Comparative Analysis of Vectorial Capacity Among Triatoma brasiliensis brasiliensis, Triatoma juazeirensis, and Their Experimental Hybrids
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 9 March 2025).
- Moncayo, A.; Silveira, A.C. Current epidemiological trends of Chagas disease in Latin America and future challenges: Epidemiology, surveillance and health policy. Mem. Inst. Oswaldo Cruz 2009, 104, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Cordovez, J.M.; Sanabria, C. Environmental changes can produce shifts in Chagas disease infection risk. Environ. Health Insights 2014, 2, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.P.; Cláudio, L.D.G.; Lima, M.M.; Albajar-Viñas, P.; Silva, R.A.E.; Alves, R.V.; Costa, V.M. Changes in the paradigm of clinical and therapeutic management of Chagas’ disease: Progress and perspectives in the pursuit of comprehensive health. Epidemiol. Serv. Saúde Rev. 2016, 25, 87–90. [Google Scholar] [CrossRef]
- Gurgel-Gonçalves, R.; Galvao, C.; Costa, J.; Peterson, A.T. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J. Trop. Med. 2012, 2012, 705326. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Dale, C.; Galvão, C.; Almeida, C.E.; Dujardin, J.P. Do the new triatomine species pose new challenges or strategies for monitoring Chagas disease? An overview from 1979–2021. Mem. Inst. Oswaldo Cruz 2021, 116, e210015. [Google Scholar] [CrossRef] [PubMed]
- Galvão, C.; Gil-Santana, H.R.; Oliveira, J. The taxonomic catalog of the Brazilian fauna: Biodiversity and geographical distribution of Triatominae (Hemiptera: Reduviidae) in Brazil. Zoologia 2024, 41, e24006. [Google Scholar] [CrossRef]
- Costa, J.; Lorenzo, M. Biology, diversity and strategies for the monitoring and control of triatomines—Chagas disease vectors. Mem. Inst. Oswaldo Cruz 2009, 104, 46–51. [Google Scholar] [CrossRef]
- Costa, J.; Marchon-Silva, V. Período de intermuda e resistência ao jejum de diferentes populações de Triatoma brasiliensis (Hemiptera, Reduviidae, Triatominae). Entomol. Vectores 1998, 5, 23–34. [Google Scholar]
- Costa, J.; Almeida, C.E.; Dontson, E.; Lins, A.; Vinhaes, M.C.; Silveira, A.C.; Beard, C.B. The epidemiologic importance of Triatoma brasiliensis as a Chagas disease vector in Brazil: A revision of domiciliary captures during 1993–1999. Mem. Inst. Oswaldo Cruz 2003, 98, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Dornak, L.L.; Almeida, C.E.; Peterson, A.T. Distributional potential of the Triatoma brasiliensis species complex at present and under scenarios of future climate conditions. Parasit Vectors 2014, 7, 238. [Google Scholar] [CrossRef]
- Dale, C.; Almeida, C.E.; Mendonça, V.J.; Oliveira, J.; da Rosa, J.A.; Galvão, C.; Costa, J. An updated and illustrated dichotomous key for the Chagas disease vectors of Triatoma brasiliensis species complex and their epidemiologic importance. ZooKeys 2018, 805, 33–43. [Google Scholar] [CrossRef]
