Characterization of a Capsule-Deficient Pasteurella multocida Isolated from Cygnus melancoryphus: Genomic, Phenotypic, and Virulence Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions, and Reagents
2.2. Whole Genome Sequencing (WGS), Genotype Determination, and Phylogeny
2.3. Transmission Electron Microscope (TEM) and Scanning Electron Microscope (SEM) Analysis
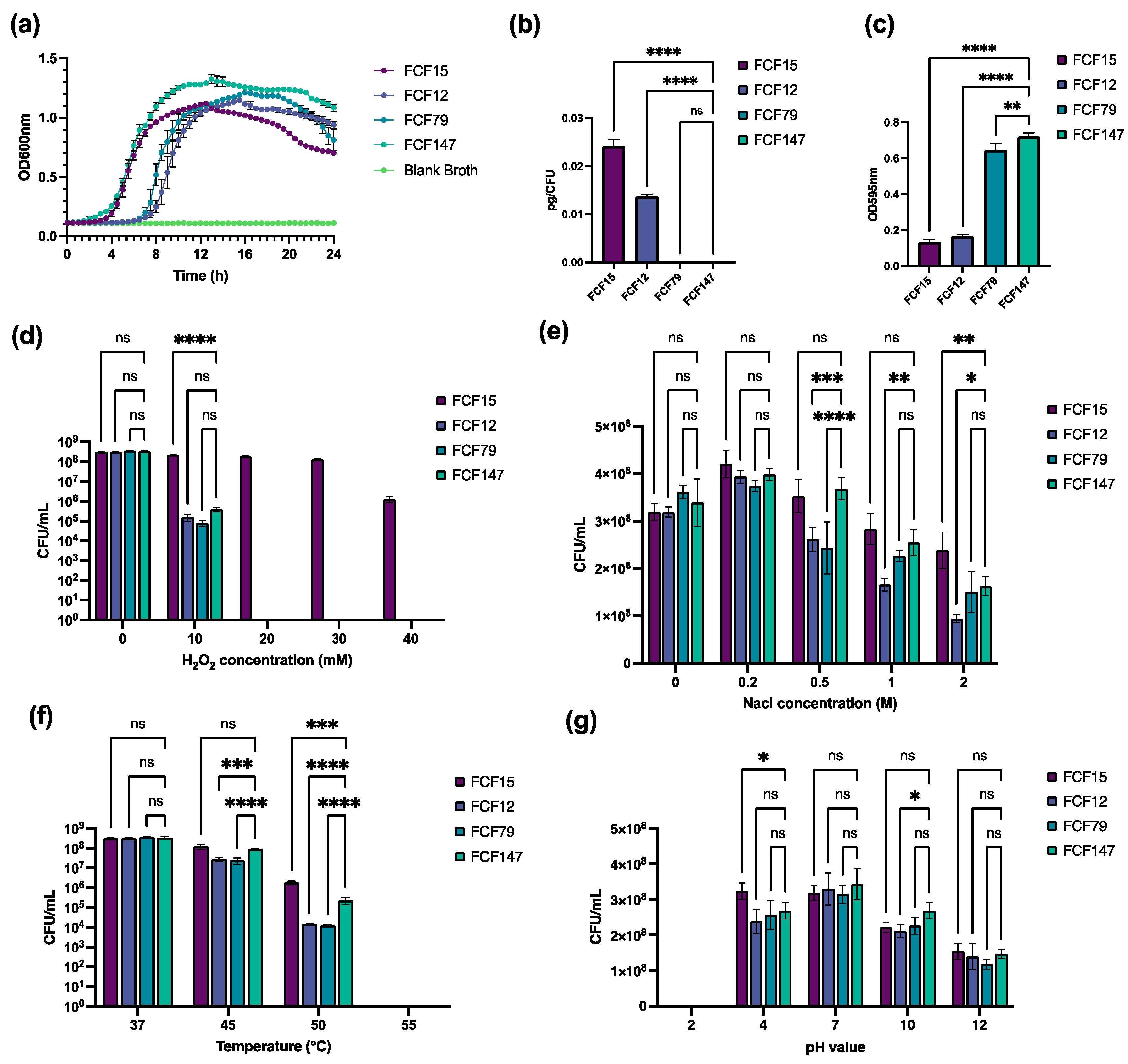
2.4. One-Step In Vitro Growth Curve
2.5. Capsule and Biofilm Quantification
2.6. Stress Tolerance Testing Assay
2.7. Determination of Minimum Inhibitory Concentration (MIC)
2.8. Animal Pathogenicity and Immune Protection Assay
2.9. Histopathological Examination
2.10. Statistical Analyses
3. Results
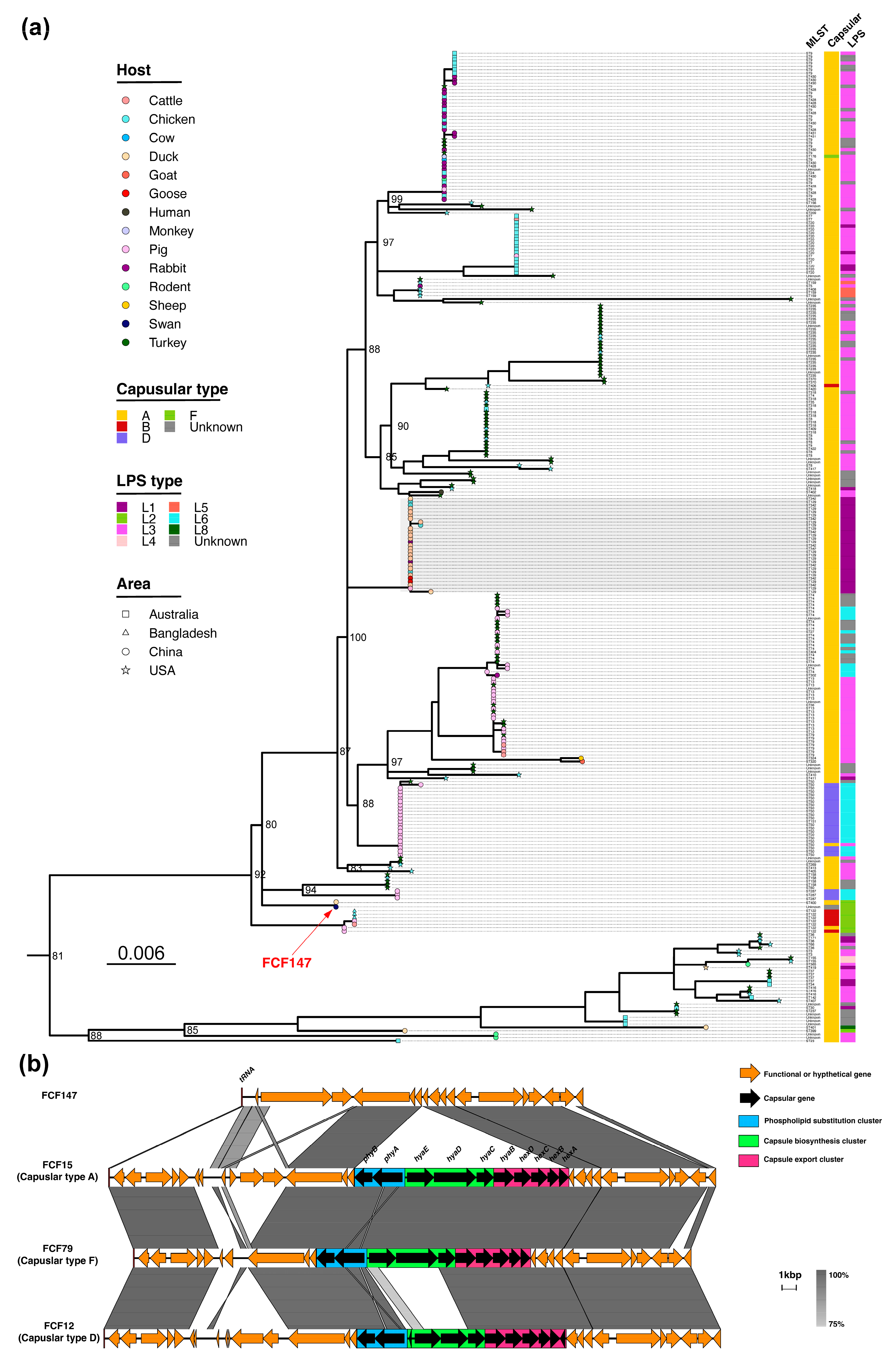
3.1. Genomic and Phylogenetic Characterization of FCF147
3.2. Loss of Capsule Locus and Associated Morphological Changes
3.3. Enhancing Biofilm Formation and Tolerance to High Temperature
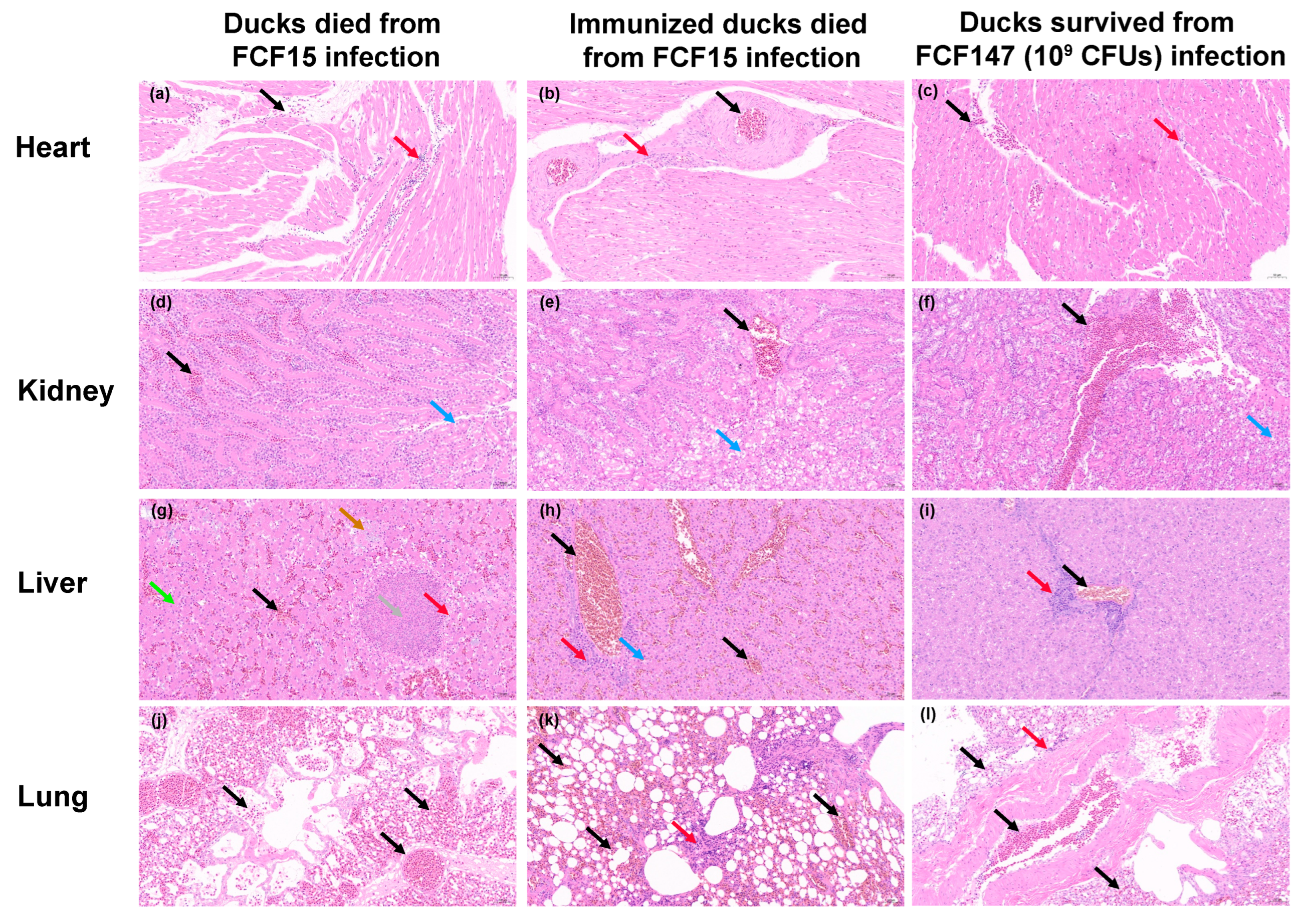
3.4. Attenuated Virulence and Limited Immunoprotection in Animal Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| MLST | The multi-locus sequence typing |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
| FBS | Fetal bovine serum |
| WGS | Whole genome sequencing |
| GO | The Gene Ontology |
| KEGG | The Kyoto Encyclopedia of Genes and Genomes |
| CARD | The Comprehensive Antibiotic Resistance Database |
| VFDB | The Virulence Factor Database |
| PHI | The Pathogen Host Interactions |
| ML | Maximum likelihood |
| GTR | General time-reversible |
| TEM | Transmission electron microscope |
| SEM | Scanning electron microscope |
| PBS | Phosphate-buffered saline |
| OD | The optical density |
| rpm | Revolutions per minute |
| CFU | colony-forming unit |
| CV | Crystal Violet |
| MIC | Minimum Inhibitory Concentration |
| CA-MHB | Cation-adjusted Mueller–Hinton broth |
| HE | Hematoxylin and eosin |
| SDs | Standard deviations |
