Qualitative Assessment of Microalgae–Bacteria Biofilm Development on K5 Carriers: Photoheterotrophic Growth in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms, Culture Medium, and Experimental Setup
2.2. K5 Carriers
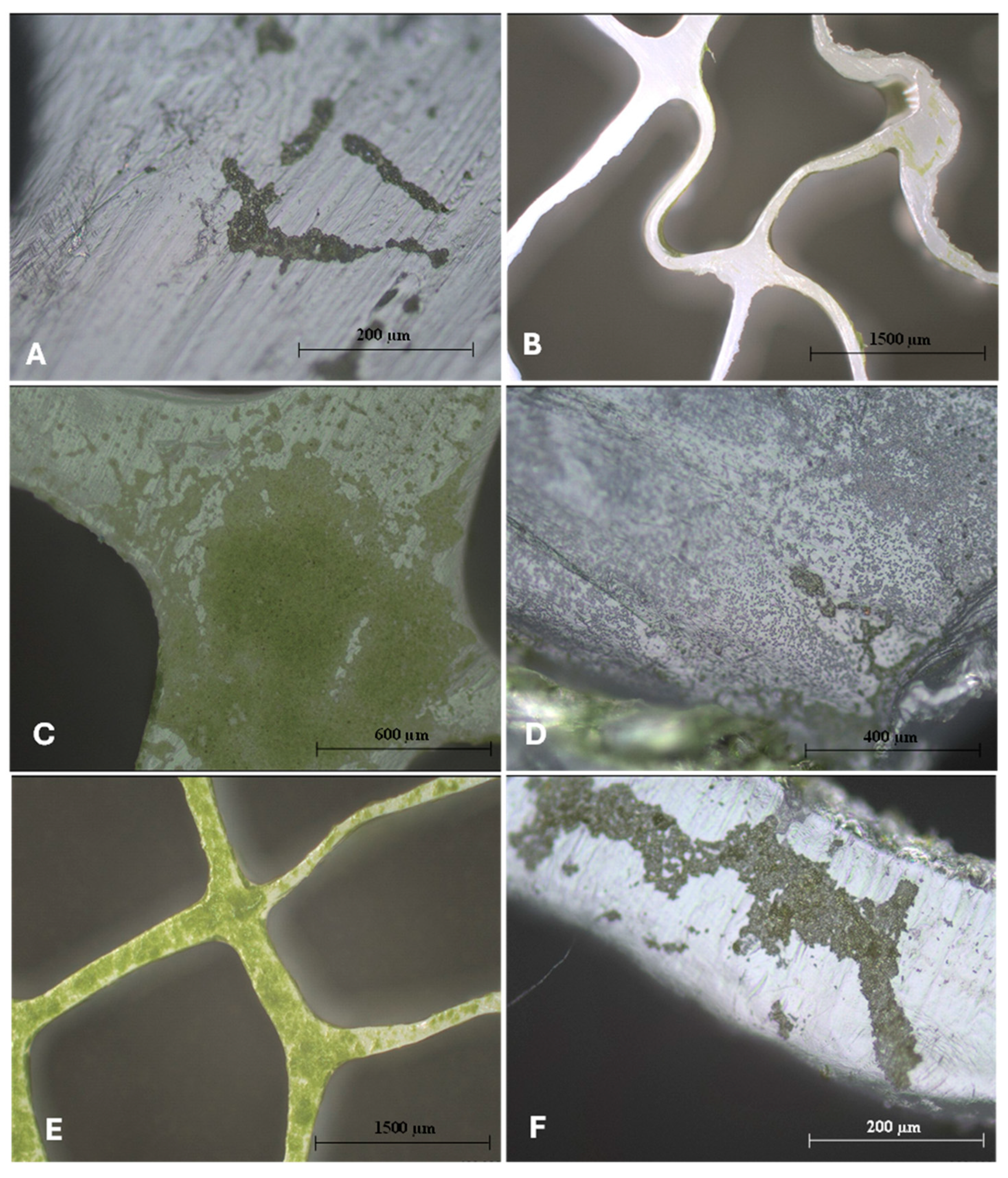
2.3. Profilometry
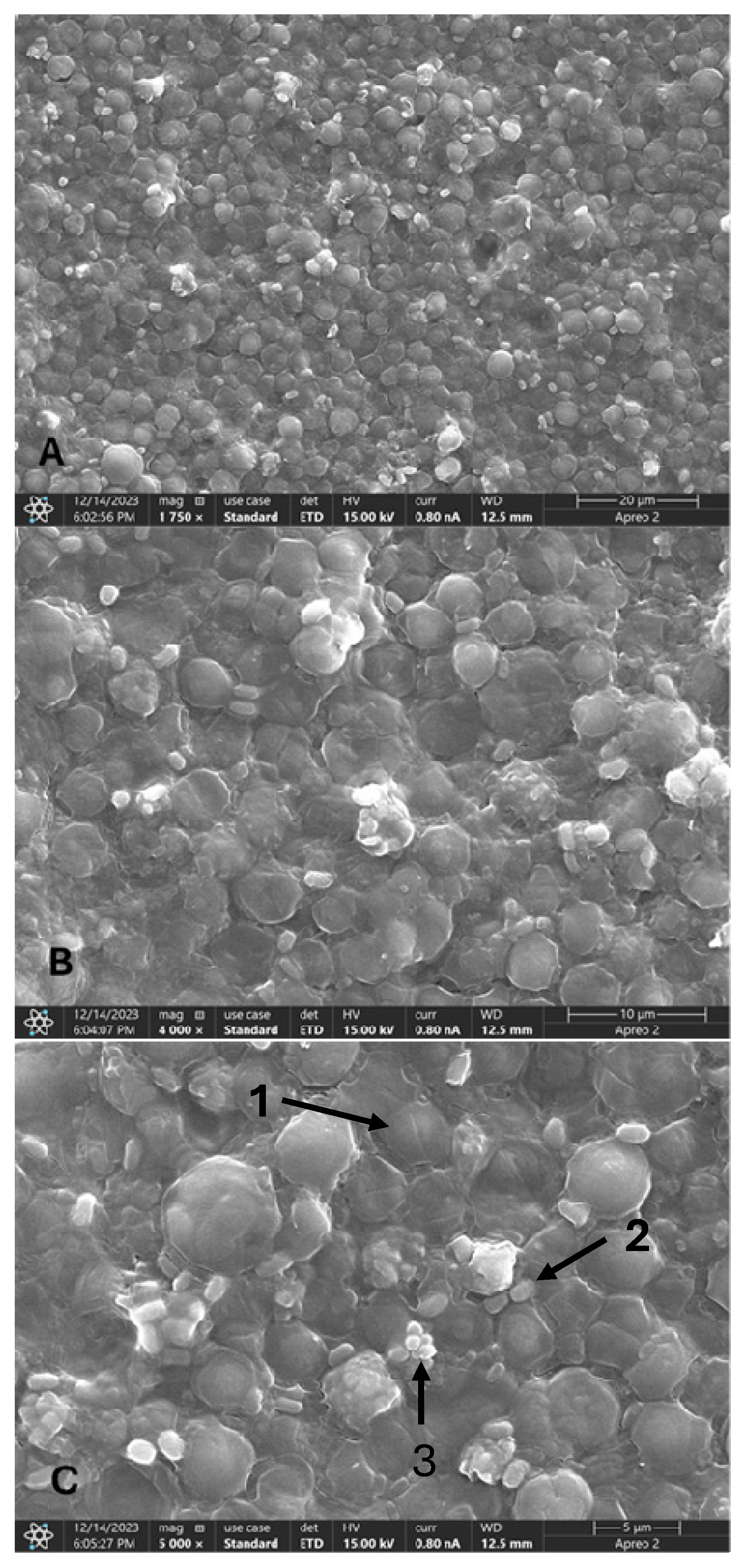
2.4. Scanning Electron Microscopy
2.5. Reproducibility of the Results and Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WW | Wastewater |
| WWTP | Wastewater Treatment Plant |
| SEM | Scanning Electron Microscopy |
| EPS | Extracellular Polymeric Substance |
| CEC | Contaminant of Emerging Concern |
| RAB | Rotating Algal Bioreactor |
| OECD | Organization for Economic Co-operation and Development |
| TSA | Tryptic Soy Agar |
| TSB | Tryptic Soy Broth |
| HDPE | High-Density Polyethylene |
References
- Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Show, P.L. Microalgal-Bacterial Consortia as Future Prospect in Wastewater Bioremediation, Environmental Management and Bioenergy Production. Indian J. Microbiol. 2021, 61, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Pires, J.; Simões, M. Wastewater polishing by consortia of Chlorella vulgaris and activated sludge native bacteria. J. Clean. Prod. 2016, 133, 348–357. [Google Scholar] [CrossRef]
- de Wilt, A.; Butkovskyi, A.; Tuantet, K.; Leal, L.H.; Fernandes, T.V.; Langenhoff, A.; Zeeman, G. Micropollutant removal in an algal treatment system fed with source separated wastewater streams. J. Hazard. Mater. 2016, 304, 84–92. [Google Scholar] [CrossRef]
- Kim, B.-H.; Ramanan, R.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Barranguet, C.; Boschker, H.T.S.; Herman, P.M.J.; Moens, T.; Heip, C.H.R. The fate of intertidal microphytobenthos carbon: An in situ 13C-labeling study. Limnol. Oceanogr. 2000, 45, 1224–1234. [Google Scholar] [CrossRef]