- Lima-Neiva, V.; Toma, H.K.; Aguiar, L.M.A.; Lopes, C.M.; Dias, L.P.; Gonçalves, T.C.M.; Costa, J. The connection between Trypanosoma cruzi transmission cycles by Triatoma brasiliensis brasiliensis: A threat to human health in an area susceptible to desertification in the Seridó, Rio Grande do Norte, Brazil. PLoS Negl. Trop. Dis. 2021, 15, e0009919. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.A.; Donnelly, M.J.; Beard, C.B.; Costa, J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Mol. Phylogenet. Evol. 2004, 32, 46–56. [Google Scholar] [CrossRef]
- Costa, J.; Bargues, M.D.; Neiva, V.L.; Lawrence, G.G.; Gumiel, M.; Oliveira, G.; Cabello, P.; Lima, M.M.; Dotson, E.; Provance, D.W.; et al. Phenotypic variability confirmed by nuclear ribosomal DNA suggests a possible natural hybrid zone of Triatoma brasiliensis species complex. Infect. Genet. Evol. 2016, 37, 77–87. [Google Scholar] [CrossRef]
- Costa, J.; Peterson, A.T.; Dujardin, J.P. Morphological evidence suggests homoploid hybridization as a possible mode of speciation in the Triatominae (Hemiptera, Heteroptera, Reduviidae). Infect. Genet. Evol. 2009, 9, 263–270. [Google Scholar] [CrossRef]
- Gumiel, M. Estudo da Variabilidade Morfológica e do Perfil Isoenzimático em Triatomíneos do Complexo Triatoma brasiliensis do Estado de Pernambuco, Brasil. Master’s Thesis, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Herrera-Aguilar, M.; Be-Barragán, L.A.; Ramirez-Sierra, M.J.; Tripet, F.; Dorn, P.; Dumonteil, E. Identification of a large hybrid zone between sympatric sibling species of Triatoma dimidiata in the Yucatan peninsula, Mexico, and its epidemiological importance. Infect. Genet. Evol. 2009, 9, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernandez, F.; Martínez-Ibarra, J.A.; Catalá, S.; Villalobos, G.; de la Torre, P.; Laclette, J.P.; Alejandre-Aguilar, R.; Espinoza, B. Natural crossbreeding between sympatric species of the Phyllosoma complex (Insecta: Hemiptera: Reduviidae) indicate the existence of only one species with morphologic and genetic variations. Am. J. Trop. Med. Hyg. 2010, 82, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Correia, N.; Paschoaletto, L.; Reigada, C.; Gonçalves, T.C.M.; Moreira, C.J.d.; Costa, J. Experimental Hybrids of the Triatoma brasiliensis Species Complex Show Higher Susceptibility to the Trypanosoma cruzi Infection Than Their Parentals. Microorganisms 2023, 11, 2850. [Google Scholar] [CrossRef]
- Gonçalves, T.C.M.; Victório, V.M.N.; Jurberg, J.; Cunha, V. Biology of Triatoma vitticeps (Stål, 1859) under laboratory conditions (Hemiptera: Reduviidae: Triatominae): I. Evolutive cycle. Mem. Inst. Oswaldo Cruz 1988, 83, 519–523. [Google Scholar] [CrossRef]
