Acclimation Time Enhances Adaptation of Heterotrophic Nitrifying-Aerobic Denitrifying Microflora to Linear Anionic Surfactant Stress
Abstract
1. Introduction
2. Material and Methods
2.1. HN-AD Microflora Domestication Process and Culture Medium Preparation
2.2. Nitrogen Removal Efficiency of HN-AD Microflora Under LAS Stress
2.3. Determination of Oxidative Stress and Cell Viability
2.4. EPS Analysis
2.5. Metagenome Sequencing
2.6. Data Analysis
3. Results and Discussion
3.1. Nitrogen Removal Performance of HN-AD Microflora Under LAS Stress
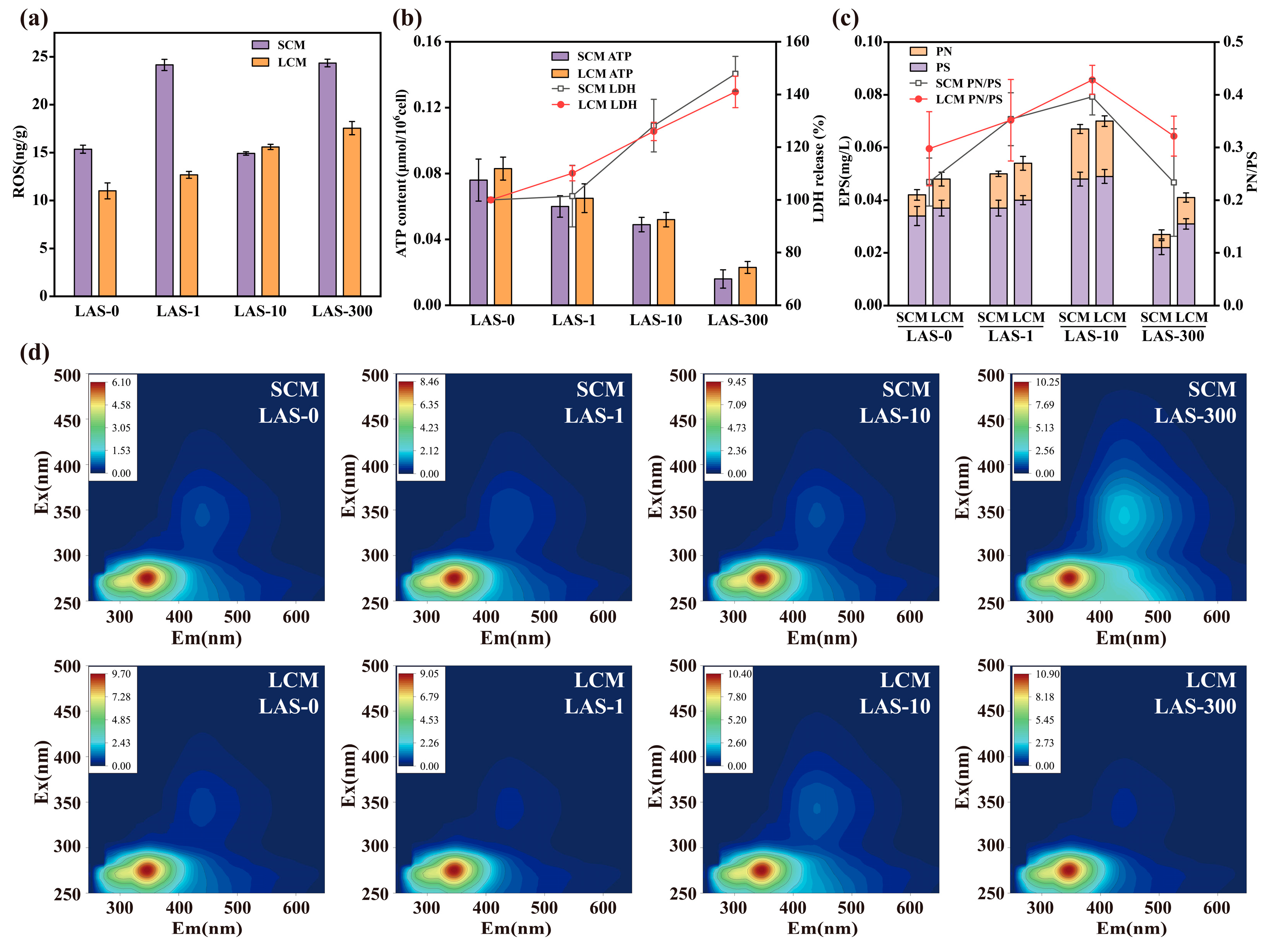
3.2. Oxidative Stress and Cell Activity
3.3. Response of EPS to LAS Stress
3.3.1. EPS Content
3.3.2. Three-Dimensional Fluorescence Characteristics of EPS
3.4. Comparative Heatmap of Stress Markers Under LAS Exposure
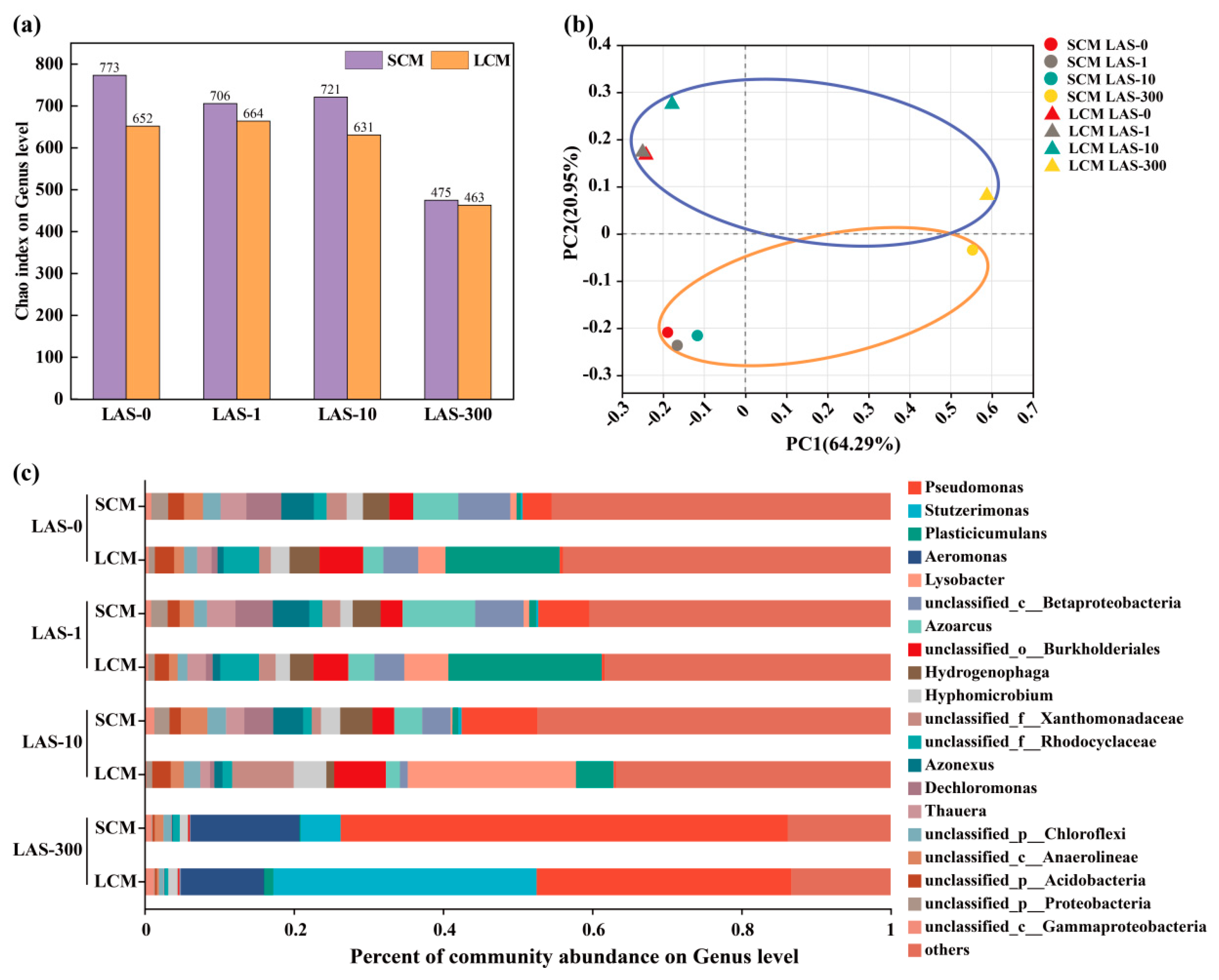
3.5. Analysis of Microbial Community Composition and Diversity
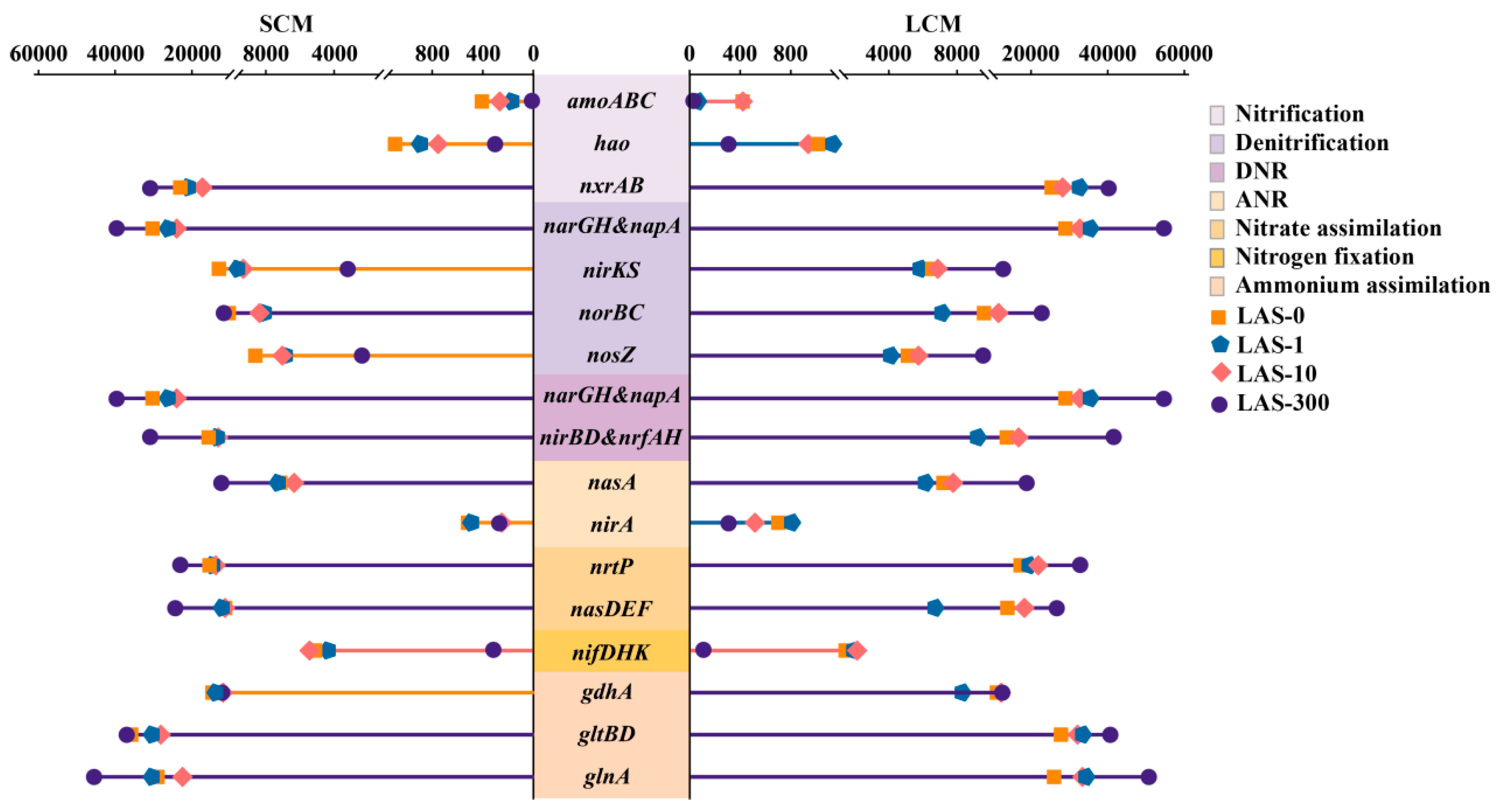
3.6. Nitrogen Metabolism
3.6.1. Changes in the Abundance of Nitrogen Cycling Functional Genes
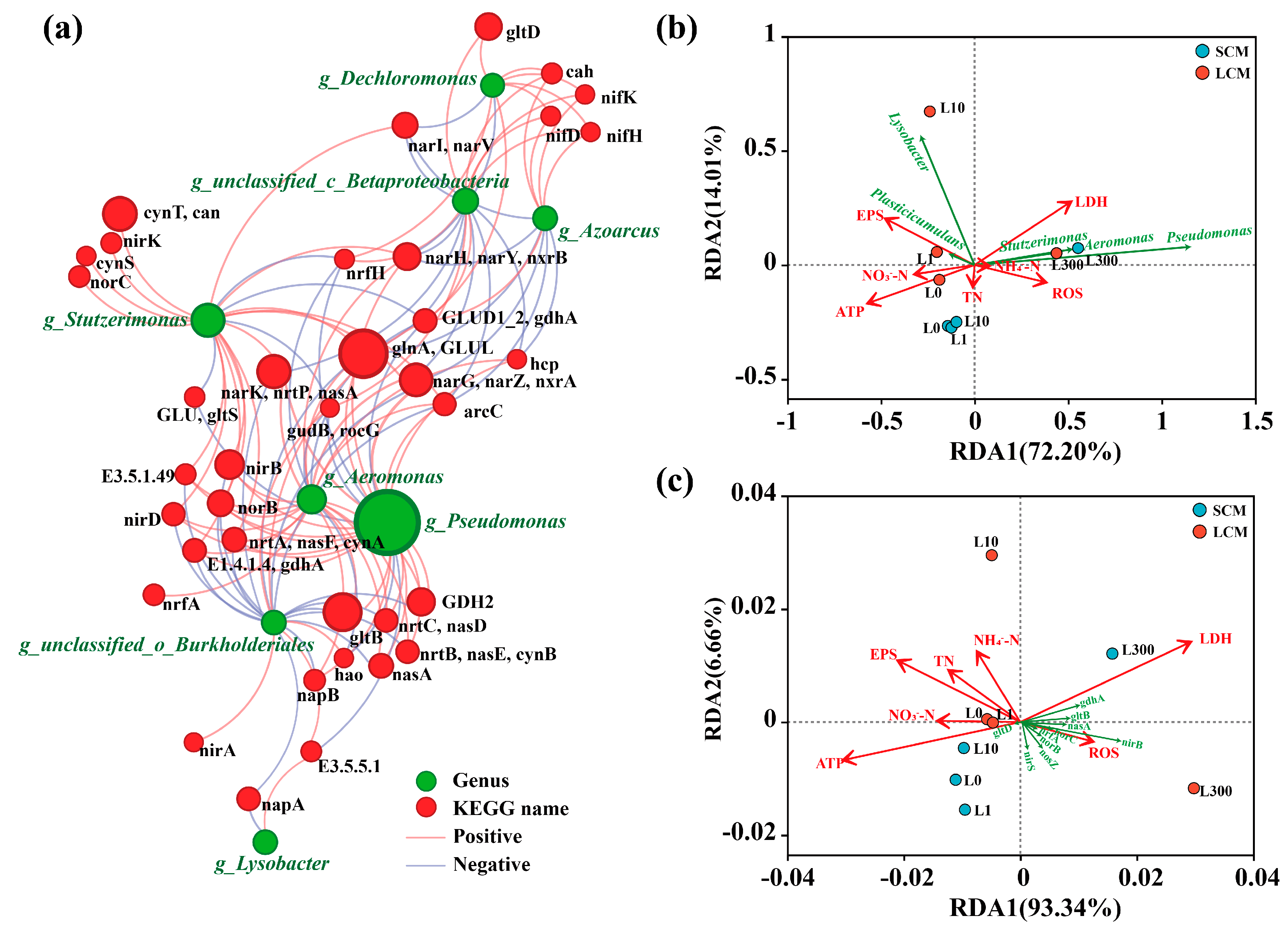
3.6.2. Regulation of Nitrogen Metabolism and Core Species
3.7. Correlations Between Environmental Variables, Bacterial Communities, and Nitrogen Functional Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Ma, B.; Huang, T.; Shi, Y. Nitrate reduction by the aerobic denitrifying actinomycete Streptomyces sp. XD-11-6-2: Performance, metabolic activity, and micro-polluted water treatment. Bioresour. Technol. 2021, 326, 124779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, X.; Xie, F.; Cui, Y.; Zhang, X.; Yue, X. Development of simultaneous nitrification-denitrification and anammox and in-situ analysis of microbial structure in a novel plug-flow membrane-aerated sludge blanket. Sci. Total Environ. 2021, 750, 142296. [Google Scholar] [CrossRef]