| ns | Not significant |
References
- Hurtado, R.; Maturrano, L.; Azevedo, V.; Aburjaile, F. Pathogenomics insights for understanding Pasteurella multocida adaptation. Int. J. Med. Microbiol. 2020, 310, 151417. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lin, L.; Wang, X.; Chen, H.; Wu, B. The public health concern of Pasteurella multocida should not be ignored. Lancet Microbe 2022, 3, e560. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, X.; Zhou, R.; Chen, H.; Wilson, B.A.; Wu, B. Pasteurella multocida: Genotypes and Genomics. Microbiol. Mol. Biol. Rev. 2019, 83, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.; Freimanis, G.L.; Orynbayev, M.; Ribeca, P.; Flannery, J.; King, D.P.; Zuther, S.; Beer, M.; Hoper, D.; Kydyrmanov, A.; et al. Mass Die-Off of Saiga Antelopes, Kazakhstan, 2015. Emerg. Infect. Dis. 2019, 25, 1169–1176. [Google Scholar] [CrossRef]
- Foggin, C.M.; Rosen, L.E.; Henton, M.M.; Buys, A.; Floyd, T.; Turner, A.D.; Tarbin, J.; Lloyd, A.S.; Chaitezvi, C.; Ellis, R.J.; et al. Pasteurella sp. associated with fatal septicaemia in six African elephants. Nat. Commun. 2023, 14, 6398. [Google Scholar] [CrossRef]
- Hashish, A.; Johnson, T.J.; Chundru, D.; Williams, M.L.; Sato, Y.; Macedo, N.R.; Clessin, A.; Gantelet, H.; Bost, C.; Tornos, J.; et al. Complete Genome Sequences of Two Pasteurella multocida Isolates from Seabirds. Microbiol. Resour. Announc. 2023, 12, e0136522. [Google Scholar] [CrossRef]
- Smith, O.M.; Snyder, W.E.; Owen, J.P. Are we overestimating risk of enteric pathogen spillover from wild birds to humans? Biol. Rev. Camb. Philos. Soc. 2020, 95, 652–679. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Cohen, J. Virus gone wild. Science 2024, 384, 615–617. [Google Scholar] [CrossRef]
- Hubalek, Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J. Wildl. Dis. 2004, 40, 639–659. [Google Scholar] [CrossRef]
