Indole Acetic Acid: A Key Metabolite That Protects Marine Sulfitobacter mediterraneus Against Oxidative Stress
Abstract
1. Introduction
2. Methods and Materials
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. ROS Detection
2.3. Detection of IAA Produced by S. mediterraneus SC1-11 Under Oxidative Stress
2.4. RNA Extraction and RT-qPCR
2.5. Protective Effect of IAA Supplementation on H2O2-Induced Stress in S. mediterraneus SC1-11
2.6. Proteomics of S. mediterraneus SC1-11 Under Oxidative Stress
2.7. Quantification of S. mediterraneus SC1-11 Amino Acids Under Oxidative Stress
2.8. Protective Effect of Amino Acid Supplementations on H2O2-Induced Effects in S. mediterraneus SC1-11
2.9. Bioinformatics Analysis
2.10. Statistical Analyses
3. Results
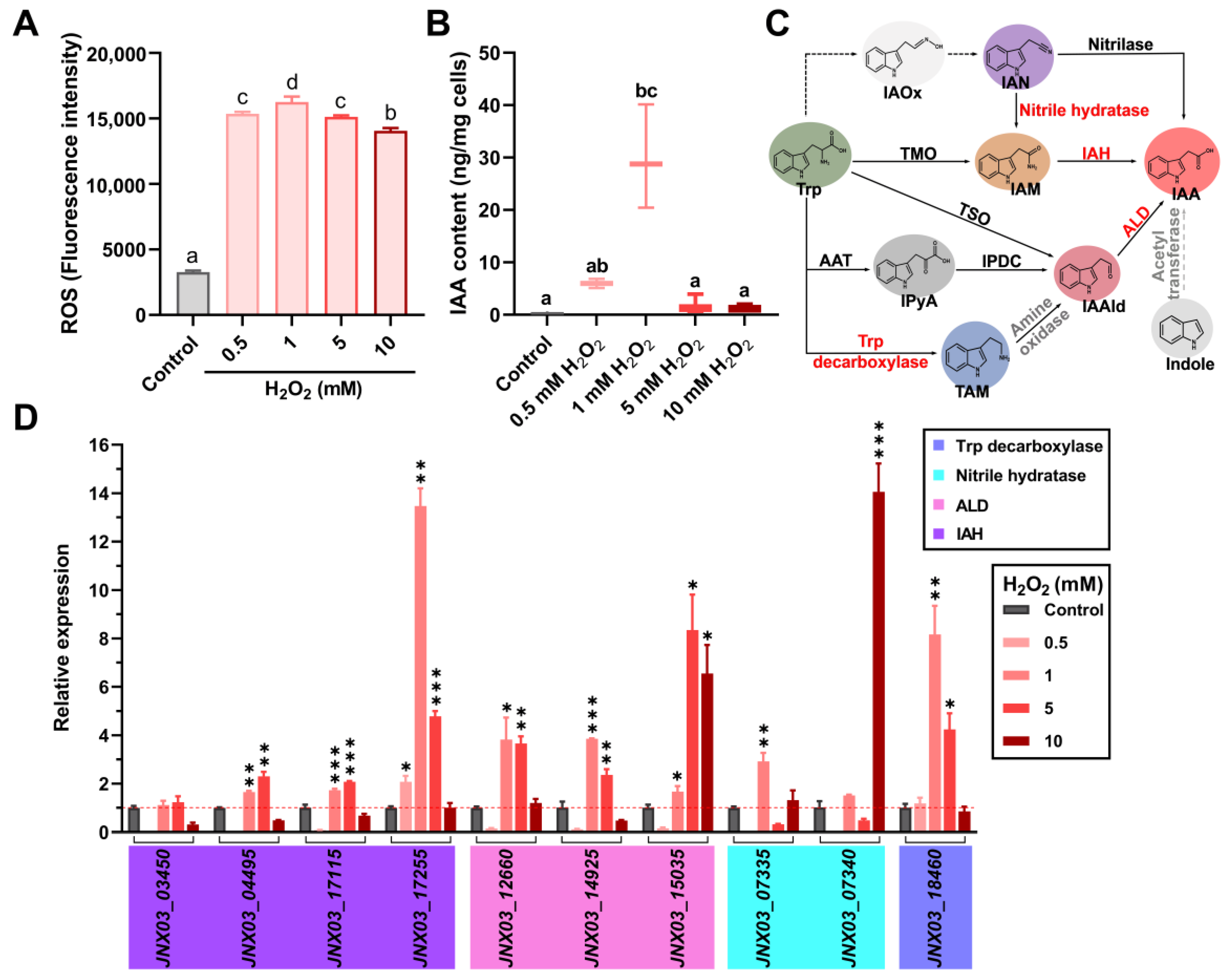
3.1. Oxidative Stress Stimulates the Biosynthesis of IAA in S. mediterraneus SC1-11
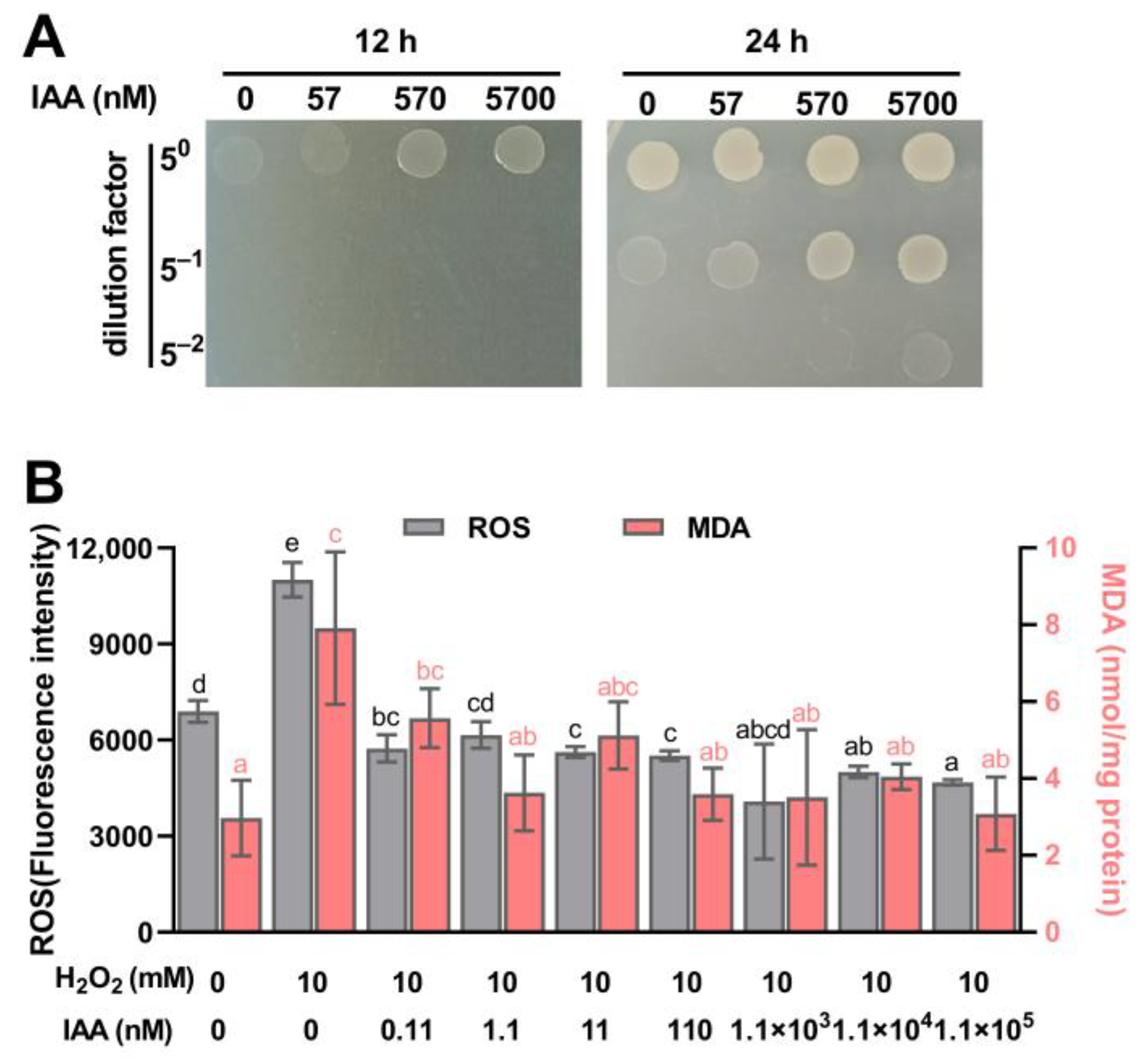
3.2. IAA Supplementation Protects S. mediterraneus SC1-11 from Oxidative Stress
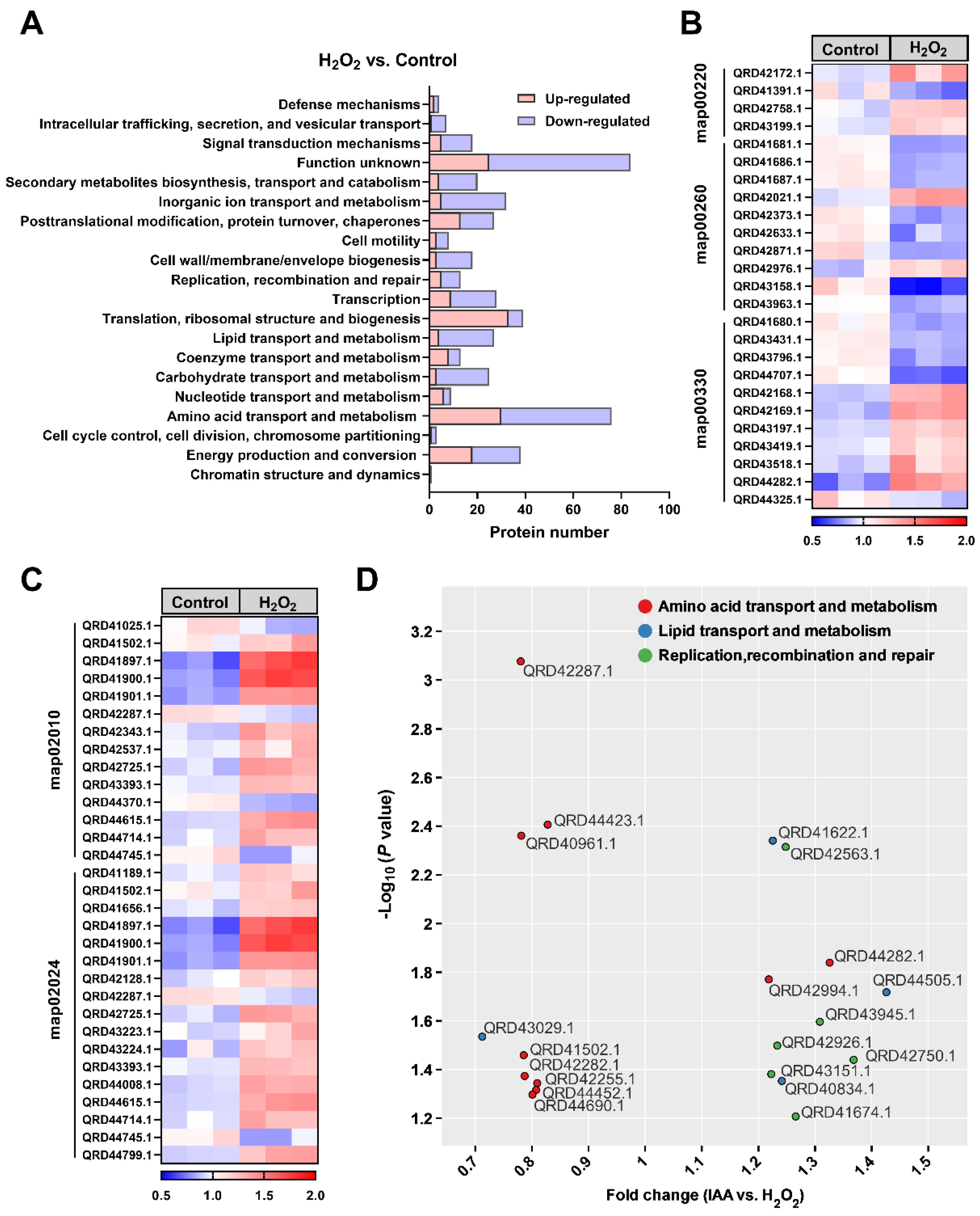
3.3. Oxidative Stress-Induced Proteomic and Metabolic Changes in SC1-11 Are Ameliorated by IAA Supplementation
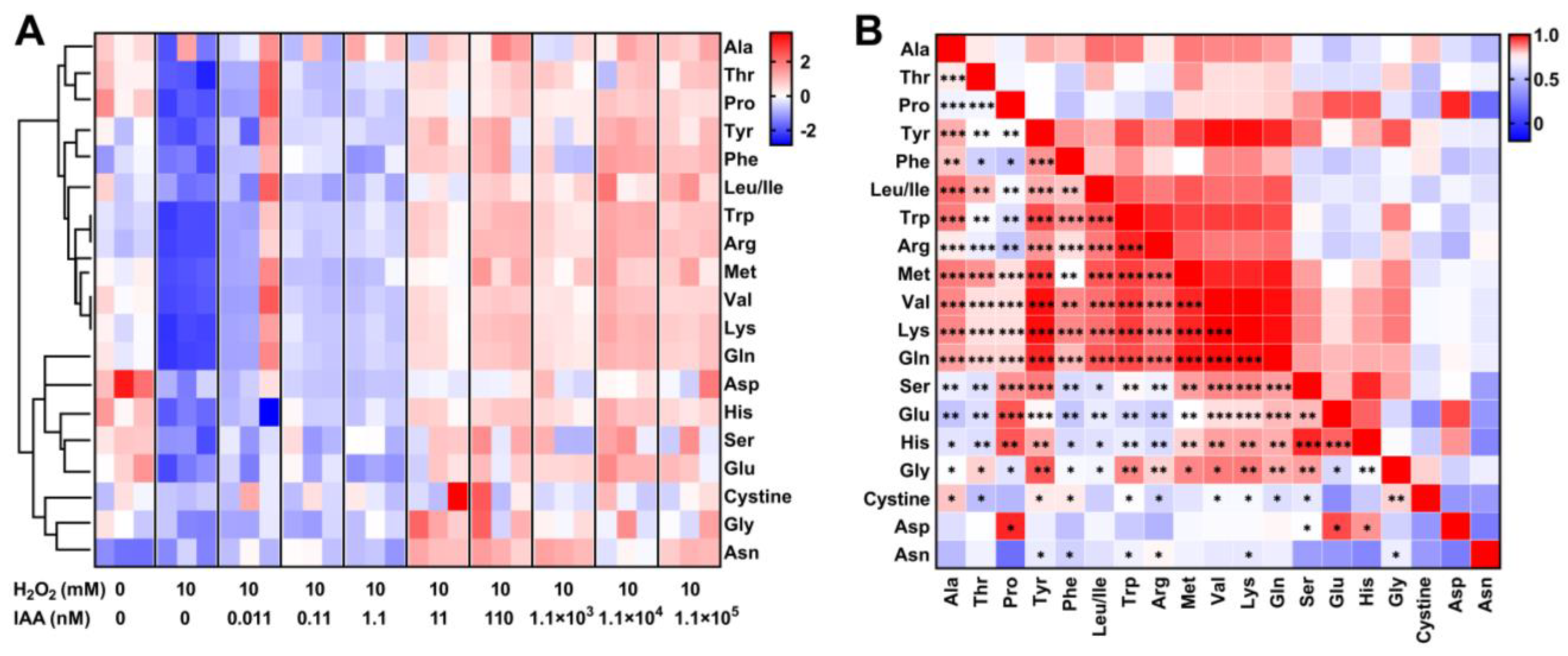
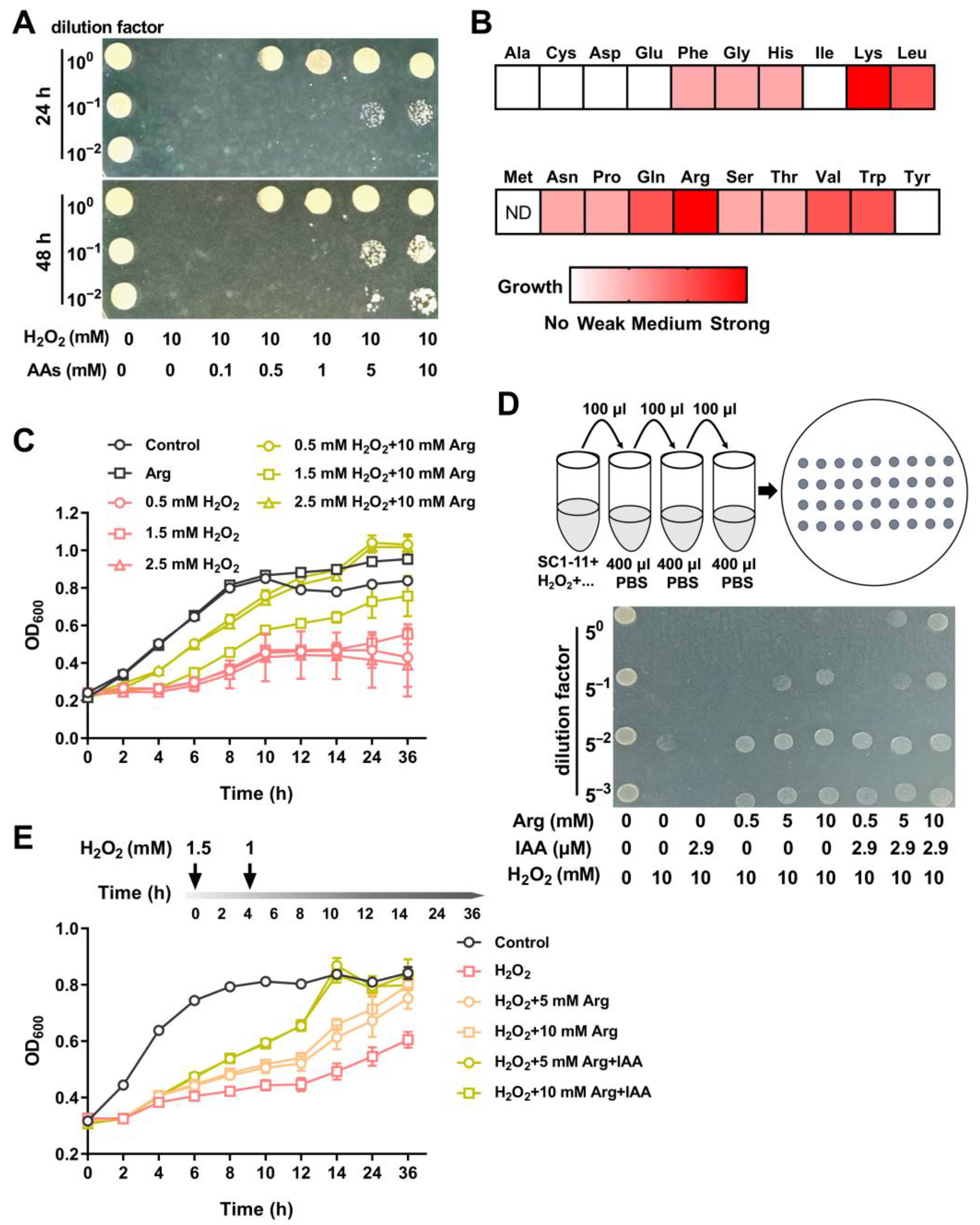
3.4. Amino Acids Supplementation Protects S. mediterraneus SC1-11 Against Oxidative Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.B.; Kazamia, E.; Helliwell, K.E.; Kudahl, U.J.; Sayer, A.; Wheeler, G.L.; Smith, A.G. Cross-exchange of b-vitamins underpins a mutualistic