- Dai, F.; De Prá, M.C.; Vanotti, M.B.; Gilmore, K.R.; Cumbie, W.E. Microbial characteristics of nitrifiers, denitrifiers and anammox bacteria on different support media to treat space mission wastewater. J. Environ. Manag. 2019, 232, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Li, Q.; Ji, M.; Yan, G.; Guo, S. Isolation and niche characteristics in simultaneous nitrification and denitrification application of an aerobic denitrifier, Acinetobacter sp. YS2. Bioresour. Technol. 2020, 302, 122799. [Google Scholar] [CrossRef]
- Chen, J.; Gu, S.; Hao, H.; Chen, J. Characteristics and metabolic pathway of Alcaligenes sp. TB for simultaneous heterotrophic nitrification-aerobic denitrification. Appl. Microbiol. Biotechnol. 2016, 100, 9787–9794. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef]
- Alvarez-Muñoz, D.; Gómez-Parra, A.; González-Mazo, E. Influence of the molecular structure and exposure concentration on the uptake and elimination kinetics, bioconcentration, and biotransformation of anionic and nonionic surfactants. Environ. Toxicol. Chem. 2010, 29, 1727–1734. [Google Scholar] [CrossRef]
- Mungray, A.K.; Kumar, P. Fate of linear alkylbenzene sulfonates in the environment: A review. Int. Biodeterior. Biodegrad. 2009, 63, 981–987. [Google Scholar] [CrossRef]
- Braga, J.K.; Varesche, M.B.A. Commercial Laundry Water Characterisation. Am. J. Anal. Chem. 2014, 5, 8–16. [Google Scholar] [CrossRef]
- Yin, C.; Li, Y.; Zhang, T.; Liu, J.; Yuan, Y.; Huang, M. Effects of exposure to anionic surfactants (SDBS and SDS) on nitrogen removal of aerobic denitrifier. Water Environ. Res. 2020, 92, 2129–2139. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Cheng, Y.; Jin, R. Linear anionic surfactant (SDBS) destabilized anammox process through sludge disaggregation and metabolic inhibition. J. Hazard. Mater. 2021, 403, 123641. [Google Scholar] [CrossRef]