- Samuel, M.D.; Goldberg, D.R.; Shadduck, D.J.; Price, J.I.; Cooch, E.G. Pasteurella multocida serotype 1 isolated from a lesser snow goose: Evidence of a carrier state. J. Wildl. Dis. 1997, 33, 332–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samuel, M.D.; Shadduck, D.J.; Goldberg, D.R. Avian cholera exposure and carriers in greater white-fronted geese breeding in Alaska, USA. J. Wildl. Dis. 2005, 41, 498–502. [Google Scholar] [CrossRef]
- Samuel, M.D.; Shadduck, D.J.; Goldberg, D.R.; Johnson, W.P. Avian cholera in waterfowl: The role of lesser snow and ross’s geese as disease carriers in the Playa Lakes Region. J. Wildl. Dis. 2005, 41, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, I.W.; Hughes, T.P. The Epidemiology of Fowl Cholera: Vi. The Spread of Epidemic and Endemic Strains of Pasteurella Avicida in Laboratory Populations of Normal Fowl. J. Exp. Med. 1932, 55, 71–78. [Google Scholar] [CrossRef][Green Version]
- Samuel, M.D.; Shadduck, D.J.; Goldberg, D.R.; Johnson, W.P. Comparison of methods to detect Pasteurella multocida in carrier waterfowl. J. Wildl. Dis. 2003, 39, 125–135. [Google Scholar] [CrossRef][Green Version]
- Guan, L.; Zhang, L.; Xue, Y.; Yang, J.; Zhao, Z. Molecular pathogenesis of the hyaluronic acid capsule of Pasteurella multocida. Microb. Pathog. 2020, 149, 104380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Chen, H.; Cheng, L.; Fu, Q.; Liu, R.; Liang, Q.; Fu, G.; Wan, C.; Huang, Y. Genomic analysis reveals the population structure and antimicrobial resistance of avian Pasteurella multocida in China. J. Antimicrob. Chemother. 2024, 79, 186–194. [Google Scholar] [CrossRef]
- Boyce, J.D.; Adler, B. The capsule is a virulence determinant in the pathogenesis of Pasteurella multocida M1404 (B:2). Infect. Immun. 2000, 68, 3463–3468. [Google Scholar] [CrossRef]
- Chung, J.Y.; Wilkie, I.; Boyce, J.D.; Townsend, K.M.; Frost, A.J.; Ghoddusi, M.; Adler, B. Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroup A. Infect. Immun. 2001, 69, 2487–2492. [Google Scholar] [CrossRef]
