Native Bacterial Communities of Two Italian Salso-Bromo-Jodic and Sulphurous Natural Mineral Waters
Abstract
1. Introduction
2. Materials and Methods
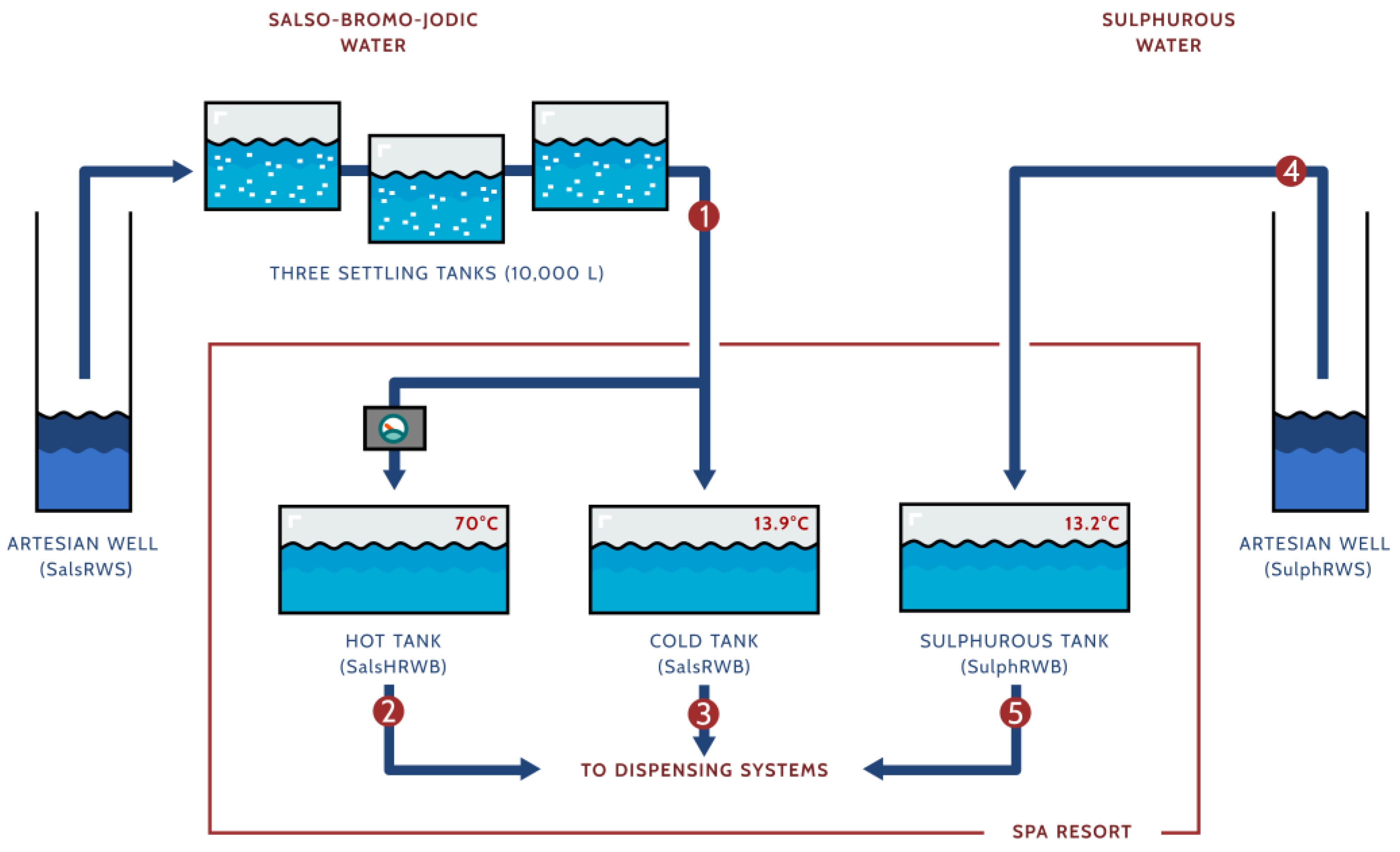
2.1. Thermal Bath Setting
2.2. Study Design
2.2.1. Samples Collection
- The exit of the third settling tank at the site of the salso-bromo-jodic natural source (SalsRWS).
- The exit of the salso-bromo-jodic storage tank at 70 °C within the spa resort (Hot tank; SalsHRWB).
- The exit of the salso-bromo-jodic storage tank at the natural source temperature within the spa resort (Cold tank; SalsRWB).
- The sulphurous natural source (SulphRWS).
- The exit of the sulphurous storage tank at the natural source temperature within the spa resort (Sulphurous tank; SulphRWB).
