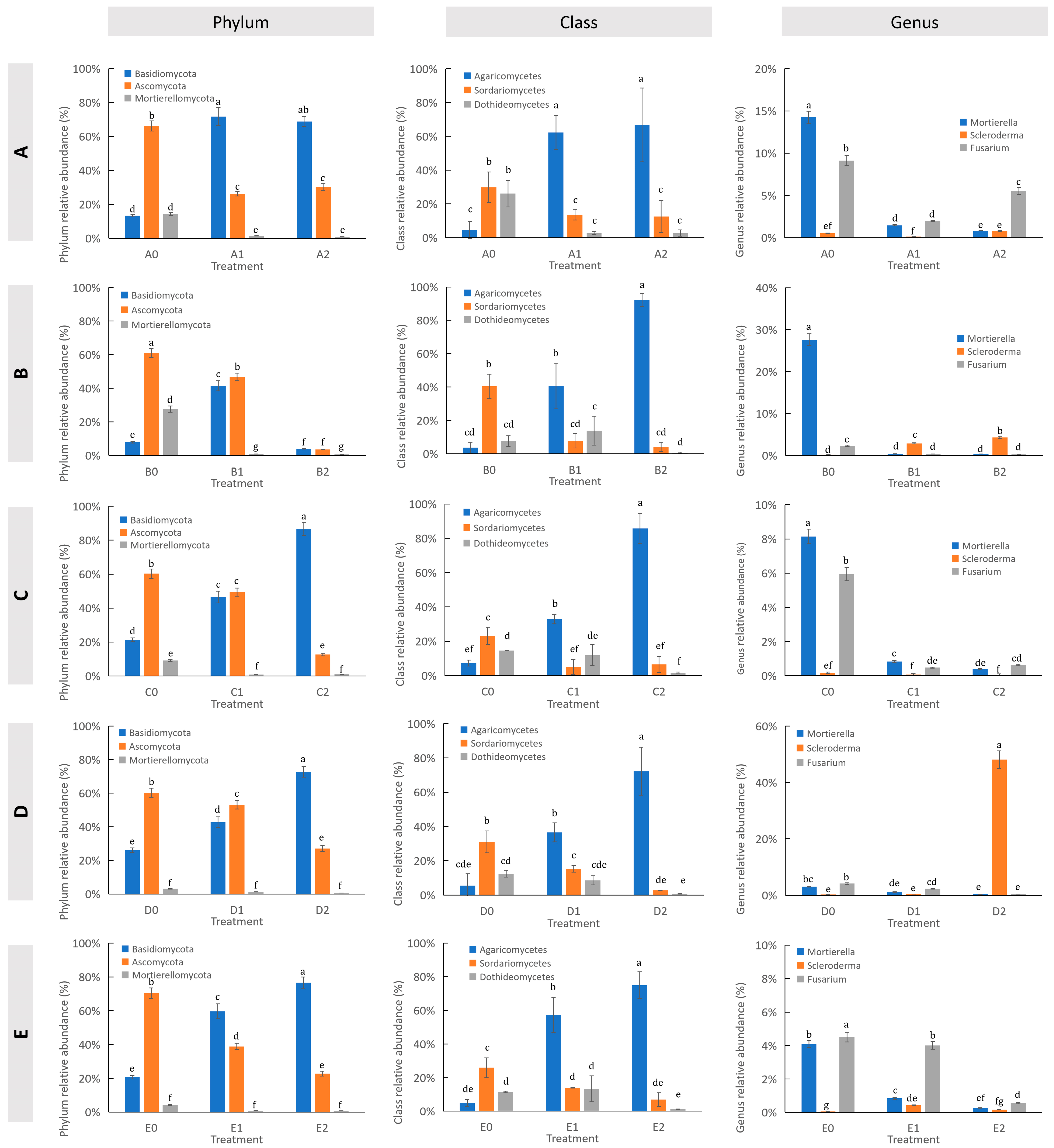
3.4. Impact of Hazel Seedling Potting on the Relative Abundance of Soil Fungi
3.5. Impact of S. bovista Inoculation on the Relative Abundance of Soil Fungi
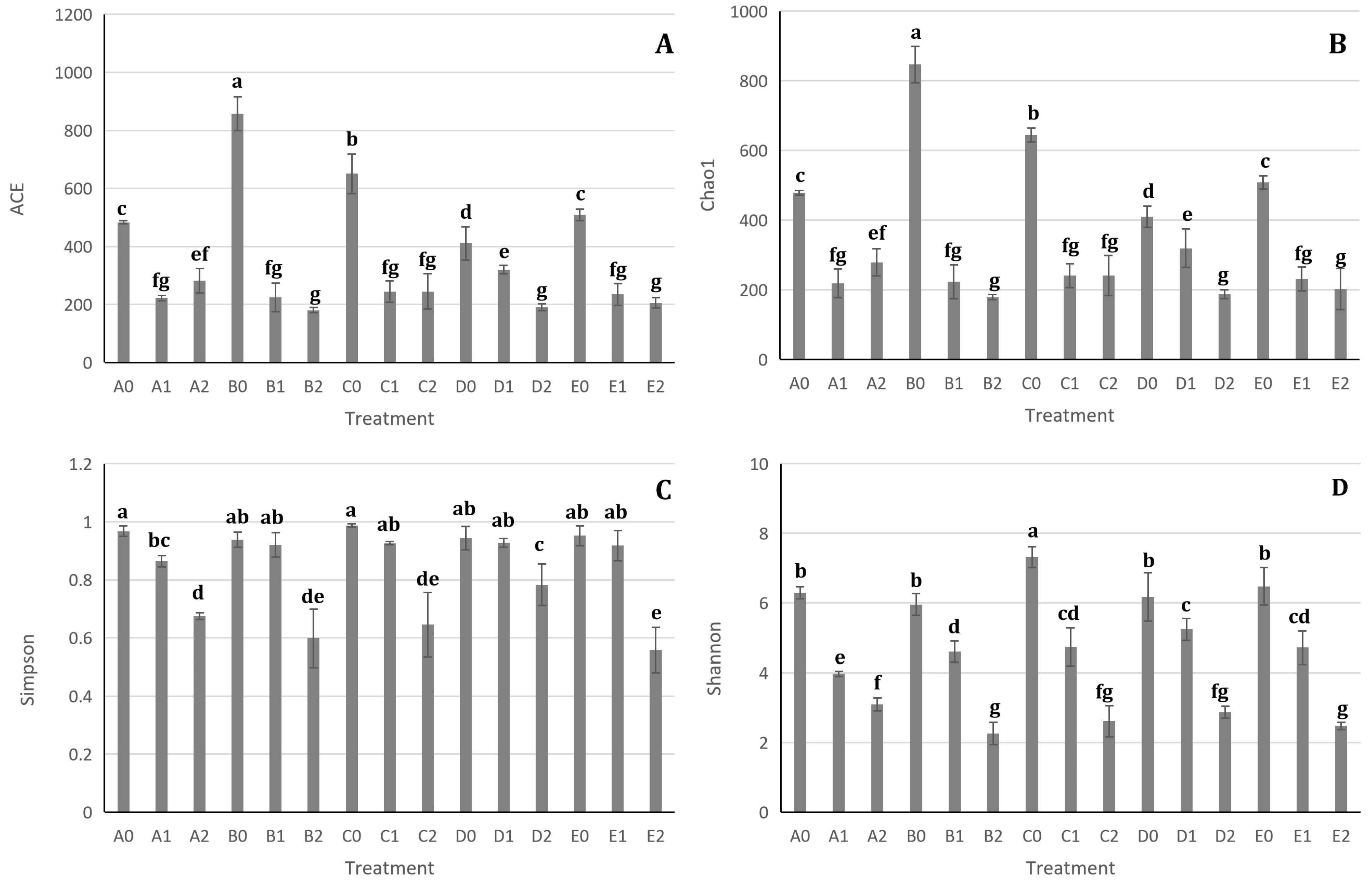
3.6. Impact of Hazel Seedling Potting and S. bovista Inoculation on Alpha Diversity of Soil Samples
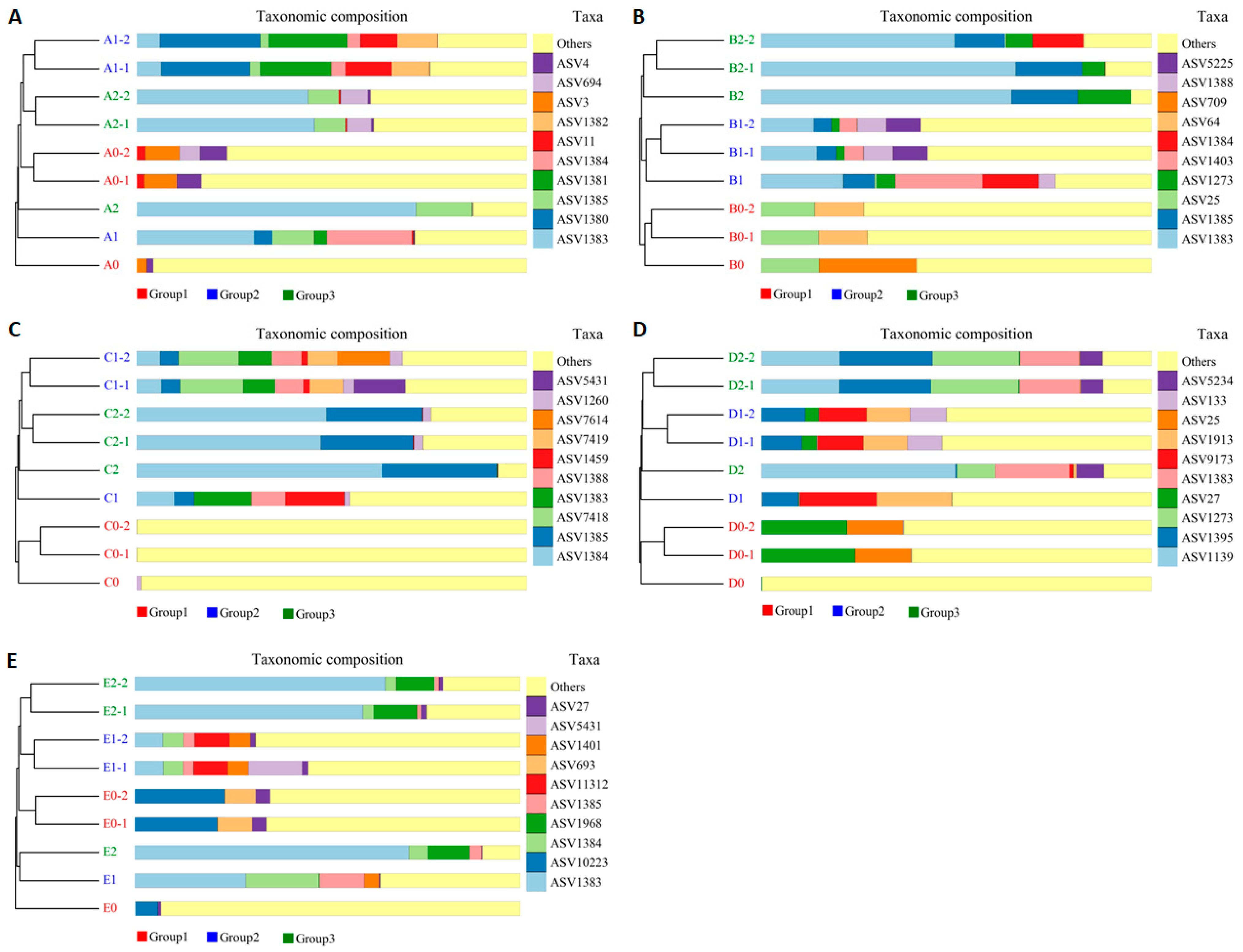
3.7. Impact of Hazel Seedling Potting and S. bovista Inoculation on Beta Diversity of Soil Samples
3.8. Fungal Phenotype Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, J.C.; Wang, X.L.; Wang, E. Mycorrhizal Symbiosis in Plant Growth and Stress Adaptation: From Genes to Ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Bodenhausen, N.; Hess, J.; Valzano-Held, A.; Waelchli, J.; Deslandes-Hérold, G.; Schlaeppi, K.; van der Heijden, M.G.A. Soil microbiome indicators can predict crop growth response to large-scale inoculation with arbuscular mycorrhizal fungi. Nat. Microbiol. 2023, 8, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Terrer, C.; Vicca, S.; Hungate, B.A.; Phillips, R.P.; Prentice, I.C. Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 2016, 353, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Q.; Sun, S.Y.; Lou, H.X.; Dong, Y.T.; He, H.L.; Mei, Q.; Liu, J.F. The ectomycorrhizal fungus Scleroderma bovista improves growth of hazelnut seedlings and plays a role in auxin signaling and transport. Front. Microbiol. 2024, 15, 1431120. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Yang, B.; Miao, R.Y.; Zhang, X.Z.; He, H.L.; Zhao, Y.B.; Liu, S.; Wu, D.Y.; Liu, J.F. Isolation, identification, and evaluation of an ectomycorrhizal fungus from a hazel orchard in China. Sci. Hortic. 2023, 309, 111679. [Google Scholar] [CrossRef]
- Santelices, R.; Palfner, G. Controlled rhizogenesis and mycorrhization of hazelnut (Corylus avellana L.) cuttings with black truffle (Tuber melanosporum Vitt.). Chil. J. Agric. Res. 2010, 70, 204–212. [Google Scholar] [CrossRef]