interaction between Ostreococcus tauri and Dinoroseobacter shibae. ISME J. 2019, 13, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin b12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; Van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Astafyeva, Y.; Gurschke, M.; Qi, M.; Bergmann, L.; Indenbirken, D.; De Grahl, I.; Katzowitsch, E.; Reumann, S.; Hanelt, D.; Alawi, M.; et al. Microalgae and bacteria interaction—Evidence for division of Diligence in the alga microbiota. Microbiol. Spectr. 2022, 10, e00633-22. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, W.; Wang, H. Physiological response and morphological changes of Heterosigma akashiwo to an algicidal compound prodigiosin. J. Hazard. Mater. 2020, 385, 121530. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, R.; Grabe, V.; Sommermann, T.; Werner, M.; Vallet, M.; Zerfaß, C.; Werz, O.; Pohnert, G. Bacteria modulate microalgal aging physiology through the induction of extracellular vesicle production to remove harmful metabolites. Nat. Microbiol. 2024, 9, 2356–2368. [Google Scholar] [CrossRef]
- Sukenik, A.; Eshkol, R.; Livne, A.; Hadas, O.; Rom, M.; Tchernov, D.; Vardi, A.; Kaplan, A. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (Cyanobacteria): A novel allelopathic mechanism. Limnol. Oceanogr. 2002, 47, 1656–1663. [Google Scholar] [CrossRef]