2.2.2. Library Preparation and Sequencing
2.2.3. Bioinformatic Analysis
2.2.4. Diversity Analyses
3. Results
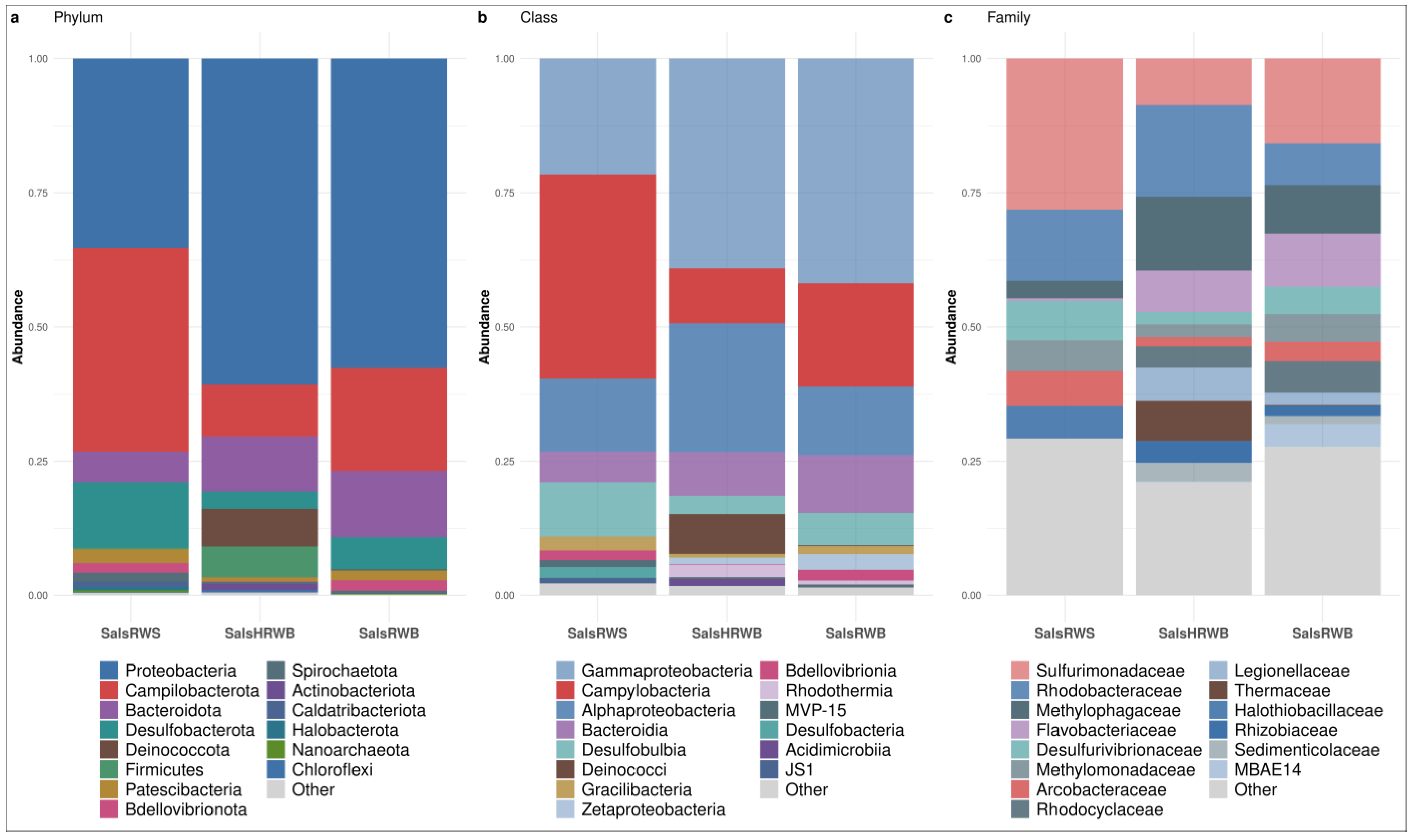
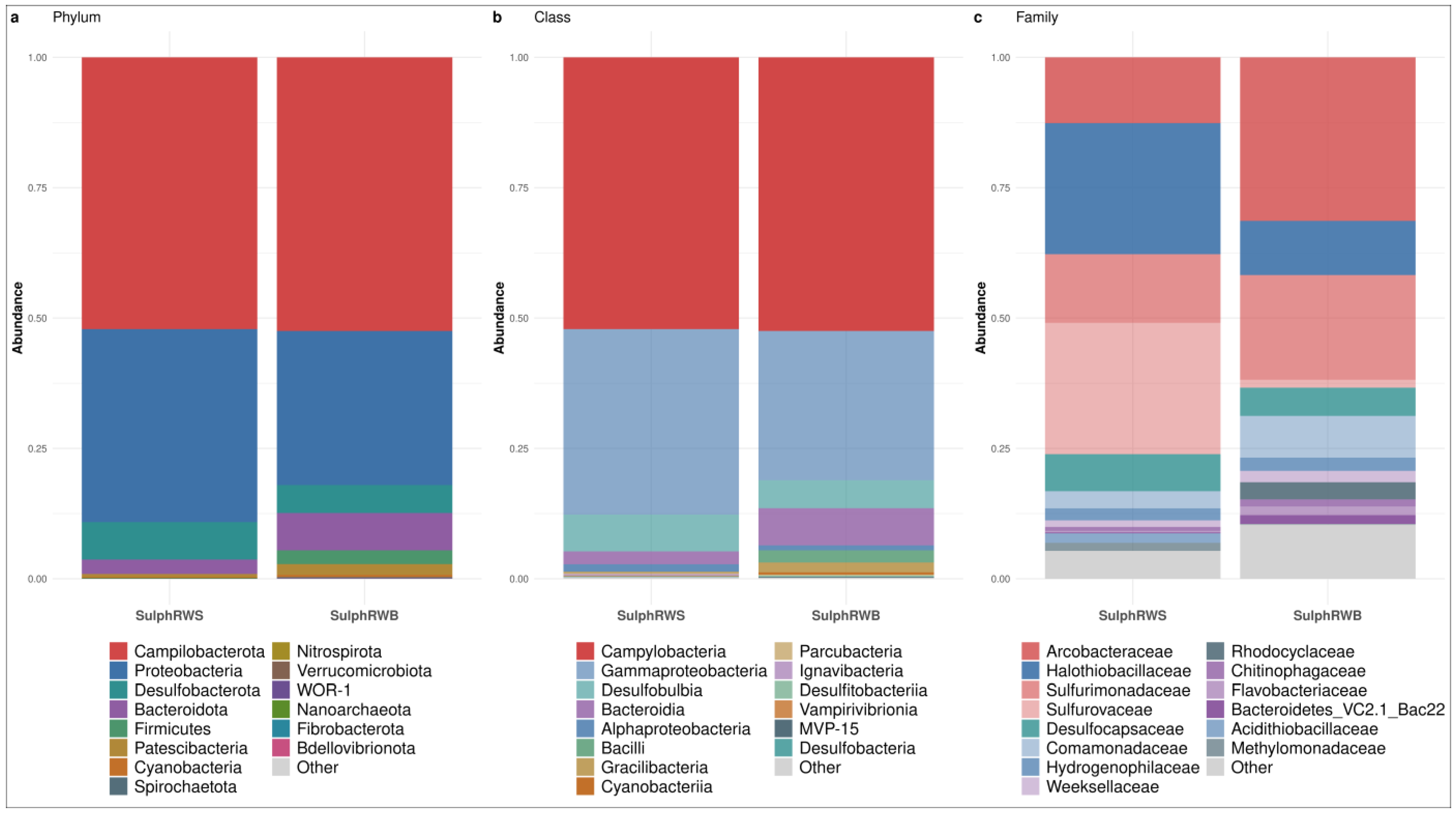
3.1. Bacterial Community Structure of the Waters
3.1.1. Bacterial Community Structure in Salso-Bromo-Jodic Waters
3.1.2. Bacterial Community Structure in Sulphurous Waters
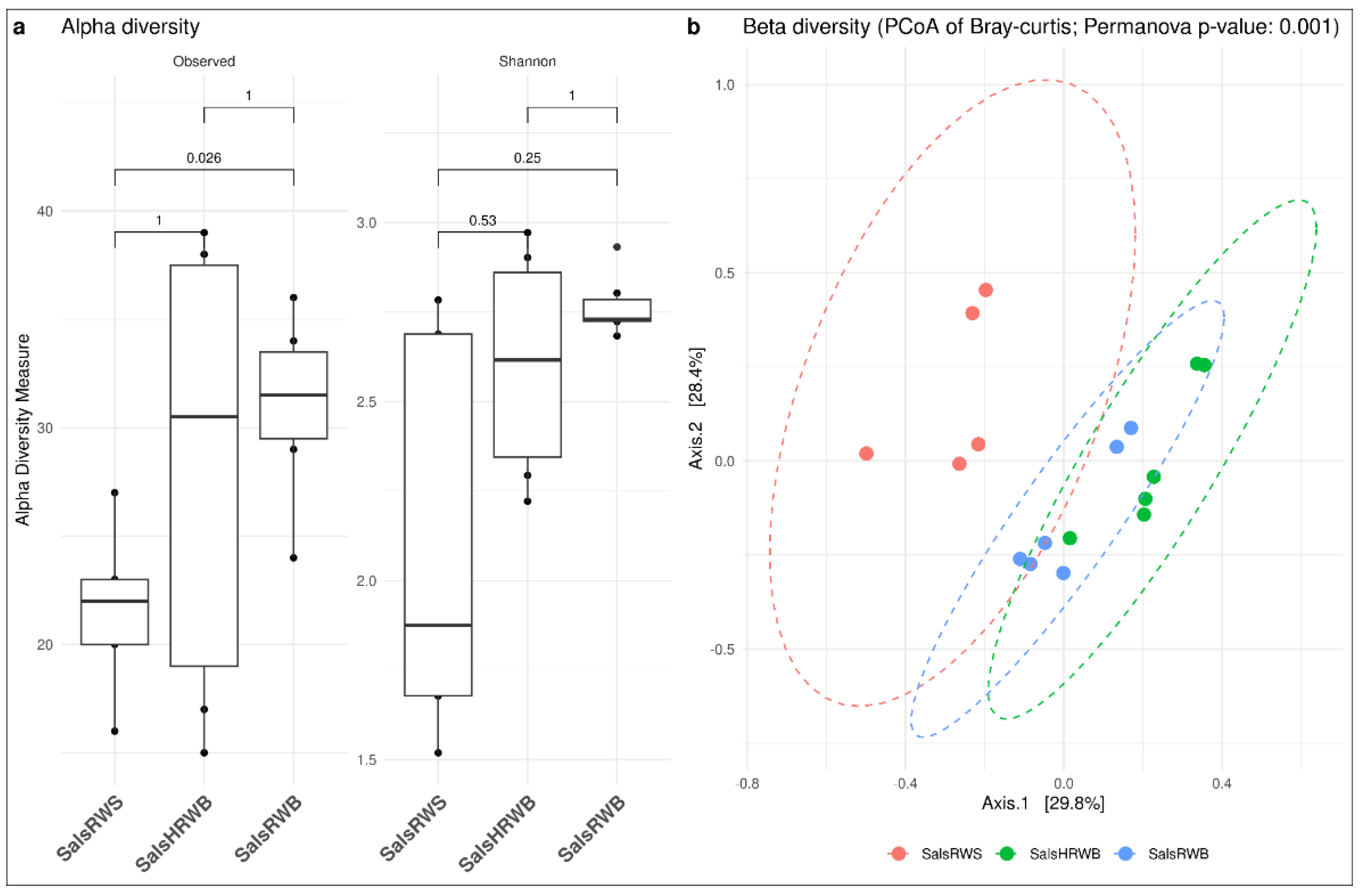
3.2. Diversity Analyses
3.2.1. Diversity Analysis in Salso-Bromo-Jodic Water
3.2.2. Diversity Analysis in Sulphurous Water
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeichner, J.; Seite, S. From probiotic to prebiotic using thermal spring water. J. Drugs Dermatol. 2018, 17, 657–662. [Google Scholar]
- Staquet, M.J.; Peguet-Navarro, J.; Richard, A.; Schmitt, D.; Rougier, A. In vitro effect of a spa water on the migratory and stimulatory capacities of human Langerhans calls. Eur. J. Dermatol. 2002, 12, LIX–LXI. [Google Scholar]
- Seite, S.; Benech, F.; Berdah, S.; Bayer, M.; Veyrat, S.; Segot, E.; Sakalikova, M.; Gibejova, L.; Zelenkova, H. Management of rosacea-prone skin: Evaluation of a skincare product containing Ambophenol, Neurosensine, and La Roche-Posay Thermal spring water as monotherapy or adjunctive therapy. J. Drugs Dermatol. 2013, 12, 920–924. [Google Scholar] [PubMed]
- Gueniche, A.; Liboutet, M.; Cheilian, S.; Fagot, D.; Juchaux, F.; Breton, L. Vitreoscilla filiformis Extract for Topical Skin Care: A Review. Front. Cell Infect. Microbiol. 2021, 11, 747663. [Google Scholar] [CrossRef] [PubMed]
