Enhanced Molybdenum Recovery Achieved by a Complex of Porous Material-Immobilized Surface-Engineered Yeast in Development of a Sustainable Biosorption Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Cultivation
2.2. Experimental Design
2.3. Immobilization Materials and Yeast Immobilization
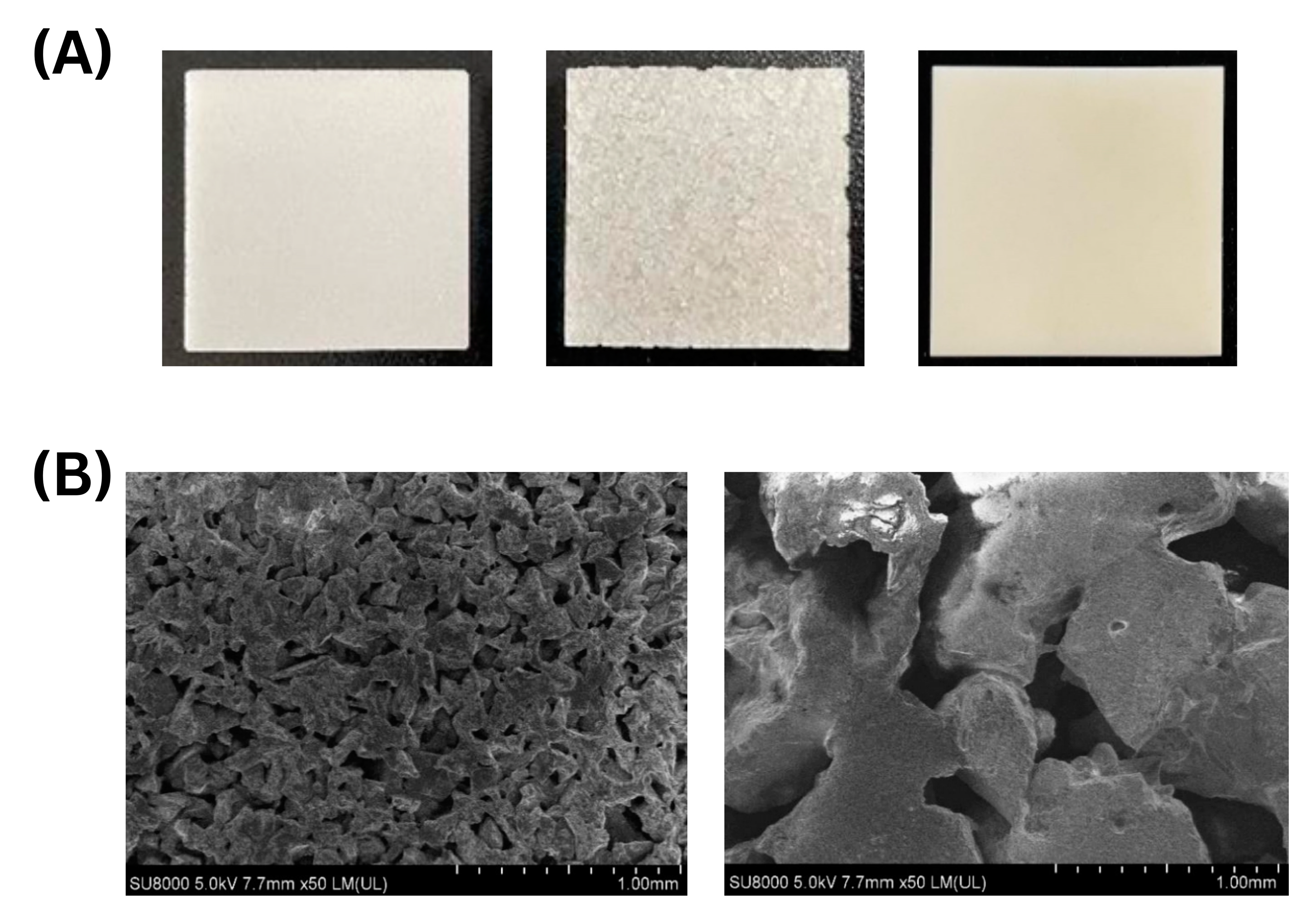
2.3.1. Ceramic Materials
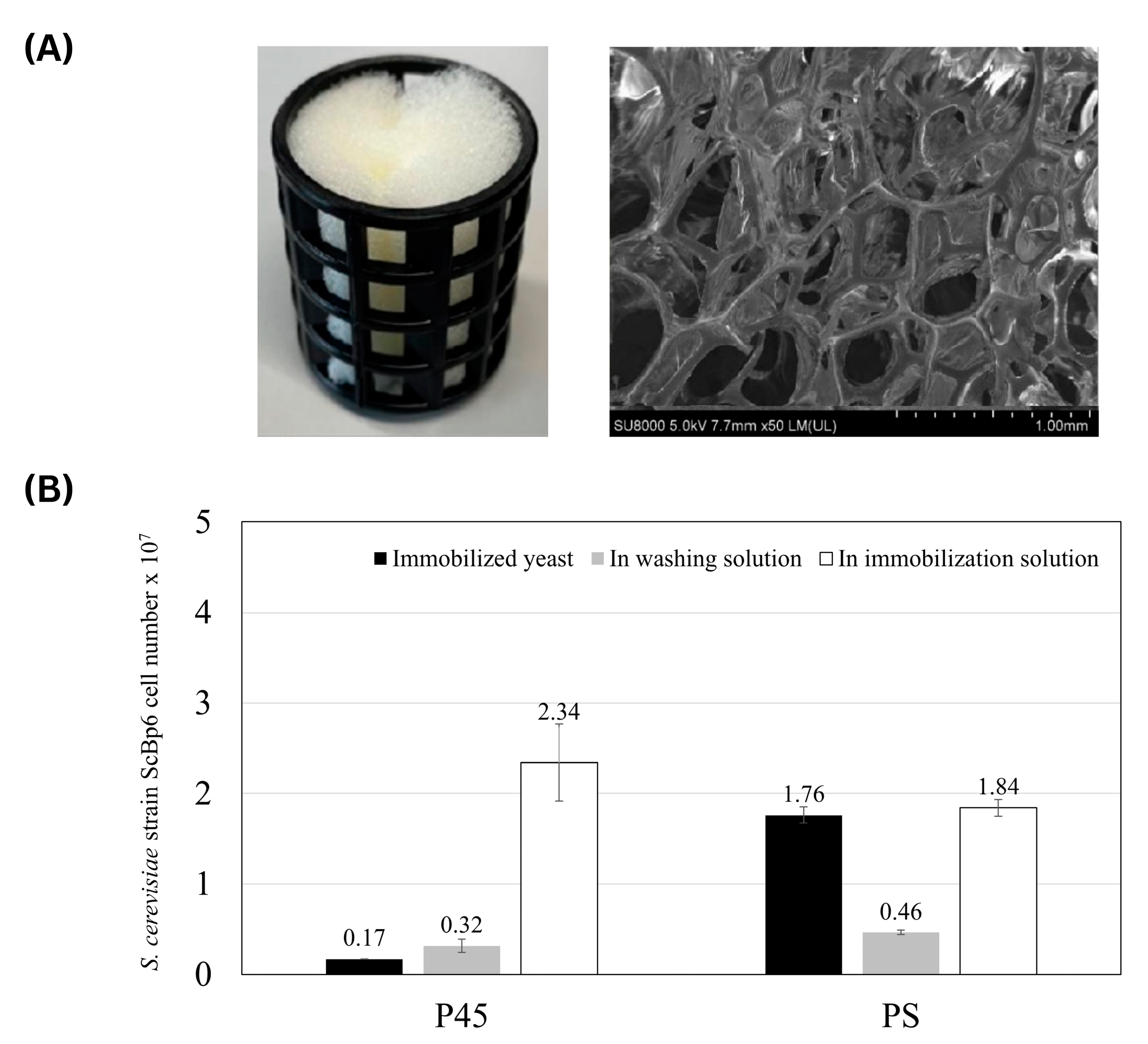
2.3.2. Polyurethane Sponges
2.3.3. Surface Characterization of Porous Material