- Li, N.; Feng, T.; Wang, Y.; Li, P.; Yin, Y.; Zhao, Z.; Hardwidge, P.R.; Peng, Y.; He, F. A single point mutation in the hyaC gene affects Pasteurella multocida serovar A capsule production and virulence. Microb. Pathog. 2021, 159, 105145. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Z.; Hu, J.; Wu, B.; Cai, X.; He, Q.; Chen, H. Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. J. Clin. Microbiol. 2009, 47, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Smallman, T.R.; Perlaza-Jimenez, L.; Wang, X.; Korman, T.M.; Kotsanas, D.; Gibson, J.S.; Turni, C.; Harper, M.; Boyce, J.D. Pathogenomic analysis and characterization of Pasteurella multocida strains recovered from human infections. Microbiol. Spectr. 2024, 12, e0380523. [Google Scholar] [CrossRef]
- Steen, J.A.; Steen, J.A.; Harrison, P.; Seemann, T.; Wilkie, I.; Harper, M.; Adler, B.; Boyce, J.D. Fis is essential for capsule production in Pasteurella multocida and regulates expression of other important virulence factors. PLoS Pathog. 2010, 6, e1000750. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, B.; Zheng, D.; Chen, L.; Yang, J. VFDB 2025: An integrated resource for exploring anti-virulence compounds. Nucleic Acids Res. 2025, 53, D871–D877. [Google Scholar] [CrossRef]
- Cuzick, A.; Seager, J.; Wood, V.; Urban, M.; Rutherford, K.; Hammond-Kosack, K.E. A framework for community curation of interspecies interactions literature. eLife 2023, 12, e84658. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Moller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wyres, K.L.; Li, J.; Fessler, A.T.; Kruger, H.; Wang, Y.; Holt, K.E.; Schwarz, S.; Wu, C. Evolution and genomic insight into methicillin-resistant Staphylococcus aureus ST9 in China. J. Antimicrob. Chemother. 