- Rijstenbil, J.W. Assessment of oxidative stress in the planktonic diatom Thalassiosira pseudonana in response to UVA and UVB radiation. J. Plankton Res. 2002, 24, 1277–1288. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Van Creveld, S.G.; Rosenwasser, S.; Schatz, D.; Koren, I.; Vardi, A. Early perturbation in mitochondria redox homeostasis in response to environmental stress predicts cell fate in diatoms. ISME J. 2015, 9, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Thamatrakoln, K.; Korenovska, O.; Niheu, A.K.; Bidle, K.D. Whole-genome expression analysis reveals a role for death-related genes in stress acclimation of the diatom Thalassiosira pseudonana. Environ. Microbiol. 2012, 14, 67–81. [Google Scholar] [CrossRef]
- Thukral, M.; Allen, A.E.; Petras, D. Progress and challenges in exploring aquatic microbial communities using non-targeted metabolomics. ISME J. 2023, 17, 2147–2159. [Google Scholar] [CrossRef]
- Bell, W.; Mitchell, R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Fasnacht, M.; Polacek, N. Oxidative stress in bacteria and the central dogma of molecular biology. Front. Mol. Biosci. 2021, 8, 671037. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Harper, C.P. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J. Exp. Bot. 2018, 69, 245–254. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Hisano, H.; Takeda-Kamiya, N.; Takebayashi, Y.; Ariizumi, T.; Gao, Y.; Ezura, H.; Sato, K.; Zhao, Y.; Hayashi, K.; et al. Agrobacterium tumefaciens enhances biosynthesis of two distinct auxins in the formation of crown galls. Plant Cell Physiol. 2019, 60, 29–37. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520.e4. [Google Scholar] [CrossRef]