- Andrade, M.V.F.; Delforno, T.P.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Dynamics and response of microbial diversity to nutritional conditions in denitrifying bioreactor for linear alkylbenzene sulfonate removal. J. Environ. Manag. 2020, 263, 110387. [Google Scholar] [CrossRef] [PubMed]
- Dereszewska, A.; Cytawa, S.; Tomczak-Wandzel, R.; Mędrzycka, K. The Effect of Anionic Surfactant Concentration on Activated Sludge Condition and Phosphate Release in Biological Treatment Plant. Pol. J. Environ. Stud. 2015, 24, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhai, T.; Zhang, L.; Zhao, T.; Xing, Z.; Liu, H. Domestication and pilot-scale culture of mixed bacteria HY-1 capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2023, 384, 129285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geng, K.; Wu, N.; Hu, G.; Fan, B.; He, J.; Qiao, W. Sustained anaerobic degradation of 4-chloro-2-methylphenoxyacetic acid by acclimated sludge in a continuous-flow reactor. Chemosphere 2023, 330, 138749. [Google Scholar] [CrossRef]
- Wang, X.; Teng, Y.; Wang, X.; Xu, Y.; Li, R.; Sun, Y.; Dai, S.; Hu, W.; Wang, H.; Li, Y.; et al. Nitrogen transfer and cross-feeding between Azotobacter chroococcum and Paracoccus aminovorans promotes pyrene degradation. ISME J. 2023, 17, 2169–2181. [Google Scholar] [CrossRef]
- Chu, X.; Awasthi, M.K.; Liu, Y.; Cheng, Q.; Qu, J.; Sun, Y. Studies on the degradation of corn straw by combined bacterial cultures. Bioresour. Technol. 2021, 320, 124174. [Google Scholar] [CrossRef]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef]
- Ali, S.; Hua, B.; Huang, J.J.; Droste, R.L.; Zhou, Q.; Zhao, W.; Chen, L. Effect of different initial low pH conditions on biogas production, composition, and shift in the aceticlastic methanogenic population. Bioresour. Technol. 2019, 289, 121579. [Google Scholar] [CrossRef]