2.4. Mo Adsorption by Immobilized Yeast
2.5. Statistical Analysis
3. Results
3.1. Yeast Immobilization
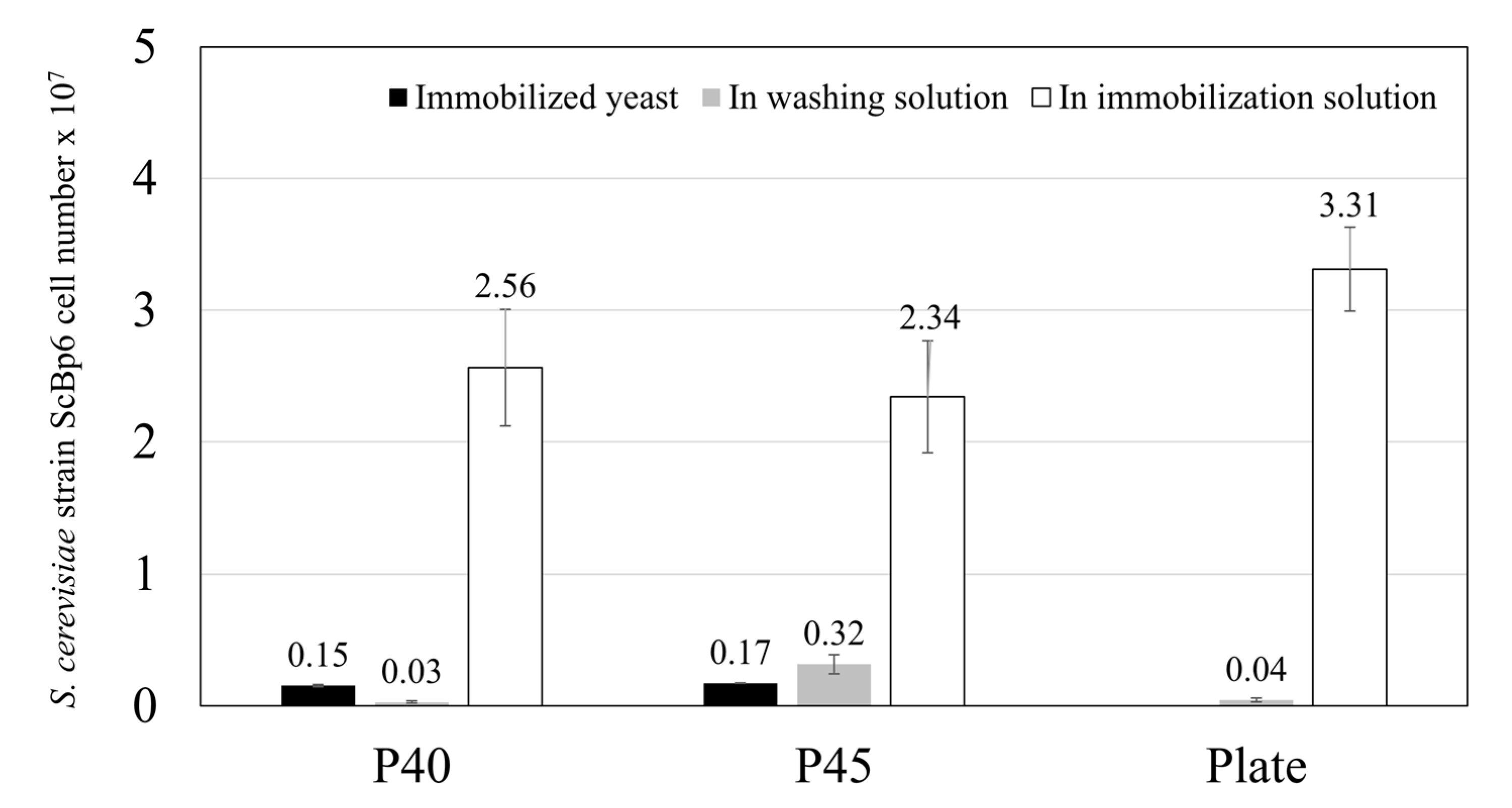
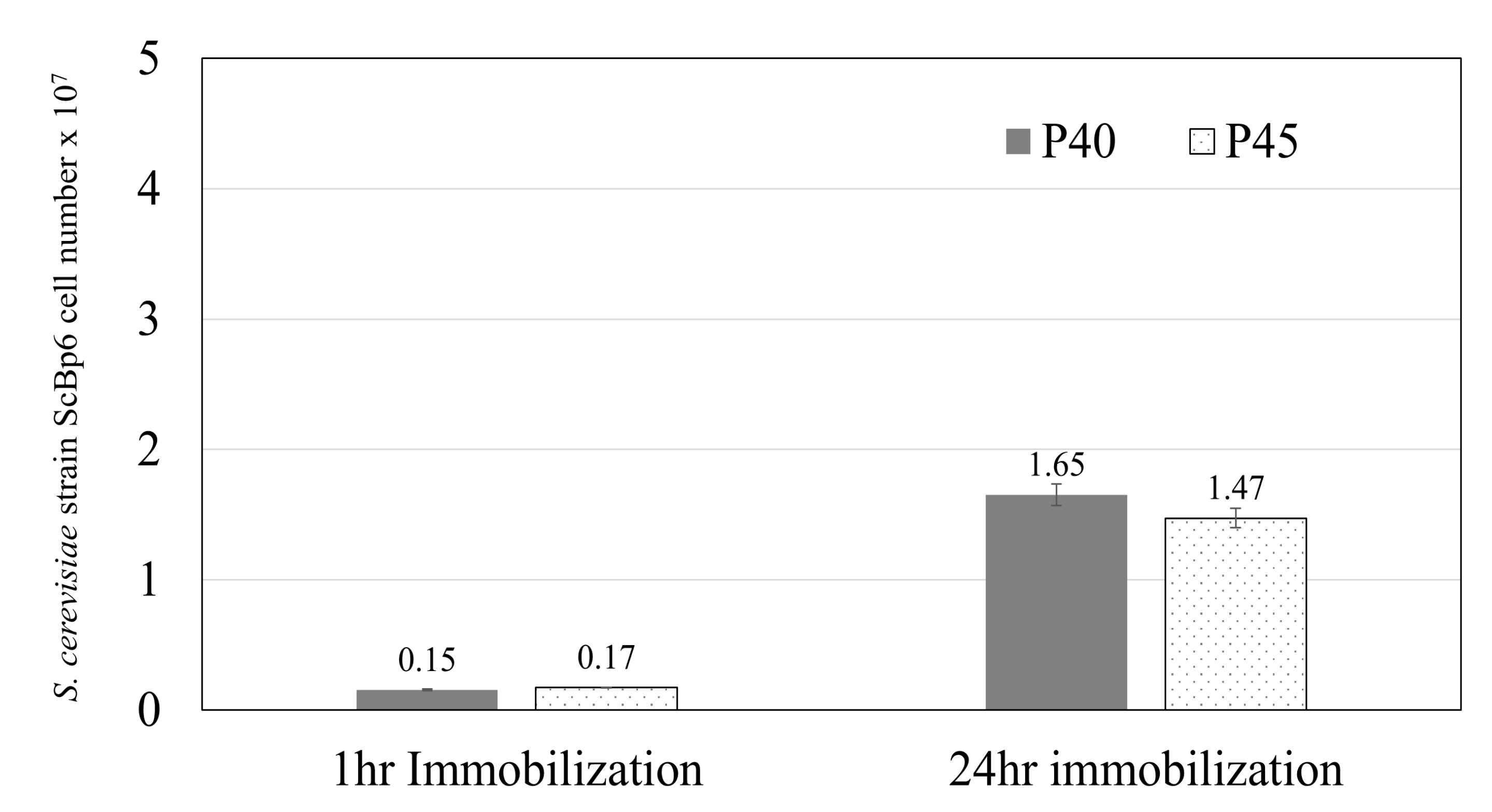
3.1.1. Yeast Immobilization on Porous Ceramics
3.1.2. Yeast Immobilization on Polyurethane Sponges
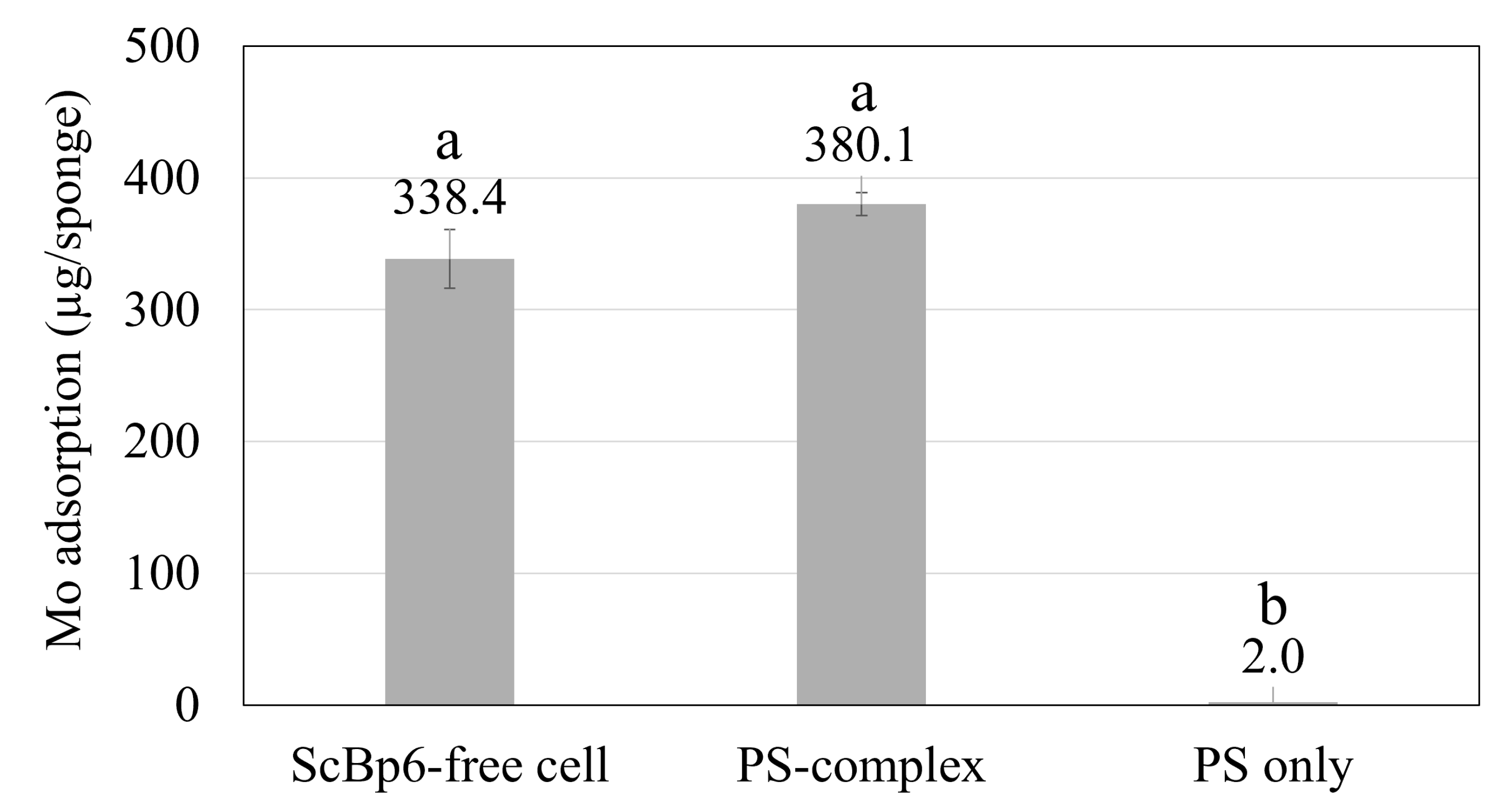
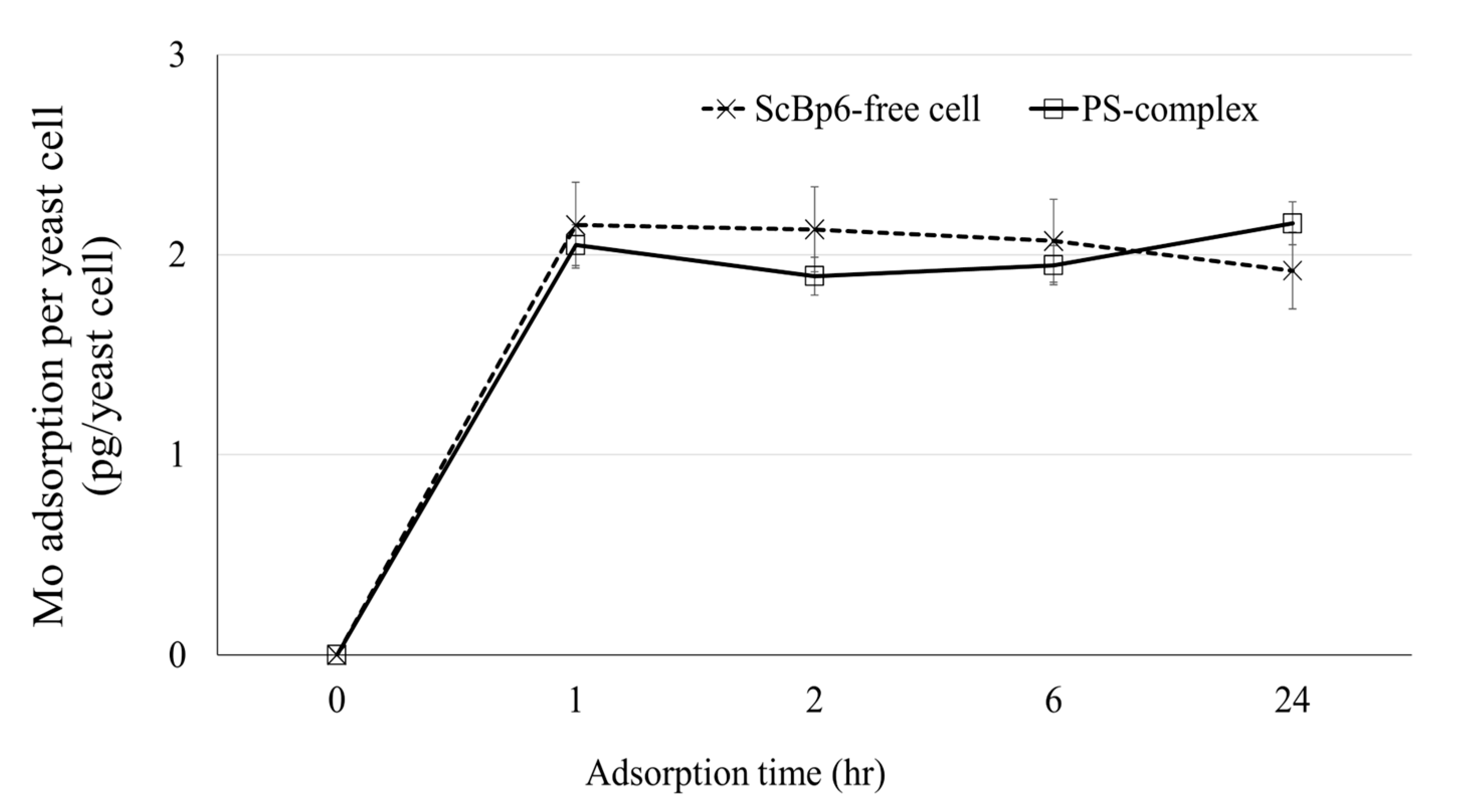
3.2. Mo Adsorption on Yeast Porous Materials Complex
4. Discussion
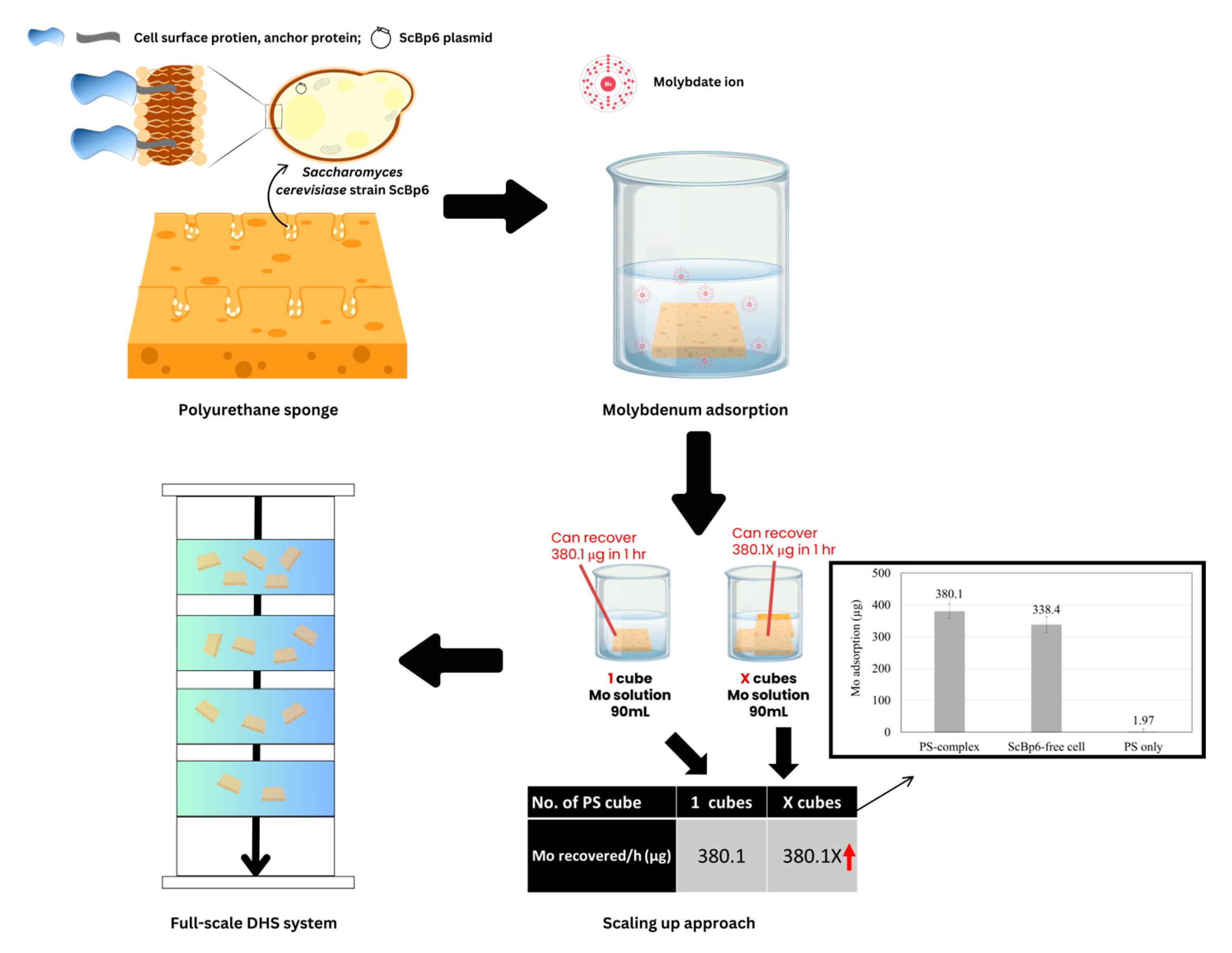