- Strati, S.; Paoletti, E.; Barbolani, E. Root length and distribution of chromium in Corylus avellana with Tuber albidum mycorrhizas. Water Air Soil Pollut. 1999, 113, 33–41. [Google Scholar] [CrossRef]
- Irmak Yilmaz, F. Seasonal changes of some microbiological properties of soils in a field of hazelnut (Corylus avellana L.) growing. Appl. Ecol. Environ. Res. 2020, 18, 253–262. [Google Scholar] [CrossRef]
- Ma, W.; Yang, Z.; Liang, L.; Ma, Q.; Wang, G.; Zhao, T. Seasonal changes in soil microbial community and co-occurrence network of species of the genus Corylus. Microorganisms 2021, 9, 2228. [Google Scholar] [CrossRef]
- Ma, W.X.; Zhen, Y.; Zhao, T.T. Effects of living cover on the soil microbial communities and ecosystem functions of hazelnut orchards. Front. Plant Sci. 2021, 12, 652493. [Google Scholar] [CrossRef] [PubMed]
- Román, M.; de Boa, E.; Woodward, S. Wild-gathered fungi for health and rural livelihoods. Proc. Nutr. Soc. 2006, 65, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wedén, C.; Pettersson, L.; Danell, E. Truffle cultivation in Sweden: Results from Quercus robur and Corylus avellana field trials on the island of Gotland. Scand. J. For. Res. 2009, 24, 37–53. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Gógán Csorbai, A.; Baciarelli Falini, L.; Bencivenga, M.; Di Massimo, G.; Donnini, D. Mycorrhization of Quercus robur L., Quercus cerris L. and Corylus avellana L. seedlings with Tuber macrosporum Vittad. Mycorrhiza 2012, 22, 639–646. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Gu, T.; Zhang, W.; Shen, Q. Continuous cropping alters the composition and function of rhizosphere soil microbial communities, leading to yield reduction in tobacco. Ann. Microbiol. 2021, 71, 1–12. [Google Scholar]