2021, 76, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cao, D.; Subhadra, B.; De Castro, C.; Speciale, I.; Inzana, T.J. Relationship between capsule production and biofilm formation by Mannheimia haemolytica, and establishment of a poly-species biofilm with other Pasteurellaceae. Biofilm 2024, 8, 100223. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2024. [Google Scholar]
- Kock, R.A.; Orynbayev, M.; Robinson, S.; Zuther, S.; Singh, N.J.; Beauvais, W.; Morgan, E.R.; Kerimbayev, A.; Khomenko, S.; Martineau, H.M.; et al. Saigas on the brink: Multidisciplinary analysis of the factors influencing mass mortality events. Sci. Adv. 2018, 4, eaao2314. [Google Scholar] [CrossRef]
- Pickering, A.C.; Vitry, P.; Prystopiuk, V.; Garcia, B.; Höök, M.; Schoenebeck, J.; Geoghegan, J.A.; Dufrêne, Y.F.; Fitzgerald, J.R. Host-specialized fibrinogen-binding by a bacterial surface protein promotes biofilm formation and innate immune evasion. PLoS Pathog. 2019, 15, e1007816. [Google Scholar] [CrossRef]
- Guo, S.; Vance, T.D.R.; Stevens, C.A.; Voets, I.K.; Davies, P.L. RTX Adhesins are Key Bacterial Surface Megaproteins in the Formation of Biofilms. Trends Microbiol. 2019, 27, 470. [Google Scholar] [CrossRef]
- Petruzzi, B.; Briggs, R.E.; Tatum, F.M.; Swords, W.E.; De Castro, C.; Molinaro, A.; Inzana, T.J. Capsular Polysaccharide Interferes with Biofilm Formation by Pasteurella multocida Serogroup A. mBio 2017, 8, e01843-17. [Google Scholar] [CrossRef]
- Petruzzi, B.; Dalloul, R.A.; LeRoith, T.; Evans, N.P.; Pierson, F.W.; Inzana, T.J. Biofilm formation and avian immune response following experimental acute and chronic avian cholera due to Pasteurella multocida. Vet. Microbiol. 2018, 222, 114–123. [Google Scholar] [CrossRef]
- Su, A.; Tong, J.; Fu, Y.; Muller, S.; Weldearegay, Y.B.; Becher, P.; Valentin-Weigand, P.; Meens, J.; Herrler, G. Infection of bovine well-differentiated airway epithelial cells by Pasteurella multocida: Actions and counteractions in the bacteria-host interactions. Vet. Res. 2020, 51, 140. [Google Scholar] [CrossRef]