4.1. Scaling up for Enhanced Mo Adsorption
4.2. Full Scale System for Practical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Mo | Molybdenum |
| MoO42− | Molybdate ion |
| P40 | Ceramic material with diameter 50–100 µm |
| P45 | Ceramic material with diameter 300–1000 µm |
| PS | Polyurethane Sponge |
| DHS | Downflow Hanging Sponge |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| SEM | Scanning Electron Microscopy |
| SD(-leu) | Synthetic Defined Medium without Leucine |
| PBS | Phosphate Buffered Saline |
| OD | Optical Density |
| ModE | Molybdate Operon Regulator Protein |
References
- Nakashima, Y.; Inoue, Y.; Yamamoto, T.; Kamichatani, W.; Kagaya, S.; Yamamoto, A.; Yarime, M.; Hara, K. Determination of molybdate in environmental water by ion chromatography coupled with a preconcentration method employing a selective chelating resin. Anal. Sci. 2012, 28, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.; Nakamura, T.; Kawasaki, N. Adsorption capability of virgin and calcined wheat bran for molybdenum present in aqueous solution and elucidating the adsorption mechanism by adsorption isotherms, kinetics, and regeneration. Environ. Chem. Eng. 2012, 6, 4459–4466. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chan, T.S.; Tu, H.W.; Wang, S.L.; You, C.F.; Chang, C.K. Rapid and efficient removal/recovery of molybdenum onto ZnFe2O4 nanoparticles. Chemosphere 2016, 148, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Considine, G.D. Van Nostrand’s Encyclopedia of Chemistry—5th Edition. J. Chem. Educ. 2005, 82, 840. [Google Scholar] [CrossRef]
- Japan Organization for Metals and Energy Security (JOGMEC). Material Flow of Mineral Resources 2019; Japan Organization for Metals and Energy Security: Tokyo, Japan, 2019; pp. 268–285. Available online: https://mric.jogmec.go.jp/wp-content/uploads/2020/05/material_flow2019.pdf (accessed on 20 October 2024).