- Underwood, G.J.C.; Boulcott, M.; Raines, C.A.; Waldron, K. Environmental effects on exopolymer production by marine benthic diatoms: Dynamics, changes in composition, and pathways of production. J. Phycol. 2004, 40, 293–304. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Otondo, A.; Kokabian, B.; Stuart-Dahl, S.; Gude, V.G. Energetic evaluation of wastewater treatment using microalgae, Chlorella vulgaris. J. Environ. Chem. Eng. 2018, 6, 3213–3222. [Google Scholar] [CrossRef]
- Kim, J.; Lingaraju, B.P.; Rheaume, R.; Lee, J.; Siddiqui, K.F. Removal of ammonia from wastewater effluent by Chlorella vulgaris. Tsinghua Sci. Technol. 2010, 15, 391–396. [Google Scholar] [CrossRef]
- Ge, S.; Qiu, S.; Tremblay, D.; Viner, K.; Champagne, P.; Jessop, P.G. Centrate wastewater treatment with Chlorella vulgaris: Simultaneous enhancement of nutrient removal, biomass and lipid production. Chem. Eng. J. 2018, 342, 310–320. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ansari, F.A.; Shriwastav, A.; Sahoo, N.K.; Rawat, I.; Bux, F. Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J. Clean. Prod. 2016, 115, 255–264. [Google Scholar] [CrossRef]
- Rani, S.; Ojha, C.S.P. Chlorella sorokiniana for integrated wastewater treatment, biomass accumulation and value-added product estimation under varying photoperiod regimes: A comparative study. J. Water Process Eng. 2021, 39, 101889. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X. Impact of the accumulation and adhesion of released oxygen during Scenedesmus obliquus photosynthesis on biofilm formation and growth. Bioresour. Technol. 2017, 244, 198–205. [Google Scholar] [CrossRef]
- Sousa, H.; Sousa, C.A.; Vale, F.; Santos, L.; Simões, M. Removal of parabens from wastewater by Chlorella vulgaris-bacteria co-cultures. Sci. Total Environ. 2023, 884, 163746. [Google Scholar] [CrossRef] [PubMed]