- Mahé, Y.F.; Martin, R.; Aubert, L.; Billoni, N.; Collin, C.; Pruche, F.; Bastien, P.; Drost, S.S.; Lane, A.T.; Meybeck, A. Induction of the skin endogenous protective mitochondrial MnSOD by Vitreoscilla filiformis extract. Int. J. Cosmet. Sci. 2006, 28, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Woolery-Lloyd, H.; Andriessen, A.; Day, D.; Gonzalez, N.; Green, L.; Grice, E.; Henry, M. Review of the microbiome in skin aging and the effect of a topical prebiotic containing thermal spring water. J. Cosmet. Dermatol. 2023, 22, 96–102. [Google Scholar] [CrossRef]
- Liang, J.; Kang, D.; Wang, Y.; Yu, Y.; Fan, J.; Takashi, E. Carbonate ion-enriched hot spring water promotes skin wound healing in nude rats. PLoS ONE 2015, 10, e0117106. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Unveiling the role of minerals and trace elements of thermal waters in skin health. Appl. Sci. 2024, 14, 6291. [Google Scholar] [CrossRef]
- Faga, A.; Nicoletti, G.; Gregotti, C.; Finotti, V.; Nitto, A.; Gioglio, L. Effects of thermal water on skin regeneration. Int. J. Mol. Med. 2012, 29, 732–740. [Google Scholar] [CrossRef]
- Nicoletti, G.; Corbella, M.; Jaber, O.; Marone, P.; Scevola, D.; Faga, A. Non-pathogenic microflora of a spring water with regenerative properties. Biomed. Rep. 2015, 3, 758–762. [Google Scholar] [CrossRef]
- Nicoletti, G.; Saler, M.; Malovini, A.; Faga, A.; Scalise, A.; Riva, F. Effects of a spring water on human skin fibroblasts in vitro cultures: Preliminary results. Acta Vulnologica 2016, 14, 196–201. [Google Scholar]
- Nicoletti, G.; Saler, M.; Pellegatta, T.; Tresoldi, M.M.; Bonfanti, V.; Malovini, A.; Faga, A.; Riva, F. Ex vivo regenerative effects of a spring water. Biomed. Rep. 2017, 7, 508–514. [Google Scholar]