- Oliveira, R.L. Principais insetos vetores e mecanismos de transmissão das doenças infecciosas e parasitárias. In Dinâmica das Doenças Infecciosas e Parasitárias, 1st ed.; Coura, J., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2013; pp. 108–130. [Google Scholar]
- Zeledón, R.; Alvarado, R.; Jirón, L.F. Observations on the feeding and defecation patterns of three triatomine species (Hemiptera: Reduviidae). Acta Trop. 1977, 34, 65–77. [Google Scholar]
- Rodrigues, V.L.C.C.; Ferraz-Filho, A.N.; Ishihata, G.K.; Silva, E.O.R. Triatoma brasiliensis (Neiva, 1911) (Hemiptera, Reduviidae): Observações Sobre seu Comportamento em Relação à Fonte Alimentar em Galinheiro Experimental. Cad. Saúde Publ. 1995, 11, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.V.; Lima, M.M. Feeding and Defecation Patterns of Nymphs of Triatoma rubrofasciata (De Geer, 1773) (Hemiptera: Reduviidae), and its Potential Role as Vector for Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 1999, 94, 127–129. [Google Scholar] [CrossRef]
- Wood, S.F. Importance of feeding and defecation times of insect vectors in transmission of Chagas diseases. J. Econ. Entomol. 1951, 44, 52–54. [Google Scholar] [CrossRef]
- Gonçalves, T.C.M.; Rocha, D.S.; Cunha, R. Feeding patterns of Triatoma vitticeps (Stal, 1859) (Hemiptera, Triatominae) in the State of Rio de Janeiro, Brazil. Rev. Saúde Publ. 2000, 34, 348–352. [Google Scholar] [CrossRef]
- Santos, C.B.D.; Leite, G.R.; Sessa, P.A.; Falqueto, A. Dynamics of feeding and defecation in Triatoma vitticeps (Stål, 1859) (Hemiptera, Reduviidae, Triatominae) and its potential in the transmission of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 2006, 101, 543–546. [Google Scholar] [CrossRef]
- Sangenis, L.; Saraiva, R.; Georg, I.; de Castro, L.; Lima, V.; Roque, A.; Xavier, S.; Santos, L.; Fernandes, F.A.; Sarquis, O.; et al. Autochthonous transmission of Chagas disease in Rio de Janeiro State, Brazil: A clinical and eco-epidemiological study. BMC Infect. Dis. 2015, 15, 4. [Google Scholar] [CrossRef]
- Rabinovich, J.E.; Leal, J.A.; Piñero, D.F. 1979. Domiciliary biting frequency and blood ingestion of the Chagas disease vector Rhodnius prolixus, Stål (Hemiptera: Reduviidae), in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, J.E.; Kitron, U.D.; Obed, Y.; Yoshioka, M.; Gottdenker, N.; Chaves, L.N. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 2011, 106, 479–494. [Google Scholar] [CrossRef]
- Rabinovich, J.E. Morphology, Life Cycle, Environmental Factors and Fitness—A Machine Learning Analysis in Kissing Bugs (Hemiptera, Reduviidae, Triatominae). Front. Ecol. Evol. 2021, 9, 651–683. [Google Scholar] [CrossRef]
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectores of Chagas’ disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 125–520. [Google Scholar]