- Shen, X.; Guan, L.; Zhang, J.; Xue, Y.; Si, L.; Zhao, Z. Study in the iron uptake mechanism of Pasteurella multocida. Vet. Res. 2025, 56, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, P.; Gao, L.; Yuan, X.; Hardwidge, P.R.; Li, T.; Li, P.; He, F.; Peng, Y.; Li, N. Deleting qseC downregulates virulence and promotes cross-protection in Pasteurella multocida. Vet. Res. 2021, 52, 140. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Z.; Shang, Y.; Song, W.; Yang, J.; Bi, H.; Wang, Z.; Xie, R.; Zhao, M.; Hua, L.; et al. Discovery of the tigecycline resistance gene cluster tmexCD3-toprJ1 in Pasteurella multocida strains isolated from pigs in China. Vet. Microbiol. 2024, 292, 110046. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.M.; Seemann, T.; Gladman, S.; Adler, B.; Harper, M.; Boyce, J.D.; Bennett, M.D. Comparative Genomic Analysis of Asian Haemorrhagic Septicaemia-Associated Strains of Pasteurella multocida Identifies More than 90 Haemorrhagic Septicaemia-Specific Genes. PLoS ONE 2015, 10, e0130296. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xiong, P.; Zhang, H.; Yang, L.; Qiu, Y.; Li, P.; Zhao, G.; Li, N.; Peng, Y. Attenuated vaccine PmCQ2Delta4555-4580 effectively protects mice against Pasteurella multocida infection. BMC Vet. Res. 2024, 20, 94. [Google Scholar] [CrossRef]
- Kubatzky, K.F.; Kloos, B.; Hildebrand, D. Signaling cascades of Pasteurella multocida toxin in immune evasion. Toxins 2013, 5, 1664–1681. [Google Scholar] [CrossRef]
- Kharb, S.; Charan, S. Mucosal immunization provides better protection than subcutaneous immunization against Pasteurella multocida (B:2) in mice preimmunized with the outer membrane proteins. Vet. Res. Commun. 2011, 35, 457–461. [Google Scholar] [CrossRef]

| Antimicrobial Agents | FCF12 | FCF15 | FCF79 | FCF147 |
|---|---|---|---|---|