- Žižková, E.; Kubeš, M.; Dobrev, P.I.; Přibyl, P.; Šimura, J.; Zahajská, L.; Záveská Drábková, L.; Novák, O.; Motyka, V. Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann. Bot. 2017, 119, 151–166. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Tong, M.; Wu, J.; Chan, L.L.; Cai, Z.; Zhou, J. Indole-3-acetic acid as a cross-talking molecule in algal-bacterial interactions and a potential driving force in algal bloom formation. Front. Microbiol. 2023, 14, 1236925. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic evidence for algal auxin production in Chlamydomonas and its role in algal-bacterial mutualism. iScience 2024, 27, 108762. [Google Scholar] [CrossRef] [PubMed]
- Khasin, M.; Cahoon, R.R.; Nickerson, K.W.; Riekhof, W.R. Molecular machinery of auxin synthesis, secretion, and perception in the unicellular chlorophyte alga Chlorella sorokiniana UTEX 1230. PLoS ONE 2018, 13, e0205227. [Google Scholar] [CrossRef]
- Matthews, J.L.; Khalil, A.; Siboni, N.; Bougoure, J.; Guagliardo, P.; Kuzhiumparambil, U.; DeMaere, M.; Le Reun, N.M.; Seymour, J.R.; Suggett, D.J.; et al. Coral endosymbiont growth is enhanced by metabolic interactions with bacteria. Nat. Commun. 2023, 14, 6864. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Bramucci, A.R.; Focardi, A.; Le Reun, N.; Willams, N.L.R.; Kuzhiumparambil, U.; Raina, J.-B.; Seymour, J.R. Widespread production of plant growth-promoting hormones among marine bacteria and their impacts on the growth of a marine diatom. Microbiome 2024, 12, 205. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Kempnich, M.W.; Appiano, M.; Mehic, S.; Yazzie, T. Specific bacterial microbiome enhances the sexual reproduction and auxospore production of the marine diatom, Odontella. PLoS ONE 2022, 17, e0276305. [Google Scholar] [CrossRef]
- Patidar, S.K. Metabolic interactions between microalgae and bacteria: Multifunctional ecological interplay and environmental applications. Algal Res. 2025, 86, 103904. [Google Scholar] [CrossRef]
- Segev, E.; Wyche, T.P.; Kim, K.H.; Petersen, J.; Ellebrandt, C.; Vlamakis, H.; Barteneva, N.; Paulson, J.N.; Chai, L.; Clardy, J.; et al. Dynamic metabolic exchange governs a marine algal-bacterial interaction. eLife 2016, 5, e17473. [Google Scholar] [CrossRef]
- Yang, Q.; Pande, G.S.J.; Wang, Z.; Lin, B.; Rubin, R.A.; Vora, G.J.; Defoirdt, T. Indole signalling and (micro)algal auxins decrease the virulence of Vibrio campbellii, a major pathogen of aquatic organisms. Environ. Microbiol. 2017, 19, 1987–2004. [Google Scholar] [CrossRef]
- Barak-Gavish, N.; Dassa, B.; Kuhlisch, C.; Nussbaum, I.; Brandis, A.; Rosenberg, G.; Avraham, R.; Vardi, A. Bacterial lifestyle switch in response to algal metabolites. Elife 2023, 12, e84400. [Google Scholar] [CrossRef] [PubMed]
- Hogle, S.L.; Brahamsha, B.; Barbeau, K.A. Direct heme uptake by phytoplankton-associated Roseobacter bacteria. mSystems 2017, 2, e00124-16. [Google Scholar] [CrossRef] [PubMed]