- Bark, K.; Sponner, A.; Kämpfer, P.; Grund, S.; Dott, W. Differences in polyphosphate accumulation and phosphate adsorption by Acinetobacter isolates from wastewater producing polyphosphate: AMP phosphotransferase. Water Res. 1992, 26, 1379–1388. [Google Scholar] [CrossRef]
- Veuillet, F.; Lacroix, S.; Bausseron, A.; Gonidec, E.; Ochoa, J.; Christensson, M.; Lemaire, R. Integrated fixed-film activated sludge ANITA™Mox process—A new perspective for advanced nitrogen removal. Water Sci. Technol. 2013, 69, 915–922. [Google Scholar] [CrossRef]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef]
- Sousa, C.A.; Sousa, H.; Vale, F.; Simoes, M. Microalgae-based bioremediation of wastewaters-Influencing parameters and mathematical growth modelling. Chem. Eng. J. 2021, 425, 131412. [Google Scholar] [CrossRef]
- Vale, F.; Sousa, C.A.; Sousa, H.; Santos, L.; Simões, M. Impact of parabens on microalgae bioremediation of wastewaters: A mechanistic study. Chem. Eng. J. 2022, 442, 136374. [Google Scholar] [CrossRef]
- Hodaifa, G.; Martínez, M.E.; Sánchez, S. Use of industrial wastewater from olive-oil extraction for biomass production of Scenedesmus obliquus. Bioresour. Technol. 2008, 99, 1111–1117. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Barata, A.; Batista, A.P.; Gouveia, L. Scenedesmus obliquus in poultry wastewater bioremediation. Environ. Technol. 2019, 40, 3735–3744. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD: Paris, France, 2011. [Google Scholar] [CrossRef]
- Simões, L.C.; Simões, M.; Oliveira, R.; Vieira, M.J. Potential of the adhesion of bacteria isolated from drinking water to materials. J. Gen. Microbiol. 2007, 47, 174–183. [Google Scholar] [CrossRef]
- Neogen. Tryptic Soy Broth (TSB)–Technical Specification Sheet. Available online: https://www.neogen.com/49891f/globalassets/pim/assets/original/10044/tss-400000733.pdf (accessed on 12 December 2024).