- Hu, J.; Yan, J.; Wu, L.; Bao, Y.; Yu, D.; Li, J. Simultaneous nitrification and denitrification of hypersaline wastewater by a robust bacterium Halomonas salifodinae from a repeated-batch acclimation. Bioresour. Technol. 2021, 341, 125818. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Yao, S.; Ni, J. High-efficient nitrogen removal by coupling enriched autotrophic-nitrification and aerobic-denitrification consortiums at cold temperature. Bioresour. Technol. 2014, 161, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Madigou, C.; Poirier, S.; Bureau, C.; Chapleur, O. Acclimation strategy to increase phenol tolerance of an anaerobic microbiota. Bioresour. Technol. 2016, 216, 77–86. [Google Scholar] [CrossRef]
- Morales, N.; Val del Río, Á.; Vázquez-Padín, J.R.; Méndez, R.; Campos, J.L.; Mosquera-Corral, A. The granular biomass properties and the acclimation period affect the partial nitritation/anammox process stability at a low temperature and ammonium concentration. Process Biochem. 2016, 51, 2134–2142. [Google Scholar] [CrossRef]
- Yang, E.; Chen, J.; Liu, K.; Jiang, J.; Wang, H.; Wu, S.; Shi, L.; Jiang, J.; Sanjaya, E.H.; Chen, H. Intensifying single-stage denitrogen by a dissolved oxygen-differentiated airlift internal circulation reactor under organic matter stress: Nitrogen removal pathways and microbial interactions. Water Res. 2023, 241, 120120. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Y.; Xu, Q.; Liu, X.; Liu, Y.; Ni, B.-J.; Yang, Q.; Wang, D.; Li, X.; Wang, Q. Free Ammonia-Based Pretreatment Promotes Short-Chain Fatty Acid Production from Waste Activated Sludge. ACS Sustain. Chem. Eng. 2018, 6, 9120–9129. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Fan, G.; Su, X.; Zhou, J.; Liu, D. Metagenomics reveals functional species and microbial mechanisms of an enriched thiosulfate-driven denitratation consortia. Bioresour. Technol. 2021, 341, 125916. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, Y.; Zhang, Q.; Zhang, W.; Li, M.; Feng, J.; Su, J.; He, J.; Zhong, M. Control of aeration time in the aniline degrading-bioreactor with the analysis of metagenomic: Aniline degradation and nitrogen metabolism. Bioresour. Technol. 