- Nicoletti, G.; Saler, M.; Tresoldi, M.M.; Faga, A.; Benedet, M.; Cristofolini, M. Regenerative effects of spring water-derived bacterial lysates on human skin fibroblast in in vitro culture: Preliminary results. J. Int. Med. Res. 2019, 47, 5777–5786. [Google Scholar] [CrossRef]
- Nicoletti, G.; Saler, M.; Tresoldi, M.M.; Villani, L.; Tottoli, E.M.; Jousson, O.; Faga, A. Effects of Comano Spring Water-derived Bacterial Lysates on Skin Regeneration: An Ex-vivo Study. Vivo 2023, 37, 2498–2509. [Google Scholar] [CrossRef] [PubMed]
- Saler, M.; Ferraro, O.; Faga, A.; Sansotta, D.; Villani, S.; Nicoletti, G. Enhancement of human native skin fibroblast proliferation in natural salso-bromo-iodic mineral water added to in vitro culture. Adv. Clin. Exp. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Éditeur Officiel du Québec: Regulation Respecting Bottled Water: Food Products Act. Chapter P-29, r.2. Updated to 1 June 2016. Available online: https://www.legisquebec.gouv.qc.ca (accessed on 20 February 2025).
- Nocera, T.; Rossi, A.B.; Mengeaud, V. Clinical development program of a new dermocosmetic range of products containing I-modulia (Aquaphilus dolomiae extract) in atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, AB62. [Google Scholar]
- Patrizi, A.; Bacquey, A.; Schmitt, A.M.; Decoster, C.J.; Phulpin, C.; Theunis, J.; Mengeaud, V. Clinical and biometrologic evaluation of a novel emollient balm containing an Aquaphilus dolomiae extract in 1- to 4-year-old children suffering from atopic dermatitis: International, multicenter, randomized versus control group study. J. Am. Acad. Dermatol. 2014, 70, AB62. [Google Scholar]
- Aries, M.-F.; Delga, H.; Levêque, M.; Castex-Rizzi, N.; Bessou-Touya, S.; Nguyen, T. Antiinflammatory and immunomodulatory effects of I-modulia, an Aquaphilus dolomiae extract, on atopic dermatitis in vitro. J. Am. Acad. Dermatol. 2014, 70, AB61. [Google Scholar]