- Wang, T.; Persson, P.; Tunlid, A. A widespread mechanism in ectomycorrhizal fungi to access nitrogen from mineral-associated proteins. Environ. Microbiol. 2021, 23, 2345–2358. [Google Scholar] [CrossRef]
- Rohland, N.; Reich, D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012, 22, 939–946. [Google Scholar] [CrossRef]
- Senst, A.; Bonsiepe, H.; Kron, S.; Schulz, I. Application of the Agilent 2100 Bioanalyzer instrument as quality control for next-generation sequencing. J. Forensic Sci. 2024, 69, 2192–2196. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Method 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Kõljalg, U.; Larsson, K.H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. UNITE: A database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 2005, 166, 1063–1068. [Google Scholar] [CrossRef]
- Chao, A.; Lee, S.M. Estimating the Number of Classes via Sample Coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Chao, A. Non-Parametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, W.; Zhang, Z. Bacterial communities in home-made Doushen with and without chili pepper. Food Res. Int. 2022, 156, 111321. [Google Scholar] [CrossRef]
- Zimowska, B.; Ludwiczuk, A.; Manganiello, G.; Wojtanowski, K.; Kot, I.; Staropoli, A.; Vinale, F.; Nicoletti, R. Fusarium and Hazelnut: A Story of Twists and Turns. Agriculture 2024, 14, 1080. [Google Scholar] [CrossRef]
- Arciuolo, R.; Santos, C.; Soares, C.; Castello, G.; Spigolon, N.; Chiusa, G.; Lima, N.; Battilani, P. Molecular characterization of Diaporthe species associated with hazelnut defects. Front. Plant Sci. 2020, 11, 611655. [Google Scholar] [CrossRef] [PubMed]