- Arabgol, R.; Vanrolleghem, P.A.; Piculell, M.; Delatolla, R. The impact of biofilm thickness-restraint and carrier type on attached growth system performance, solids characteristics and settleability. Environ. Sci. Water Res. Technol. 2020, 6, 2843–2855. [Google Scholar] [CrossRef]
- T.M.I. Keyence VK-X1100 Optical Profilometer. Available online: https://tmi.utexas.edu/facilities/instrumentation/keyence-vk-x1100-optical-profilometer (accessed on 12 December 2024).
- Burmaster, D.; Kainer, A.; Lu, Y. Effects of surface roughness on wax deposition. Geoenergy Sci. Eng. 2023, 231, 212383. [Google Scholar] [CrossRef]
- Vale, F.; Sousa, C.A.; Sousa, H.; Simões, L.C.; McBain, A.J.; Simões, M. Bacteria and microalgae associations in periphyton—Mechanisms and biotechnological opportunities. FEMS Microbiol. Rev. 2023, 47, fuad047. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef]
- Sekar, R.; Venugopalan, V.P.; Satpathy, K.K.; Nair, K.V.K.; Rao, V.N.R. Laboratory studies on adhesion of microalgae to hard substrates. In Proceedings of the Asian Pacific Phycology in the 21st Century: Prospects and Challenges, Hong Kong, China, 21–25 June 1999; pp. 109–116. [Google Scholar]
- Irving, T.E.; Allen, D.G. Species and material considerations in the formation and development of microalgal biofilms. Appl. Microbiol. Biotechnol. 2011, 92, 283–294. [Google Scholar] [CrossRef]