- Pukall, R.; Buntefuss, D.; Frühling, A.; Rohde, M.; Kroppenstedt, R.M.; Burghardt, J.; Lebaron, P.; Bernard, L.; Stackebrandt, E. Sulfitobacter mediterraneus sp. nov., a new sulfite-oxidizing member of the alpha-proteobacteria. Int. J. Syst. Bacteriol. 1999, 49 Pt 2, 513–519. [Google Scholar] [CrossRef]
- Qu, L.; Feng, X.; Chen, Y.; Li, L.; Wang, X.; Hu, Z.; Wang, H.; Luo, H. Metapopulation Structure of Diatom-Associated Marine Bacteria. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhao, J. Isolation, Characterization and Implications of the Bacterial Communities Associated with Established Cultures of Chattonella marina (Raphidophyceae) and Skeletonema costatum (Bacillariophyceae). Acta Oceanol. Sin. 2019, 38, 128–135. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Cheng, K.; Cai, Z.; Chen, G.; Zhou, J. Microbial community composition and metabolic potential during a succession of algal blooms from Skeletonema sp. to Phaeocystis sp. Front. Microbiol. 2023, 14, 1147187. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Pratush, A.; Ye, X.; Xie, J.; Wei, H.; Sun, C.; Hu, Z. Acclimation of culturable bacterial communities under the stresses of different organic compounds. Front. Microbiol. 2018, 9, 225. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Olin-Sandoval, V.; Yu, J.S.L.; Miller-Fleming, L.; Alam, M.T.; Kamrad, S.; Correia-Melo, C.; Haas, R.; Segal, J.; Peña Navarro, D.A.; Herrera-Dominguez, L.; et al. Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism. Nature 2019, 572, 249–253. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Diagnosing oxidative stress in bacteria: Not as easy as you might think. Curr. Opin. Microbiol. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Schmacht, M.; Lorenz, E.; Senz, M. Microbial production of glutathione. World J. Microbiol. Biotechnol. 2017, 33, 106. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Thominet, D.; Vanacker, H.; Miginiac-Maslow, M.; Gakière, B.; Noctor, G. H2O2-activated up-regulation of glutathione in Arabidopsis involves induction of genes encoding enzymes involved in cysteine synthesis in the chloroplast. Mol. Plant 2009, 2, 344–356. [Google Scholar] [CrossRef]
- Krüger, A.; Vowinckel, J.; Mülleder, M.; Grote, P.; Capuano, F.; Bluemlein, K.; Ralser, M. Tpo1-mediated spermine and spermidine export controls cell cycle delay and times antioxidant protein expression during the oxidative stress response. EMBO Rep. 2013, 14, 1113–1119. [Google Scholar] [CrossRef]
- Gutsche, K.A.; Tran, T.B.T.; Vogel, R.F. Production of volatile compounds by Lactobacillus sakei from branched chain α-keto acids. Food Microbiol. 2012, 29, 224–228. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Lylyk, M.P.; Vytvytska, O.M.; Lushchak, V.I. Assessment of antioxidant properties of alpha-keto acids in vitro and in vivo. Eur. Food Res. Technol. 2016, 242, 179–188. [Google Scholar] [CrossRef]