- Arciuolo, R.; Chiusa, G.; Castello, G.; Camardo Leggieri, M.; Spigolon, N.; Battilani, P. Diaporthe spp. is confirmed as the main fungus associated with defective Turkish hazelnuts. Plant Health Prog. 2022, 23, 440–448. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, W.; Wang, J.F.; Guo, J.S.; Zhou, C. Root exudate-mediated assemblage of rhizo-microbiome enhances Fusarium wilt suppression in chrysanthemum. Microbiol. Res. 2024, 292, 128031. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Z.; Wang, S.; Fei, J.; Qu, Z.; Wu, H.; Zhao, M.; Yang, H. Protective role of Mortierella alpina-derived lipids in resisting root rot in Panax ginseng. Rhizosphere 2024, 32, 100994. [Google Scholar] [CrossRef]
- Chang, L.; Chen, H.; Tang, X.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Advances in improving the biotechnological application of oleaginous fungus Mortierella alpina. Appl. Microbiol. Biotechnol. 2021, 105, 6275–6289. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef]
- Hu, Q.P. Analysis on the Characteristics of Eight Phialocephala and the Inoculation Effect of the Two in Blueberry Seedling. Master’s Thesis, Beijing Forestry University, Bejing, China, 2019. [Google Scholar]
- An, C.R.; Li, Y.; Liu, C.H.; Chong, H.Y.; Wen, G.Q.; Nie, F.; Duan, R.Y. Effects of inoculating endophytic fungi on the growth and physiological indexes of blueberry seedlings. China Fruits 2022, 7, 16–22. [Google Scholar]
- Schlegel, M.; Münsterkötter, M.; Güldener, U.; Bruggmann, R.; Duò, A.; Hainaut, M.; Henrissat, B.; Sieber, C.M.; Hoffmeister, D.; Grünig, C.R. Globally distributed root endophyte Phialocephala subalpina links pathogenic and saprophytic lifestyles. BMC Genomics 2016, 17, 1015. [Google Scholar] [CrossRef]
- You, Y.H.; Yoon, H.; Kang, S.M.; Woo, J.R.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; Kim, J.G. Cadophora malorum Cs-8-1 as a new fungal strain producing gibberellins isolated from Calystegia soldanella. J. Basic Microbiol. 2013, 53, 630–634. [Google Scholar] [CrossRef]