- Liang, W.; Yang, B.; Bin, L.; Hu, Y.; Fan, D.; Chen, W.; Li, P.; Tang, B. Intensifying the simultaneous removal of nitrogen and phosphorus of an integrated aerobic granular sludge-membrane bioreactor by Acinetobacter junii. Bioresour. Technol. 2024, 397, 130474. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, L.; Yang, K.; Wang, H. Investigation of rapid granulation in SBRs treating aniline-rich wastewater with different aniline loading rates. Sci. Total Environ. 2019, 646, 841–849. [Google Scholar] [CrossRef]
- Zeng, W.; Li, P.; Huang, Y.; Xia, A.; Zhu, X.; Zhu, X.; Liao, Q. How Interfacial Properties Affect Adhesion: An Analysis from the Interactions between Microalgal Cells and Solid Substrates. Langmuir 2022, 38, 3284–3296. [Google Scholar] [CrossRef]
- Soriano-Jerez, Y.; Macías-de la Rosa, A.; García-Abad, L.; López-Rosales, L.; Maza-Márquez, P.; García-Camacho, F.; Bressy, C.; Cerón-García, M.C.; Molina-Grima, E. Transparent antibiofouling coating to improve the efficiency of Nannochloropsis gaditana and Chlorella sorokiniana culture photobioreactors at the pilot-plant scale. Chemosphere 2024, 347, 140669. [Google Scholar] [CrossRef]