2022, 344, 126281. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z.; Lv, Z.; Zhang, L.; Xin, F.; Li, Y.; Liu, G.; Dong, W.; Wei, P.; Jia, H. Enhanced chloramphenicol-degrading biofilm formation in microbial fuel cells through a novel synchronous acclimation strategy. J. Clean. Prod. 2021, 317, 128376. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Wei, H.; Wang, J.; Zhou, W.; Zhou, P.; Li, D. Environmental concentrations of anionic surfactants in lake surface microlayers enhance the toxicity of Microcystis blooms: Insight from photosynthesis, interspecies competition, and MC production. Water Res. 2023, 244, 120430. [Google Scholar] [CrossRef]
- Ran, T.; Wei, T.; Xia, L.; Rong, W.; Zhou, Y. Dissolved oxygen dependence of Microbe-Derived dissolved organic nitrogen dynamics during membrane biofilm-based linear alkylbenzene sulfonate mineralization. Chem. Eng. J. 2024, 489, 151409. [Google Scholar] [CrossRef]
- Zhou, M.; Ye, H.; Zhao, X. Isolation and characterization of a novel heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas stutzeri KTB for bioremediation of wastewater. Biotechnol. Bioprocess Eng. 2014, 19, 231–238. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhang, M.; Zheng, C.; Yang, L.; Yang, L. Review of the mechanisms involved in dissimilatory nitrate reduction to ammonium and the efficacies of these mechanisms in the environment. Environ. Pollut. 2024, 345, 123480. [Google Scholar] [CrossRef]
- Yu, N.; Zhao, C.; Ma, B.; Li, S.; She, Z.; Guo, L.; Zhang, Q.; Zhao, Y.; Jin, C.; Gao, M. Impact of ampicillin on the nitrogen removal, microbial community and enzymatic activity of activated sludge. Bioresour. Technol. 2019, 272, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, Y.; Sen, B.; Wang, G. Reactive oxygen species and their applications toward enhanced lipid accumulation in oleaginous microorganisms. Bioresour. Technol. 