- Costa, J.; Correia, N.C.; Neiva, V.L.; Goncalves, T.C.M.; Felix, M. Revalidation and re description of Triatoma brasiliensis macromelasoma Galvão, 1956 and an identification key for the Triatoma brasiliensis complex (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 2013, 108, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dias, E. Observações sobre eliminação de dejeções e tempo de sucção em alguns triatomíneos sul-americanos. Mem. Inst. Oswaldo Cruz 1956, 54, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Galvão, C.; Rocha, D.S.; Cunha, V.; Jurberg, J. Tempo de alimentação e defecação das ninfas de Triatoma melanosoma Martínez, Olmedo & Carcavallo (Hemiptera, Reduviidae) em diferentes condições de temperatura e umidade. Rev. Bras. Zool. 2001, 18, 233–242. [Google Scholar]
- Almeida, C.E.; Francischetti, C.N.; Pacheco, R.S.; Costa, J. Triatoma rubrovaria (Blanchard, 1843) (Hemiptera-Reduviidae-Triatominae) III: Patterns of feeding, defecation and resistance to starvation. Mem. Inst. Oswaldo Cruz 2003, 98, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.N.; Gontijo, N.F.; Lazzari, C.R.; Pereira, M.H. Interação entre os Insetos Hematófagos e seus Hospedeiros Vertebrados. In Tópicos Avançados em Entomologia Molecular, 1st ed.; Silva-Neto, M.A.C., Winter, C.E., Termignoni, C., Eds.; Universidade Federal do Rio Grande do Sul: Rio Grande do Sul, Brazil, 2012. [Google Scholar]
- Guarneri, A.A.; Diotaiuti, L.; Gontijo, N.F.; Gontijo, A.F.; Pereira, M.H. Comparison of feeding behaviour of Triatoma infestans, Triatoma brasiliensis and Triatoma pseudomaculata in different hosts by electronic monitoring of the cibarial pump. J. Insect Physiol. 2000, 46, 1121–1127. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Silva-Cardoso, L.; Atella, G. Interação Parasito-Vetor (Tripanossomatídeos). In Tópicos Avançados em Entomologia Molecular, 1st ed.; Silva-Neto, M.A.C., Winter, C.E., Termignoni, C., Eds.; Universidade Federal do Rio Grande do Sul: Rio Grande do Sul, Brazil, 2012. [Google Scholar]
- Lima-Neiva, V. Aspectos Biológicos e Potencial Vetorial de Triatoma sherlocki Papa, Jurberg Carcavallo, Cerqueira & Barata, 2002 (Hemiptera: Reduviidae: Triatominae) em Condições de Laboratório. Master’s Thesis, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Kollien, A.H.; Schaub, G.A. The Development of Trypanosoma cruzi in Triatominae. Parasitol. Today 2000, 16, 381–387. [Google Scholar] [CrossRef]
- Soares, R.P.P.; Evangelista, L.G.; Laranja, L.S.; Diotaiuti, L. Population Dynamics and Feeding Behavior of Triatoma brasiliensis and Triatoma pseudomaculata, Main Vectors of Chagas Disease in Northeastern Brazil. Mem. Inst. Oswaldo Cruz 2000, 95, 151–155. [Google Scholar] [CrossRef]