- Droumpali, A.; Hübner, J.; Gram, L.; Taboryski, R. Fabrication of Microstructured Surface Topologies for the Promotion of Marine Bacteria Biofilm. Micromachines 2021, 12, 926. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Lai, Z.; Zhang, X.; Jiang, Z.; Zhang, X. Analyzing microalgal biofilm structures formed under different light conditions by evaluating cell–cell interactions. J. Colloid Interface Sci. 2021, 583, 563–570. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Wang, Y.; Guan, L.; Zeng, Z.; Jiang, Z.; Zhang, X. Cell Surface Energy Affects the Structure of Microalgal Biofilm. Langmuir 2020, 36, 3057–3063. [Google Scholar] [CrossRef]
- Russel, M.; Meixue, Q.; Alam, M.A.; Lifen, L.; Daroch, M.; Blaszczak-Boxe, C.; Gupta, G. Investigating the potentiality of Scenedesmus obliquus and Acinetobacter pittii partnership system and their effects on nutrients removal from synthetic domestic wastewater. Bioresour. Technol. 2020, 299, 122571. [Google Scholar] [CrossRef]
- Rokhbakhsh-Zamin, F.; Sachdev, D.; Kazemi-Pour, N.; Engineer, A.; Pardesi, K.; Zinjarde, S.; Dhakephalkar, P.; Chopade, B. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011, 21, 556–566. [Google Scholar] [CrossRef]
- Sousa, H.; Simões, L.C.; Sousa, C.A.; Simões, M. Influence of bacterial load on the development and performance of a photo-heterotrophic biofilm community for wastewater polishing. Chem. Eng. J. 2024. under review. [Google Scholar]