- Chen, Y.L.; Kang, L.H.; Malajczuk, N.; Dell, B. Selecting ectomycorrhizal fungi for inoculating plantations in south China: Effect of Scleroderma on colonization and growth of exotic Eucalyptus globulus, Europhylla, Pinus elliottii, and P. radiata. Mycorrhiza 2006, 16, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Li, Y.; Zhang, X. Scleroderma species enhance plant growth and drought tolerance through improved nutrient uptake and antioxidant enzyme activities. Plant Soil 2020, 447, 123–136. [Google Scholar]
- Hirooka, Y.; Rossman, A.Y.; Chaverri, P. Thelonectria and Cylindrocladiella species associated with dying Quercus leaves in Japan and their potential as leaf saprotrophs. Mycologia 2012, 104, 1323–1336. [Google Scholar]
- Serrano, K.; Bezrutczyk, M.; Goudeau, D. Spatial co-transcriptomics reveals discrete stages of the arbuscular mycorrhizal symbiosis. Nat. Plants 2024, 10, 673–688. [Google Scholar] [CrossRef]
- Back, C.G.; Lee, C.Y.; Seo, G.S.; Jung, H.Y. Characterization of species of Cladobotryum which cause cobweb disease in edible mushrooms grown in Korea. Mycobiology 2012, 40, 189–194. [Google Scholar] [CrossRef]
- McKay, G.J.; Egan, D.; Morris, E.; Scott, C.; Brown, A.E. Genetic and morphological characterization of Cladobotryum species causing cobweb disease of mushrooms. Appl. Environ. Microbiol. 1999, 65, 606–610. [Google Scholar] [CrossRef]
- Gong, X.; Feng, Y.; Dang, K.; Jiang, Y.; Qi, H.; Feng, B. Linkages of microbial community structure and root exudates: Evidence from microbial nitrogen limitation in soils of crop families. Sci. Total Environ. 2023, 881, 163536. [Google Scholar] [CrossRef]
- Gallego, A.; Imseng, N.; Bonfill, M. Development of a hazel cell culture-based paclitaxel and baccatin III production process on a benchtop scale. J. Biotechnol. 2015, 195, 93–102. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, H. Paclitaxel’s dual role: Anticancer activity and antimicrobial effects. J. Antimicrob. Chemother. 2023, 78, 1012–1020. [Google Scholar]