- Galvão, C.; Jurberg, J.; Cunha, V.; De Mello, R.P. Biologia do Triatoma nitida Usinger 1939 em Laboratorio (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 1995, 90, 657–663. [Google Scholar] [CrossRef]
- Kollien, A.H.; Schaub, G.A. The Development of Trypanosoma cruzi (Trypanosomatidae) in the Reduviid Bug Triatoma infestans (Insecta): Influence of Starvation. J. Euk. Microbiol. 1998, 45, 59–63. [Google Scholar] [CrossRef]
- Gonçalves, T.C.M.; Cunha, V.; Oliveira, E.D.; Jurberg, J. Alguns aspectos da biologia de Triatoma pseudomaculata Corrêa & Espínola, 1964, em condições de laboratório (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 1997, 92, 275–280. [Google Scholar]
- Jurberg, J.; Costa, J.M. The resistance of fasting and nutritional aspects of Triatoma lecticularia (Stål, 1859) (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 1989, 84, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.P.; Lemos, F.J.A.; Silva, J.R. Digestão em Insetos. In Tópicos Avançados em Entomologia Molecular, 1st ed.; Silva-Neto, M.A.C., Winter, C.E., Termignoni, C., Eds.; Universidade Federal do Rio Grande do Sul: Rio Grande do Sul, Brazil, 2012. [Google Scholar]
- Rocha, D.S.; Galvão, C.; Jurberg, J. Biologia do Rhodnius pictipes Stal, 1872 em condições de laboratório (Hemiptera, Reduviidae, Triatominae). Mem. Inst. Oswaldo Cruz 1994, 89, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J.; Sherlock, I.A. Factors controlling the volume of feces produced by triatomine vectors of Chagas’ disease. Acta Trop. 1983, 40, 351–358. [Google Scholar]
- Silva, I.G.; Luquetti, A.O.; Silva, H.H.G.D. Importância do método de obtenção das dejeções de triatomíneos na avaliação da suscetibilidade triatomínica para Trypanosoma cruzi. Rev. Soc. Bras. Med. Trop. 1993, 26, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Crocco, L.B.; Catalá, S.S. Feeding and defecation patterns in Triatoma sordida. Mem. Inst. Oswaldo Cruz 1996, 91, 409–413. [Google Scholar] [CrossRef]
- Pietrokovsky, S.; Bottazzi, V.; Schweigmann, N.; Haedo, A.; Wisnivesky-Colli, C. Comparison of the blood meal size among Triatoma infestans, T. guasayana and T. sordida (Hemiptera: Reduviidae) of Argentina under laboratory conditions. Mem. Inst. Oswaldo Cruz 1996, 91, 241–242. [Google Scholar] [CrossRef]
- Heitzmann-Fontenelle, T. Bionomia de Triatoma pseudomaculata Correa & Espinola, 1964, em laboratório. Mem. Inst. Butantan 1972, 36, 251–262. [Google Scholar]


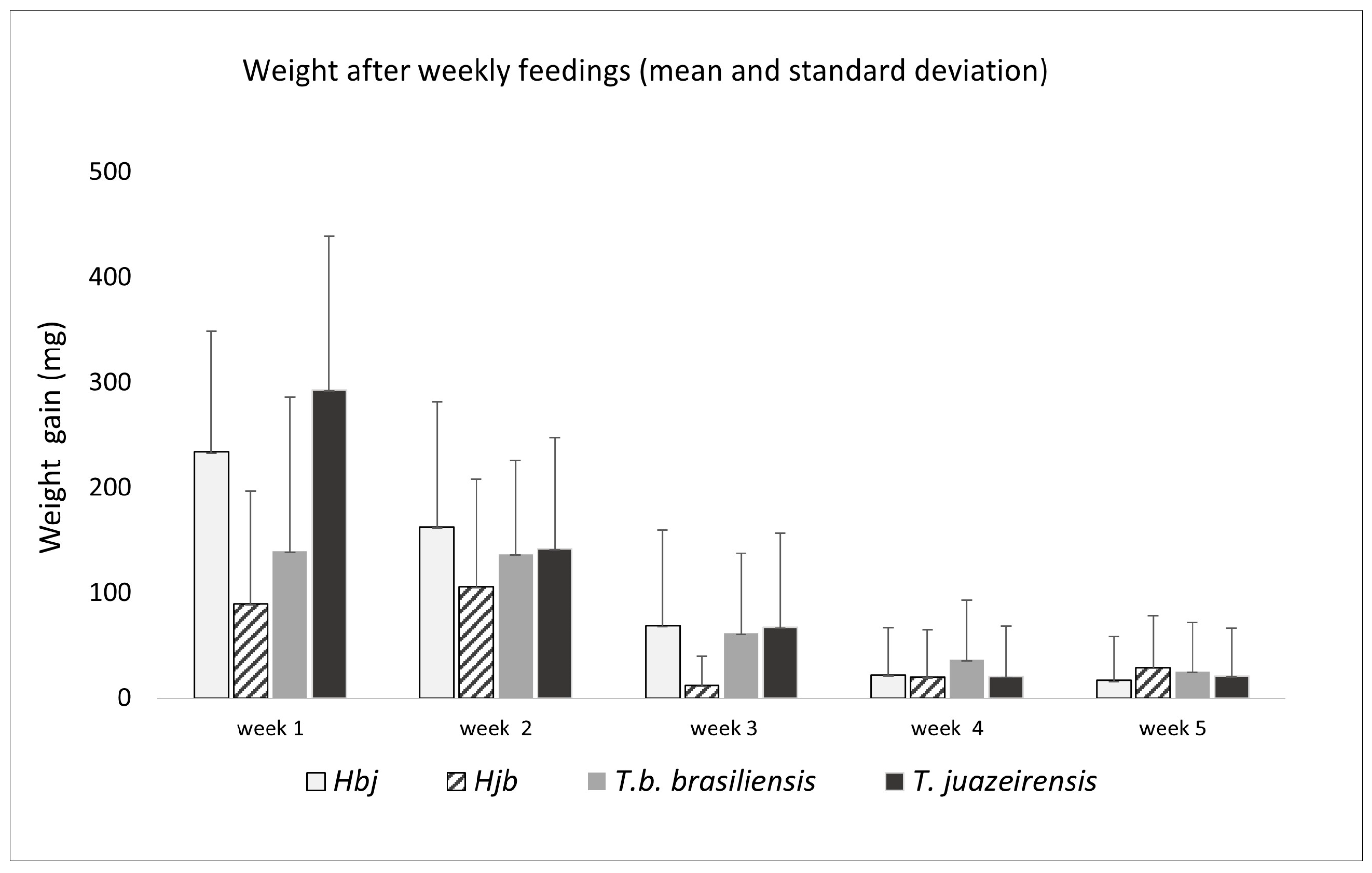


| Species | N | Weeks | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||
| Hbj | 30 | 100% | 87% | 63% | 33% | 27% |
| Hjb | 12 | 83% | 100% | 50% | 50% | 50% |
| T. b. brasiliensis | 30 | 77% | 93% | 77% | 57% | 57% |
| T. juazeirensis | 30 | 97% | 93% | 47% | 37% | 30% |