- Benedict, R.G.; Carlson, D.A. Aerobic heterotrophic bacteria in activated sludge. Water Res. 1971, 5, 1023–1030. [Google Scholar] [CrossRef]
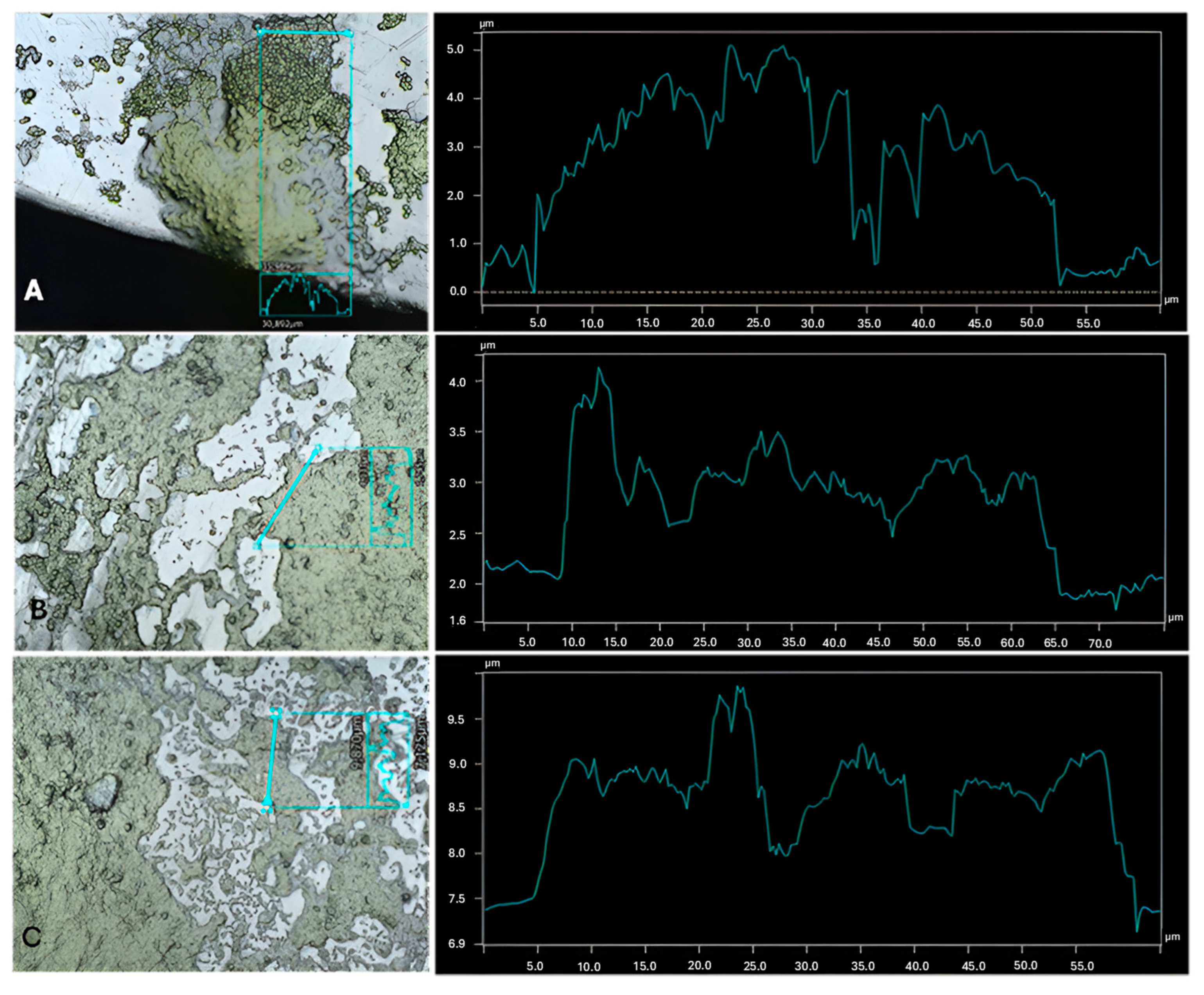
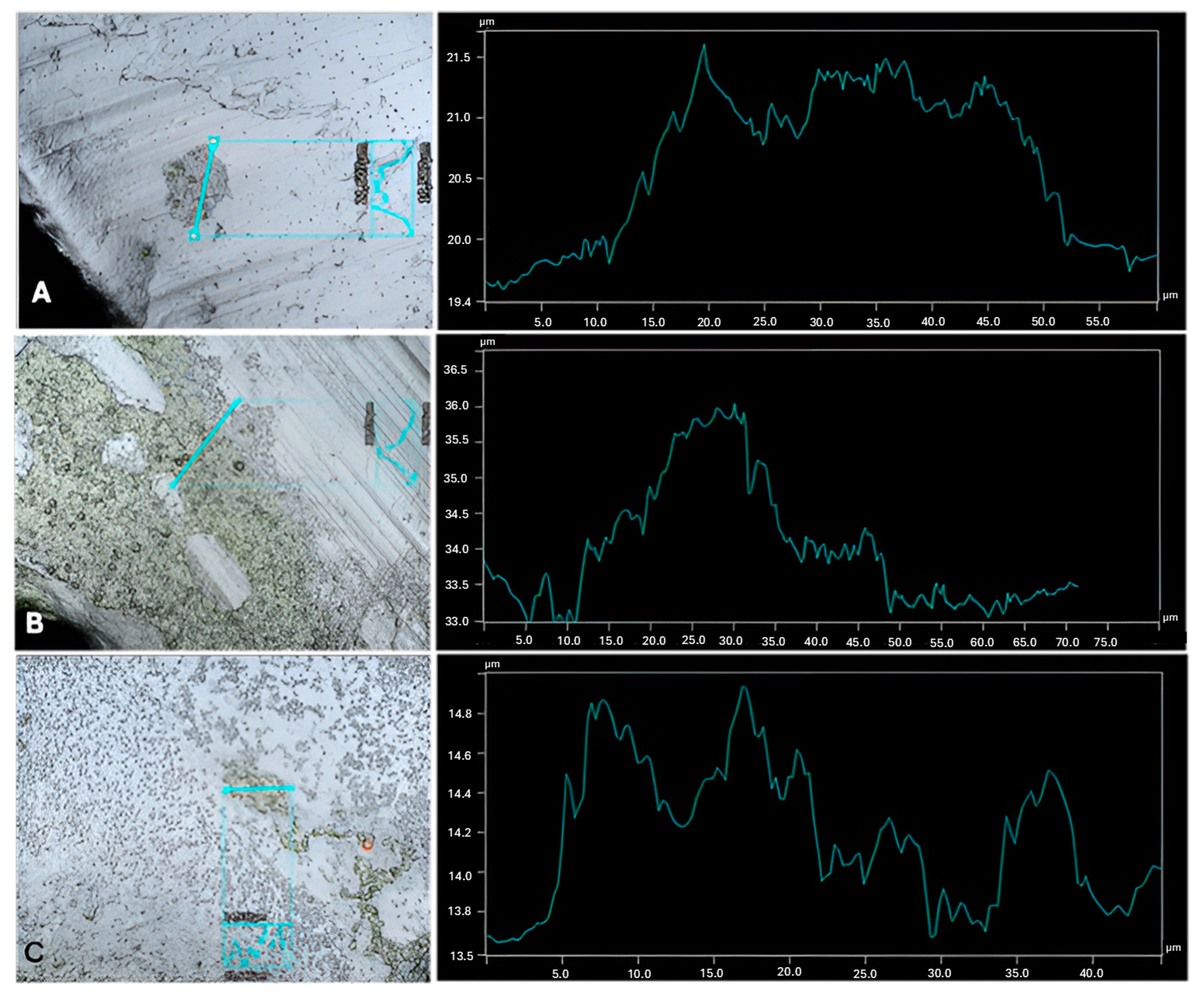

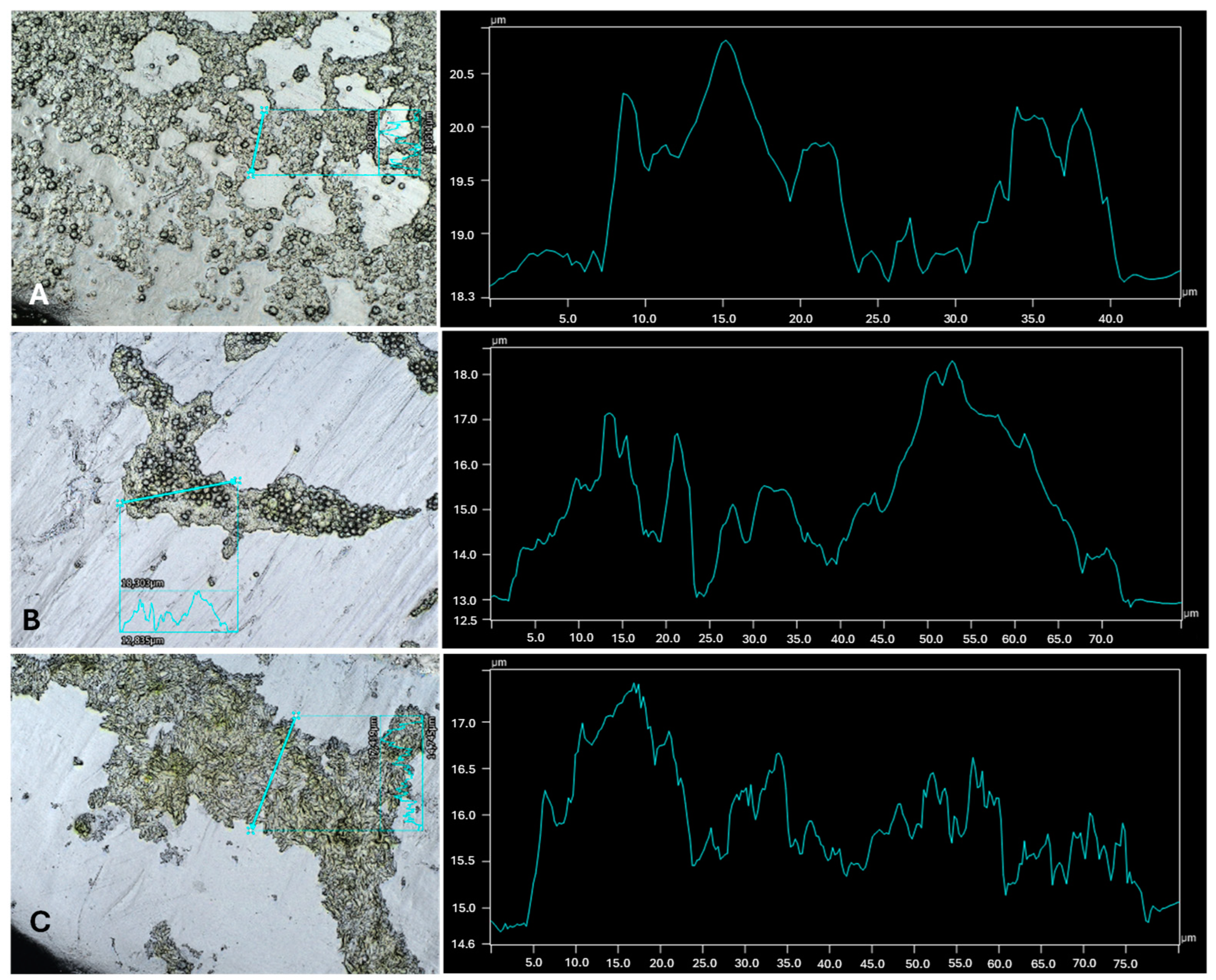
| Cultures | Highest Thickness (µm) | Average Thickness (µm) | Biggest Peak (µm) |
|---|---|---|---|
| C. vulgaris | 3.06 ± 0.9 a | 2.42 ± 0.7 a | 1.79 ± 0.7 a,b |
| C. vulgaris with A. calcoaceticus | 1.91 ± 0.1 a,b | 1.20 ± 0.1 b | 0.95 ± 0.1 a |
| C. vulgaris with R. fascians | 2.29 ± 0.4 a | 1.37 ± 0.3 b | 1.27 ± 0.3 a |
| C. vulgaris with Leucobacter sp. | 1.54 ± 0.2 b | 1.03 ± 0.1 b | 0.89 ± 0.2 c |
| C. sorokiniana | 2.55 ± 0.7 a | 1.07 ± 0.1 b | 1.21 ± 0.3 a |
| C. sorokiniana with A. calcoaceticus | 4.51 ± 0.8 c | 2.80 ± 0.9 a | 2.36 ± 0.5 a,b |
| C. sorokiniana with R. fascians | 3.44 ± 0.5 a,c | 2.39 ± 0.6 a | 2.14 ± 0.5 a,b |
| C. sorokiniana with Leucobacter sp. | 2.51 ± 0.3 a | 2.09 ± 0.4 a | 1.46 ± 0.3 a |
| S. obliquus | 3.25 ± 0.3 a,c | 1.93 ± 0.3 a | 1.72 ± 0.2 a |
| S. obliquus with A. calcoaceticus | 3.18 ± 0.6 a,c | 1.58 ± 0.5 a | 1.59 ± 0.2 a |
| S. obliquus with R. fascians | 1.78 ± 0.3 b | 1.38 ± 0.2 a | 1.12 ± 0.2 a |
| S. obliquus with Leucobacter sp. | 3.15 ± 0.3 a,c | 2.21 ± 0.2 a | 1.44 ± 0.2 a |