- Aries, M.-F.; Duplan, H.; Hernandez-Pigeon, H.; Galliano, M.F.; Castex-Rizzi, N.; Bessou-Touya, S.; Nguyen, T. I-modulia, an Aquaphilus dolomiae extract, stimulates innate immune response through Toll-like receptor activation. J. Am. Acad. Dermatol. 2014, 70, AB63. [Google Scholar]
- Mahe, Y.F.; Perez, M.-J.; Tacheau, C.; Fanchon, C.; Martin, R.; Rousset, F.; Seite, S. A new Vitreoscilla filiformis extract grown on spa water-enriched medium activates endogenous cutaneous antioxidant and antimicrobial defenses through a potential Toll-like receptor 2/protein kinase C, zeta transduction pathway. Clin. Cosmet. Investig. Dermatol. 2013, 6, 191–196. [Google Scholar]
- Gueniche, A.; Knaudt, B.; Schuck, E.; Volz, T.; Bastien, P.; Martin, R.; Röcken, M.; Breton, L.; Biedermann, T. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled clinical study. Br. J. Dermatol. 2008, 159, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Cristofolini, M.; Jousson, O. Una componente ignorata delle acque termali: Il microbiota nascosto. La Clin. Termal. 2017, 64, 1–4. [Google Scholar]
- Saler, M.; Ferraro, O.; Faga, A.; Sansonetta, D.; Villani, S.; Nicoletti, G. Natural Sulphurous Mineral Water Induced Proliferation En-Hancement of Native Human Skin Fibroblast In-Vitro Cultures; In-Vivo, Stanford University Highwire Press: Stanford, CA, USA, 2025; Original Article, Submitted. [Google Scholar]
- Terme di Rivanazzano, Pavia (PV), Lombardia. Terme di Rivanazzano. Available online: https://www.federterme.it/spa/Lombardia/TermediRivanazzano(PV)Lombardia/106/ (accessed on 20 February 2025).