2020, 307, 123234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y. Roles of ATP-dependent N-acylhomoserine lactones (AHLs) and extracellular polymeric substances (EPSs) in aerobic granulation. Chemosphere 2012, 88, 1058–1064. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, S.; Wu, L.; Guo, Y.; Shi, W.; Lens, P.N.L. Micro(nano)plastic size and concentration co-differentiate the treatment performance and toxicity mechanism in aerobic granular sludge systems. Chem. Eng. J. 2023, 457, 141212. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G. Microbial extracellular polymeric substances (EPS) acted as a potential reservoir in responding to high concentrations of sulfonamides shocks during biological wastewater treatment. Bioresour. Technol. 2020, 313, 123654. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Wei, T.; Zhou, Y. Response of antibiotic resistance genes expression and distribution on extracellular polymeric substances and microbial community in membrane biofilm during greywater treatment. Bioresour. Technol. 2024, 393, 130146. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, K.; Xu, H.; Li, Y.; Xue, Q.; Xue, W.; Zhang, A.; Wen, X.; Xu, G.; Huang, X. Spectroscopic fingerprints profiling the polysaccharide/protein/humic architecture of stratified extracellular polymeric substances (EPS) in activated sludge. Water Res. 2023, 235, 119866. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Niu, B.; Sun, H.; Zhou, X. Insight into response characteristics and inhibition mechanisms of anammox granular sludge to polyethylene terephthalate microplastics exposure. Bioresour. Technol. 2023, 385, 129355. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Cao, J.; Feng, G.; Luo, J.; Feng, Q.; Ni, B.; Fang, F. Fast identification of fluorescent components in three-dimensional excitation-emission matrix fluorescence spectra via deep learning. Chem. Eng. J. 2022, 430, 132893. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen removal by heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas sp. DM02: Removal performance, mechanism and immobilized application for real aquaculture wastewater treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, W.; Feng, Y.; Zhu, X.; Zhou, H.; Tan, Z.; Li, X. Impact resistance of different factors on ammonia removal by heterotrophic nitrification–aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef]
- Liu, T.; Wang, B.; Liu, M.; Jiang, K.; Wang, L. Stutzerimonas frequens strain TF18 with superior heterotrophic nitrification-aerobic denitrification ability for the treatment of aquaculture effluent. Process Biochem. 2023, 130, 156–165. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, P.; Hao, B.; Yu, Z. Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresour. Technol. 2011, 102, 9866–9869. [Google Scholar] [CrossRef]
- Jin, R.; Liu, T.; Liu, G.; Zhou, J.; Huang, J.; Wang, A. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by the Marine Origin Bacterium Pseudomonas sp. ADN-42. Appl. Biochem. Biotechnol. 2014, 175, 2000–2011. [Google Scholar] [CrossRef]
- Hu, R.; Liu, S.; Huang, W.; Nan, Q.; Strong, P.J.; Saleem, M.; Zhou, Z.; Luo, Z.; Shu, F.; Yan, Q.; et al. Evidence for Assimilatory Nitrate Reduction as a Previously Overlooked Pathway of Reactive Nitrogen Transformation in Estuarine Suspended Particulate Matter. Environ. Sci. Technol. 2022, 56, 14852–14866. [Google Scholar] [CrossRef]
- Lu, J.; Tan, Y.; Tian, S.; Qin, Y.; Zhou, M.; Hu, H.; Zhao, X.; Wang, Z.; Hu, B. Effect of carbon source on carbon and nitrogen metabolism of common heterotrophic nitrification-aerobic denitrification pathway. Chemosphere 2024, 361, 142525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Han, P.; Zhao, M.; Yu, Y.; Sun, D.; Hou, L.; Liu, M.; Zhao, Q.; Tang, X.; Klümper, U.; et al. Biotransformation of lincomycin and fluoroquinolone antibiotics by the ammonia oxidizers AOA, AOB and comammox: A comparison of removal, pathways, and mechanisms. Water Res. 2021, 196, 117003. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Wu, J.; Pang, W.; Hu, Z. Bioaugmented constructed wetlands for efficient saline wastewater treatment with multiple denitrification pathways. Bioresour. Technol. 2021, 335, 125236. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Huang, T.; Zhang, H.; Wen, G.; Li, K. Aerobic denitrifying bacterial community with low C/N ratio remove nitrate from micro-polluted water: Metagenomics unravels denitrification pathways. Sci. Total Environ. 2024, 951, 175457. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Chen, P.; Zhao, W.; Li, S.; Zhang, K. Acclimation Time Enhances Adaptation of Heterotrophic Nitrifying-Aerobic Denitrifying Microflora to Linear Anionic Surfactant Stress. Microorganisms 2025, 13, 1031. https://doi.org/10.3390/microorganisms13051031
Han H, Chen P, Zhao W, Li S, Zhang K. Acclimation Time Enhances Adaptation of Heterotrophic Nitrifying-Aerobic Denitrifying Microflora to Linear Anionic Surfactant Stress. Microorganisms. 2025; 13(5):1031. https://doi.org/10.3390/microorganisms13051031
Chicago/Turabian StyleHan, Huihui, Peizhen Chen, Wenjie Zhao, Shaopeng Li, and Keyu Zhang. 2025. "Acclimation Time Enhances Adaptation of Heterotrophic Nitrifying-Aerobic Denitrifying Microflora to Linear Anionic Surfactant Stress" Microorganisms 13, no. 5: 1031. https://doi.org/10.3390/microorganisms13051031
APA StyleHan, H., Chen, P., Zhao, W., Li, S., & Zhang, K. (2025). Acclimation Time Enhances Adaptation of Heterotrophic Nitrifying-Aerobic Denitrifying Microflora to Linear Anionic Surfactant Stress. Microorganisms, 13(5), 1031. https://doi.org/10.3390/microorganisms13051031