- Maqbool, A.; Horler, R.S.P.; Muller, A.; Wilkinson, A.J.; Wilson, K.S.; Thomas, G.H. The substrate-binding protein in bacterial ABC transporters: Dissecting roles in the evolution of substrate specificity. Biochem. Soc. Trans. 2015, 43, 1011–1017. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Wang, F.; Zhang, L.; Zhou, S.; Che, X.; Yu, D.; Liu, X.; Li, Z.; Sun, H.; et al. Structural and biochemical characterization of the key components of an auxin degradation operon from the rhizosphere bacterium Variovorax. PLoS Biol. 2023, 21, e3002189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Cai, R.; Cai, G.; Aweya, J.J.; Xie, J.; Chen, Z.; Wang, H. Indole Acetic Acid: A Key Metabolite That Protects Marine Sulfitobacter mediterraneus Against Oxidative Stress. Microorganisms 2025, 13, 1014. https://doi.org/10.3390/microorganisms13051014
Gan Y, Cai R, Cai G, Aweya JJ, Xie J, Chen Z, Wang H. Indole Acetic Acid: A Key Metabolite That Protects Marine Sulfitobacter mediterraneus Against Oxidative Stress. Microorganisms. 2025; 13(5):1014. https://doi.org/10.3390/microorganisms13051014
Chicago/Turabian StyleGan, Yongliang, Runlin Cai, Guanjing Cai, Jude Juventus Aweya, Jianmin Xie, Ziming Chen, and Hui Wang. 2025. "Indole Acetic Acid: A Key Metabolite That Protects Marine Sulfitobacter mediterraneus Against Oxidative Stress" Microorganisms 13, no. 5: 1014. https://doi.org/10.3390/microorganisms13051014
APA StyleGan, Y., Cai, R., Cai, G., Aweya, J. J., Xie, J., Chen, Z., & Wang, H. (2025). Indole Acetic Acid: A Key Metabolite That Protects Marine Sulfitobacter mediterraneus Against Oxidative Stress. Microorganisms, 13(5), 1014. https://doi.org/10.3390/microorganisms13051014