- International Molybdenum Association (IMOA). Global Production Use; International Molybdenum Association: London, UK, 2017; Available online: http://www.imoa.info (accessed on 20 October 2024).
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. Molybdenum resources: Their depletion and safeguarding for future generations. Resour. Conserv. Recycl. 2018, 134, 61–69. [Google Scholar] [CrossRef]
- Kar, B.B.; Datta, P.; Misra, V.N. Spent catalyst: Secondary source for molybdenum recovery. Hydrometallurgy 2004, 72, 87–92. [Google Scholar] [CrossRef]
- Volesky, B. Biosorbents for metal recovery. Trends Biotechnol. 1987, 5, 96–101. [Google Scholar] [CrossRef]
- Dorfler, R.R.; Laferty, J.M. Review of molybdenum recovery processes. JOM 1981, 33, 48–54. [Google Scholar] [CrossRef]
- Stephanie, A.; Chien, M.-F.; Ikeda, N.; Inoue, C. Molybdate recovery using immobilized bioengineered Saccharomyces cerevisiae. Hydrometallurgy 2020, 198, 105491. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Braibant, M.; Gilot, P.; Content, J. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 2000, 24, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Medof, M.E.; Nagarajain, S.; Tykocinski, M.E. Cell-surface engineering with GPI-anchored proteins. FASEB J. 1996, 10, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Grunden, A.M.; Ray, R.M.; Rosentel, J.K.; Healy, F.G.; Shanmugam, K.T. Repression of the Escherichia coli modABCD (molybdate transport) operon by ModE. J. Bacteriol. 1996, 178, 735–744. [Google Scholar] [CrossRef]
- Delgado, M.J.; Tresierra-Ayala, A.; Talbi, C.; Bedmar, E.J. Functional characterization of the Bradyrhizobium japonicum modA and modB genes involved in molybdenum transport. Microbiology 2006, 152, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.Y.; Guo, Y.; Yang, X.-Q.; Zhang, W.; Huang, X.-Q. Surface display of metal binding domain derived from PbrR on Escherichia coli specifically increases lead (II) adsorption. Biotechnol. Lett. 2018, 40, 837–845. [Google Scholar] [CrossRef]
- Thai, C.K.; Dai, H.; Sastry, M.S.R.; Sarikaya, M.; Schwartz, D.T.; Baneyx, F. Identification and characterization of Cu2O− and ZnO− binding polypeptides by Escherichia coli cell surface display: Toward an understanding of metal oxide binding. Biotechnol. Bioeng. 2004, 87, 129–254. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Deak, T. Environmental Factors Influencing Yeasts. In Biodiversity and Ecophysiology of Yeasts; The Yeast Handbook; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 155–174. [Google Scholar] [CrossRef]
- Chien, M.-F.; Ikeda, N.; Kubota, K.; Inoue, C. Construction of a cell surface engineered yeast aims to selectively recover molybdenum, a rare metal. Solid State Phenom. 2017, 262, 421–424. [Google Scholar] [CrossRef]
- Jhariya, U.; Chien, M.-F.; Umetsu, M.; Kamitakahara, M. New insights into immobilized bacterial systems for removal of heavy metals from wastewater. Int. J. Environ. Sci. Technol. 2025, 1–16. [Google Scholar] [CrossRef]
- Wong, P.K.; Lam, K.C.; So, C.M. Removal and recovery of Cu(lI) from industrial effluent by immobilized cells of Pseudomonas putida II-11. Appl. Microbiol. Biotechnol. 1993, 39, 127–131. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, T.; Pan, Y.; Shi, M.; Ding, D.; Ma, Z.; Liu, J.; Yuan, Y.; Fei, L.; Sun, Y. Recovering rare earth elements via immobilized red algae from ammonium-rich wastewater. Environ. Sci. Ecotechnol. 2022, 12, 100204. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Otani, T.; Hariu, T.; Jin, T.; Tagawa, T.; Morikawa, M.; Li, Y.-Y. Evaluation of sewage treatment performance and duckweed biomass production efficiency in a primary sedimentation basin + duckweed pond + downflow hanging sponge (PSB + DWP + DHS) system. J. Water Process Eng. 2024, 65, 105818. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Polyurethane sponge modified by alginate and activated carbon with abilities of oil absorption, and selective cationic and anionic dyes clean-up. J. Clean. Prod. 2021, 312, 127513. [Google Scholar] [CrossRef]