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamada, K.D.; Tomii, K.; Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 2018, 34, 2490–2492. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 February 2025).
- Oltresentieri. Available online: https://www.oltresentieri.com/ (accessed on 20 February 2025).
- Oliveira, A.S.; Vaz, C.V.; Silva, A.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-De-Oliveira, J.; Pereira, C.; Cruz, M.T. In vitro evaluation of potential benefits of a silica-rich thermal water (Monfortinho Thermal Water) in hyperkeratotic skin conditions. Int. J. Biometeorol. 2020, 64, 1957–1968. [Google Scholar] [CrossRef]
- Logue, J.B.; Robinson, C.T.; Meier, C.; der Meer, J.R.V. Relationship between sediment organic matter, bacteria composition, and the ecosystem metabolism of alpine streams. Limnol. Oceanogr. 2004, 49, 2001–2010. [Google Scholar] [CrossRef]
- Han, Y.; Perner, M. The globally widespread genus Sulfurimonas: Versatile energy metabolisms and adaptations to redox clines. Front. Microbiol. 2015, 6, 989. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A.; Brock, T. Brock Biology of Microorganisms, 14th ed.; Brock, T., Ed.; Pearson: London, UK, 2015. [Google Scholar]
- Kuever, J. The Family Desulfovibrionaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 107–133. [Google Scholar]
- Kelly, D.P.; Wood, A.P. Halothiobacillaceae fam. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–9. [Google Scholar] [CrossRef]
- Boden, R. Reclassification of Halothiobacillus hydrothermalis and Halothiobacillus halophilus to Guyparkeria gen. nov. in the Thioalkalibacteraceae fam. nov., with emended descriptions of the genus Halothiobacillus and family Halothiobacillaceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 3919–3928. [Google Scholar]
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Sievert, S.M.; Simon, J.; Campbell, B.J.; Hanson, T.E.; et al. Comparative Genomic Analysis of the Class Epsilonproteobacteria and Proposed Reclassification to Epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2017, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hetharua, B.; Lin, L.; Xu, H.; Zheng, T.; He, Z.; Tian, Y. Mangrove sediment microbiome: Adaptive microbial assemblages and their routed biogeochemical processes in yunxiao mangrove national nature reserve, China. Microb. Ecol. 2019, 78, 57–69. [Google Scholar] [CrossRef]
- Marziah, Z.; Mahdzir, A.; Musa, M.N.; Jaafar, A.B.; Azhim, A.; Hara, H. Abundance of sulfur-degrading bacteria in a benthic bacterial community of shallow sea sediment in the off-Terengganu coast of the South China Sea. Microbiologyopen 2016, 5, 967–978. [Google Scholar] [PubMed]
- Wang, L.; Cheung, M.K.; Liu, R.; Wong, C.K.; Kwan, H.S.; Hwang, J.-S. Diversity of Total Bacterial Communities and Chemoautotrophic Populations in Sulfur-Rich Sediments of Shallow-Water Hydrothermal Vents off Kueishan Island, Taiwan. Microb. Ecol. 2017, 73, 571–582. [Google Scholar] [CrossRef]
- Wright, K.E.; Williamson, C.; Grasby, S.E.; Spear, J.R.; Templeton, A.S. Metagenomic evidence for sulfur lithotrophy by Epsilonproteobacteria as the major energy source for primary productivity in a sub-aerial arctic glacial deposit, Borup Fiord Pass. Front. Microbiol. 2013, 4, 63. [Google Scholar]