| Species | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triatoma b. brasiliensis | Triatoma juazeirensis | Hbj | Hjb | |||||||||||||||||
| Amplitude | Amplitude | Amplitude | Amplitude | |||||||||||||||||
| Weeks | N | SD | Min | Max | n | SD | Min | Max | n | SD | Min | Max | n | SD | Min | Max | ||||
| 1 | 30 | 48.5 | 36.1 | 8.1 | 156.4 | 30 | 56.2 | 34.9 | 12.6 | 121 | 30 | 43.1 | 31.6 | 9.4 | 169.2 | 12 | 65.7 | 47.2 | 22.8 | 184.3 |
| 2 | 30 | 42.7 | 32.7 | 2.8 | 150.6 | 30 | 28.7 | 15 | 0.3 | 64.4 | 30 | 43.1 | 25 | 11 | 129.9 | 12 | 47.2 | 42.1 | 11.1 | 166.9 |
| 3 | 30 | 43.4 | 47.1 | 3.1 | 213.8 | 30 | 35.6 | 16.2 | 14.5 | 72.2 | 30 | 31 | 25.6 | 6.8 | 99.4 | 12 | 66.4 | 64.3 | 2.5 | 154.1 |
| 4 | 30 | 41.2 | 42.7 | 3.8 | 140.6 | 30 | 12.1 | 7.4 | 5 | 29.7 | 30 | 26.6 | 21.2 | 4.5 | 64.6 | 12 | 30.6 | 18.2 | 10 | 58.5 |
| 5 | 30 | 27.4 | 26.4 | 0.3 | 81.3 | 30 | 21.3 | 17.4 | 0.3 | 46.5 | 30 | 20.3 | 17.2 | 2.9 | 50.4 | 12 | 28.4 | 17.2 | 10.9 | 58.5 |
| Ft × Td | Ft × W | Td × W | ||||
|---|---|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | Correlation | p-Value | |
| T. b. brasiliensis | −0.803 | 0.085 | −0.671 | 0.117 | 0.894 | 0.036 |
| T. juazeirensis | −0.600 | 0.236 | −0.738 | 0.077 | 0.738 | 0.077 |
| HBJ | −0.949 | 0.020 * | −0.939 | 0.023 * | 1 | 0.019 ** |
| HJB | −0.130 | 0.805 | −0.920 | 0.025 * | −0.105 | 0.801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, N.C.; Moreira, C.J.d.C.; Firmino, F.O.; Rocha, D.d.S.; Oliveira-Correia, J.P.S.; Galvão, C.; Costa, J. Comparative Analysis of Vectorial Capacity Among Triatoma brasiliensis brasiliensis, Triatoma juazeirensis, and Their Experimental Hybrids. Microorganisms 2025, 13, 1025. https://doi.org/10.3390/microorganisms13051025
Correia NC, Moreira CJdC, Firmino FO, Rocha DdS, Oliveira-Correia JPS, Galvão C, Costa J. Comparative Analysis of Vectorial Capacity Among Triatoma brasiliensis brasiliensis, Triatoma juazeirensis, and Their Experimental Hybrids. Microorganisms. 2025; 13(5):1025. https://doi.org/10.3390/microorganisms13051025
Chicago/Turabian StyleCorreia, Nathália Cordeiro, Carlos José de Carvalho Moreira, Fernanda Oliveira Firmino, Dayse da Silva Rocha, João Paulo Sales Oliveira-Correia, Cleber Galvão, and Jane Costa. 2025. "Comparative Analysis of Vectorial Capacity Among Triatoma brasiliensis brasiliensis, Triatoma juazeirensis, and Their Experimental Hybrids" Microorganisms 13, no. 5: 1025. https://doi.org/10.3390/microorganisms13051025
APA StyleCorreia, N. C., Moreira, C. J. d. C., Firmino, F. O., Rocha, D. d. S., Oliveira-Correia, J. P. S., Galvão, C., & Costa, J. (2025). Comparative Analysis of Vectorial Capacity Among Triatoma brasiliensis brasiliensis, Triatoma juazeirensis, and Their Experimental Hybrids. Microorganisms, 13(5), 1025. https://doi.org/10.3390/microorganisms13051025