- Yang, C.; Song, L.L.; Miao, Z.; Jiang, L.Y.; Li, T.; Zhi, X.Y.; Hao, X.J.; Cao, H. Discovery of novel obovatol-based phenazine analogs as potential antifungal agents: Synthesis and biological evaluation in vitro. Z. Naturforschung B 2021, 76, 173–179. [Google Scholar] [CrossRef]
- Kovács, B.; Béni, Z.; Dékány, M.; Bózsity, N.; Zupkó, I.; Hohmann, J.; Ványolós, A. Isolation and structure determination of antiproliferative secondary metabolites from the potato earthball mushroom, Scleroderma bovista (Agaricomycetes). Int. J. Med. Mushroom 2018, 20, 411–418. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Li, Y.; Yu, H.; He, H.; Cheng, Y.; Sun, S.; Liu, J. Synergistic Recruitment of Symbiotic Fungi by Potting and Scleroderma bovista Inoculation Suppresses Pathogens in Hazel Rhizosphere Microbiomes. Microorganisms 2025, 13, 1063. https://doi.org/10.3390/microorganisms13051063
Peng C, Li Y, Yu H, He H, Cheng Y, Sun S, Liu J. Synergistic Recruitment of Symbiotic Fungi by Potting and Scleroderma bovista Inoculation Suppresses Pathogens in Hazel Rhizosphere Microbiomes. Microorganisms. 2025; 13(5):1063. https://doi.org/10.3390/microorganisms13051063
Chicago/Turabian StylePeng, Cheng, Yuqing Li, Hengshu Yu, Hongli He, Yunqing Cheng, Siyu Sun, and Jianfeng Liu. 2025. "Synergistic Recruitment of Symbiotic Fungi by Potting and Scleroderma bovista Inoculation Suppresses Pathogens in Hazel Rhizosphere Microbiomes" Microorganisms 13, no. 5: 1063. https://doi.org/10.3390/microorganisms13051063
APA StylePeng, C., Li, Y., Yu, H., He, H., Cheng, Y., Sun, S., & Liu, J. (2025). Synergistic Recruitment of Symbiotic Fungi by Potting and Scleroderma bovista Inoculation Suppresses Pathogens in Hazel Rhizosphere Microbiomes. Microorganisms, 13(5), 1063. https://doi.org/10.3390/microorganisms13051063