- Edwin, T.; Mera, M.; Komala, P.S.; Zukarnaini. The capacity of polyurethane sponge to adsorb nitrate, ammonium, and phosphate. IOP Conf. Ser. Earth Environ. Sci. 2023, 1173, 012073. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Dharmawan, F.; Palmer, C.G. Roles of polyurethane foam in aerobic moving and fixed bed bioreactors. Bioresour. Technol. 2010, 101, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Ivshina, I.B.; Kuyukina, M.S.; Litvinenko, L.V.; Golysheva, A.A.; Kostrikina, N.A.; Sorokin, V.V.; Mulyukin, A.L. Bioaccumulation of molybdate ions by alkanotrophic Rhodococcus leads to significant alterations in cellular ultrastructure and physiology. Ecotoxicol. Environ. Saf. 2024, 274, 116190. [Google Scholar] [CrossRef]
- Uemura, S.; Okubo, T.; Maeno, K.; Takahashi, M.; Kubota, K.; Harada, H. Evaluation of water distribution and oxygen mass transfer in sponge support media for a Down-flow hanging sponge reactor. Int. J. Environ. Res. 2016, 10, 265–272. [Google Scholar]
- Cao, L.T.T.; Kodera, H.; Abe, K.; Imachi, H.; Aoi, Y.; Kindaichi, T.; Ozaki, N.; Ohashi, A. Biological oxidation of Mn(II) coupled with nitrification for removal and recovery of minor metals by downflow hanging sponge reactor. Water Res. 2015, 68, 545–553. [Google Scholar] [CrossRef]
- Younes, S.M.; El-Tabl, A.S.; Daba, M.S.; El-Saied, A.B.; Faheem, S.M. Development of down flow hanging sponge reactor as a new process and technology for sewage sludge treatment. Eur. Chem. Bull. 2023, 12, 1931–1960. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jittayasotorn, T.; Kojima, K.; Stephanie, A.; Nakamura, K.; Bacosa, H.P.; Kubota, K.; Kamitakahara, M.; Inoue, C.; Chien, M.-F. Enhanced Molybdenum Recovery Achieved by a Complex of Porous Material-Immobilized Surface-Engineered Yeast in Development of a Sustainable Biosorption Technology. Microorganisms 2025, 13, 1034. https://doi.org/10.3390/microorganisms13051034
Jittayasotorn T, Kojima K, Stephanie A, Nakamura K, Bacosa HP, Kubota K, Kamitakahara M, Inoue C, Chien M-F. Enhanced Molybdenum Recovery Achieved by a Complex of Porous Material-Immobilized Surface-Engineered Yeast in Development of a Sustainable Biosorption Technology. Microorganisms. 2025; 13(5):1034. https://doi.org/10.3390/microorganisms13051034
Chicago/Turabian StyleJittayasotorn, Thiti, Kentaro Kojima, Audrey Stephanie, Kaho Nakamura, Hernando P. Bacosa, Kengo Kubota, Masanobu Kamitakahara, Chihiro Inoue, and Mei-Fang Chien. 2025. "Enhanced Molybdenum Recovery Achieved by a Complex of Porous Material-Immobilized Surface-Engineered Yeast in Development of a Sustainable Biosorption Technology" Microorganisms 13, no. 5: 1034. https://doi.org/10.3390/microorganisms13051034
APA StyleJittayasotorn, T., Kojima, K., Stephanie, A., Nakamura, K., Bacosa, H. P., Kubota, K., Kamitakahara, M., Inoue, C., & Chien, M.-F. (2025). Enhanced Molybdenum Recovery Achieved by a Complex of Porous Material-Immobilized Surface-Engineered Yeast in Development of a Sustainable Biosorption Technology. Microorganisms, 13(5), 1034. https://doi.org/10.3390/microorganisms13051034