| A. calcoaceticus | 0.61 ± 0.2 d | 0.67 ± 0.4 c | 0.43 ± 0.2 d |
| R. fascians | 1.01 ± 0.2 e | 0.71 ± 0.1 c | 0.62 ± 0.1 d |
| Leucobacter sp. | 0.43 ± 0.1 f | 0.32 ± 0.1 d | 0.27 ± 0.1 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, H.; Kinney, K.A.; Sousa, C.A.; Simões, M. Qualitative Assessment of Microalgae–Bacteria Biofilm Development on K5 Carriers: Photoheterotrophic Growth in Wastewater. Microorganisms 2025, 13, 1060. https://doi.org/10.3390/microorganisms13051060
Sousa H, Kinney KA, Sousa CA, Simões M. Qualitative Assessment of Microalgae–Bacteria Biofilm Development on K5 Carriers: Photoheterotrophic Growth in Wastewater. Microorganisms. 2025; 13(5):1060. https://doi.org/10.3390/microorganisms13051060
Chicago/Turabian StyleSousa, Henrique, Kerry A. Kinney, Cátia A. Sousa, and Manuel Simões. 2025. "Qualitative Assessment of Microalgae–Bacteria Biofilm Development on K5 Carriers: Photoheterotrophic Growth in Wastewater" Microorganisms 13, no. 5: 1060. https://doi.org/10.3390/microorganisms13051060
APA StyleSousa, H., Kinney, K. A., Sousa, C. A., & Simões, M. (2025). Qualitative Assessment of Microalgae–Bacteria Biofilm Development on K5 Carriers: Photoheterotrophic Growth in Wastewater. Microorganisms, 13(5), 1060. https://doi.org/10.3390/microorganisms13051060