| Ampicillin | <0.25 | <0.25 | 0.5 | <0.25 |
| Amoxicillin | 0.5 | 0.5 | 0.5 | 0.5 |
| Kanamycin | 0.5 | 8 | 4 | 1 |
| Streptomycin | 1 | 4 | 1 | 1 |
| Erythromycin | 1 | 2 | 2 | 2 |
| Tilmicosin | 2 | 4 | 2 | 1 |
| Chloramphenicol | 0.5 | 1 | 1 | 0.5 |
| Florfenicol | 1 | 0.5 | 1 | 0.5 |
| Tetracycline | 1 | 16 | 4 | 2 |
| Tigecycline | <0.25 | <0.25 | <0.25 | <0.25 |
| Sulfadiazine | 2 | >128 | 4 | 4 |
| Ciprofloxacin | 0.5 | 1 | 1 | 1 |
| Enrofloxacin | <0.25 | 0.5 | 1 | 0.5 |
| Rifampin | <0.25 | <0.25 | <0.25 | <0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Chen, H.; Wang, W.; Liang, Q.; Fu, Q.; Liu, R.; Fu, G.; Wan, C.; Huang, Y.; Cheng, L. Characterization of a Capsule-Deficient Pasteurella multocida Isolated from Cygnus melancoryphus: Genomic, Phenotypic, and Virulence Insights. Microorganisms 2025, 13, 1024. https://doi.org/10.3390/microorganisms13051024
Jiang N, Chen H, Wang W, Liang Q, Fu Q, Liu R, Fu G, Wan C, Huang Y, Cheng L. Characterization of a Capsule-Deficient Pasteurella multocida Isolated from Cygnus melancoryphus: Genomic, Phenotypic, and Virulence Insights. Microorganisms. 2025; 13(5):1024. https://doi.org/10.3390/microorganisms13051024
Chicago/Turabian StyleJiang, Nansong, Hongmei Chen, Weiwei Wang, Qizhang Liang, Qiuling Fu, Rongchang Liu, Guanghua Fu, Chunhe Wan, Yu Huang, and Longfei Cheng. 2025. "Characterization of a Capsule-Deficient Pasteurella multocida Isolated from Cygnus melancoryphus: Genomic, Phenotypic, and Virulence Insights" Microorganisms 13, no. 5: 1024. https://doi.org/10.3390/microorganisms13051024
APA StyleJiang, N., Chen, H., Wang, W., Liang, Q., Fu, Q., Liu, R., Fu, G., Wan, C., Huang, Y., & Cheng, L. (2025). Characterization of a Capsule-Deficient Pasteurella multocida Isolated from Cygnus melancoryphus: Genomic, Phenotypic, and Virulence Insights. Microorganisms, 13(5), 1024. https://doi.org/10.3390/microorganisms13051024