- Sun, Y.; Song, Z.; Zhang, H.; Liu, P.; Hu, X. Seagrass vegetation affect the vertical organization of microbial communities in sediment. Mar. Environ. Res. 2020, 162, 105174. [Google Scholar]
- Nakagawa, T.; Takai, K.; Suzuki, Y.; Hirayama, H.; Konno, U.; Tsunogai, U.; Horikoshi, K. Geomicrobiological exploration and characterization of a novel deep-sea hydrothermal system at the TOTO caldera in the Mariana Volcanic Arc. Environ. Microbiol. 2006, 8, 37–49. [Google Scholar] [CrossRef]
- Dahle, H.; Roalkvam, I.; Thorseth, I.H.; Pedersen, R.B.; Steen, I.H. The versatile in situ gene expression of an Epsilonproteobacteria-dominated biofilm from a hydrothermal chimney. Environ. Microbiol. Rep. 2013, 5, 282–290. [Google Scholar] [PubMed]
- Meier, D.V.; Pjevac, P.; Bach, W.; Hourdez, S.; Girguis, P.R.; Vidoudez, C.; Amann, R.; Meyerdierks, A. Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J. 2017, 11, 1545–1558. [Google Scholar]
- Galushko, A.; Kuever, J. Desulfocapsaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Song, J.; Hwang, J.; Kang, I.; Cho, J.-C. A sulfate-reducing bacterial genus, Desulfosediminicola gen. nov., comprising two novel species cultivated from tidal-flat sediments. Sci. Rep. 2021, 11, 19978. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, L.; Xie, S.; Alain, K.; Wang, Z.; Wang, J.; Liu, D.; Shao, Z. Disproportionation of inorganic sulfur compounds by mesophilic chemolithoautotrophic campylobacterota. mSystems 2023, 8, e0095422. [Google Scholar] [PubMed]
- Cofield, C. Simulating Early Ocean Vents Shows Life’s Building Blocks Form Under Pressure. NASA. 2020. Available online: https://astrobiology.nasa.gov (accessed on 20 February 2025).
- Manos, J. The human microbiome in disease and pathology. Apmis 2022, 130, 690–705. [Google Scholar]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.-M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S3), 7–15. [Google Scholar] [CrossRef]
- Whiting, C.; Abdel Azim, S.; Friedman, A. The skin microbiome and its significance for dermatologists. Am. J. Clin. Dermatol. 2024, 25, 169–177. [Google Scholar]

| Parameter | Salso-Bromo-Jodic | Sulphurous |
|---|---|---|
| Temperature at the spring | 13.9 °C | 13.2 °C |
| pH | 7.7 | 7.4 |
| Conductivity at 20 °C | 8100 μS/cm | 1805 μS/cm |
| Fixed residue at 180 °C | 12,400 mg/L | 1334 mg/L |
| Oxidability | 48.0 mg/L (O2) | 39.0 mg/L (O2) |
| Silica (SiO2) | 50.5 mg/L | 19.9 mg/L |
| Bicarbonates (HCO3) | 814 mg/L | 705 mg/L |
| Chlorides (Cl) | 7300 mg/L | 192 mg/L |
| Sulphates (SO4) | 35.7 mg/L | 283 mg/L |
| Sodium (Na) | 4490 mg/L | 379.1 mg/L |
| Potassium (K) | 24.5 mg/L | 4.7 mg/L |
| Calcium (Ca) | 143 mg/L | 75 mg/L |
| Magnesium (Mg) | 78.5 mg/L | 31.2 mg/L |
| Dissolved iron (Fe) | 3.37 mg/L | <0.01 mg/L |
| Ammonium ion (NH4) | 22.4 mg/L | 3.15 mg/L |
| Total phosphorus (P) | 0.13 mg/L | <0.02 mg/L |
| Sulphide level (H2S) | not detectable | 11.9 mg/L |
| Strontium (Sr) | 15.8 mg/L | 1.964 mg/L |
| Lithium (Li) | 0.417 mg/L | 0.066 mg/L |
| Aluminum (Al) | <0.01 mg/L | <0.01 mg/L |
| Bromides (Br) | 37.1 mg/L | 1.0 mg/L |
| Iodides (I) | 13.9 mg/L | 0.5 mg/L |
| Antimony (Sb) | <0.0005 mg/L | <0.0005 mg/L |
| Arsenic (As) | 0.001 mg/L | <0.001 mg/L |
| Barium (Ba) | 3.6 mg/L | <0.1 mg/L |
| Boron (B) | 54.1 mg/L | 3.24 mg/L |
| Cadmium (Cd) | <0.0001 mg/L | <0.0001 mg/L |
| Chromium (Cr) | 0.001 mg/L | <0.001 mg/L |
| Copper (Cu) | <0.1 mg/L | <0.1 mg/L |
| Cyanides (CN) | <0.001 mg/L | <0.001 mg/L |
| Fluorides (F) | 1.4 mg/L | 0.26 mg/L |
| Lead (Pb) | <0.001 mg/L | <0.001 mg/L |
| Manganese (Mn) | 0.06 mg/L | 0.041 mg/L |
| Mercury (Hg) | <0.0001 mg/L | <0.0001 mg/L |
| Nickel (Ni) | <0.001 mg/L | 0.001 mg/L |
| Nitrates (NO3) | <1.0 mg/L | <1.0 mg/L |
| Nitrites (NO2) | <0.002 mg/L | 0.007 mg/L |
| Selenium (Se) | 0.005 mg/L | <0.001 mg/L |
| Anionic surfactant agents | <50 μg/L | <50 μg/L |
| Mineral oils—dissolved or emulsified hydrocarbons | 41 μg/L | 34 μg/L |
| Benzene | <0.1 μg/L | <0.1 μg/L |
| Benzo[a]pyrene | not detectable | <0.003 μg/L |
| Benzo[b]fluoranthene | not detectable | <0.003 μg/L |
| Benzo[k]fluoranthene | not detectable | <0.003 μg/L |
| Benzo[ghi]perylene | not detectable | <0.003 μg/L |
| Dibenz[a,h]anthracene | not detectable | <0.003 μg/L |
| Indeno[1,2,3-cd]pyrene | not detectable | <0.003 μg/L |
| Acenaphthene | not detectable | <0.003 μg/L |
| Fluorene | not detectable | <0.003 μg/L |
| Phenanthrene | not detectable | <0.003 μg/L |
| Anthracene | not detectable | <0.003 μg/L |
| Fluoranthene | not detectable | <0.003 μg/L |
| Pyrene | not detectable | <0.003 μg/L |
| Benzo[a]anthracene | not detectable | <0.003 μg/L |
| Chrysene | not detectable | <0.003 μg/L |
| Chloroform | not detectable | <0.1 μg/L |
| Dibromochloromethane | not detectable | <0.1 μg/L |
| Bromodichloromethane | not detectable | <0.1 μg/L |
| Bromoform | not detectable | <0.1 μg/L |
| Trichloroethylene | not detectable | <0.1 μg/L |
| Tetrachloroethylene | not detectable | <0.1 μg/L |
| 1,2-Dichloroethane | not detectable | <0.1 μg/L |
| 1,1-Dichloroethylene | not detectable | <0.1 μg/L |
| 1,2-Dichloroethylene | not detectable | <0.1 μg/L |
| 1,1,1-Trichloroethane | not detectable | <0.1 μg/L |
| Carbon tetrachloride | not detectable | <0.1 μg/L |
| Total hardness | 59.6 °F | 31.6 °F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuka, A.; Mileto, I.; Saler, M.; Petazzoni, G.; Corbella, M.; Baldanti, F.; Faga, A.; Nicoletti, G. Native Bacterial Communities of Two Italian Salso-Bromo-Jodic and Sulphurous Natural Mineral Waters. Microorganisms 2025, 13, 1038. https://doi.org/10.3390/microorganisms13051038
Kuka A, Mileto I, Saler M, Petazzoni G, Corbella M, Baldanti F, Faga A, Nicoletti G. Native Bacterial Communities of Two Italian Salso-Bromo-Jodic and Sulphurous Natural Mineral Waters. Microorganisms. 2025; 13(5):1038. https://doi.org/10.3390/microorganisms13051038
Chicago/Turabian StyleKuka, Angela, Irene Mileto, Marco Saler, Greta Petazzoni, Marta Corbella, Fausto Baldanti, Angela Faga, and Giovanni Nicoletti. 2025. "Native Bacterial Communities of Two Italian Salso-Bromo-Jodic and Sulphurous Natural Mineral Waters" Microorganisms 13, no. 5: 1038. https://doi.org/10.3390/microorganisms13051038
APA StyleKuka, A., Mileto, I., Saler, M., Petazzoni, G., Corbella, M., Baldanti, F., Faga, A., & Nicoletti, G. (2025). Native Bacterial Communities of Two Italian Salso-Bromo-Jodic and Sulphurous Natural Mineral Waters. Microorganisms, 13(5), 1038. https://doi.org/10.3390/microorganisms13051038






