Alteration of the Rhizosphere Microbiota and Growth Performance of Barley Infected with Fusarium graminearum and Screening of an Antagonistic Bacterial Strain (Bacillus amyloliquefaciens)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Materials
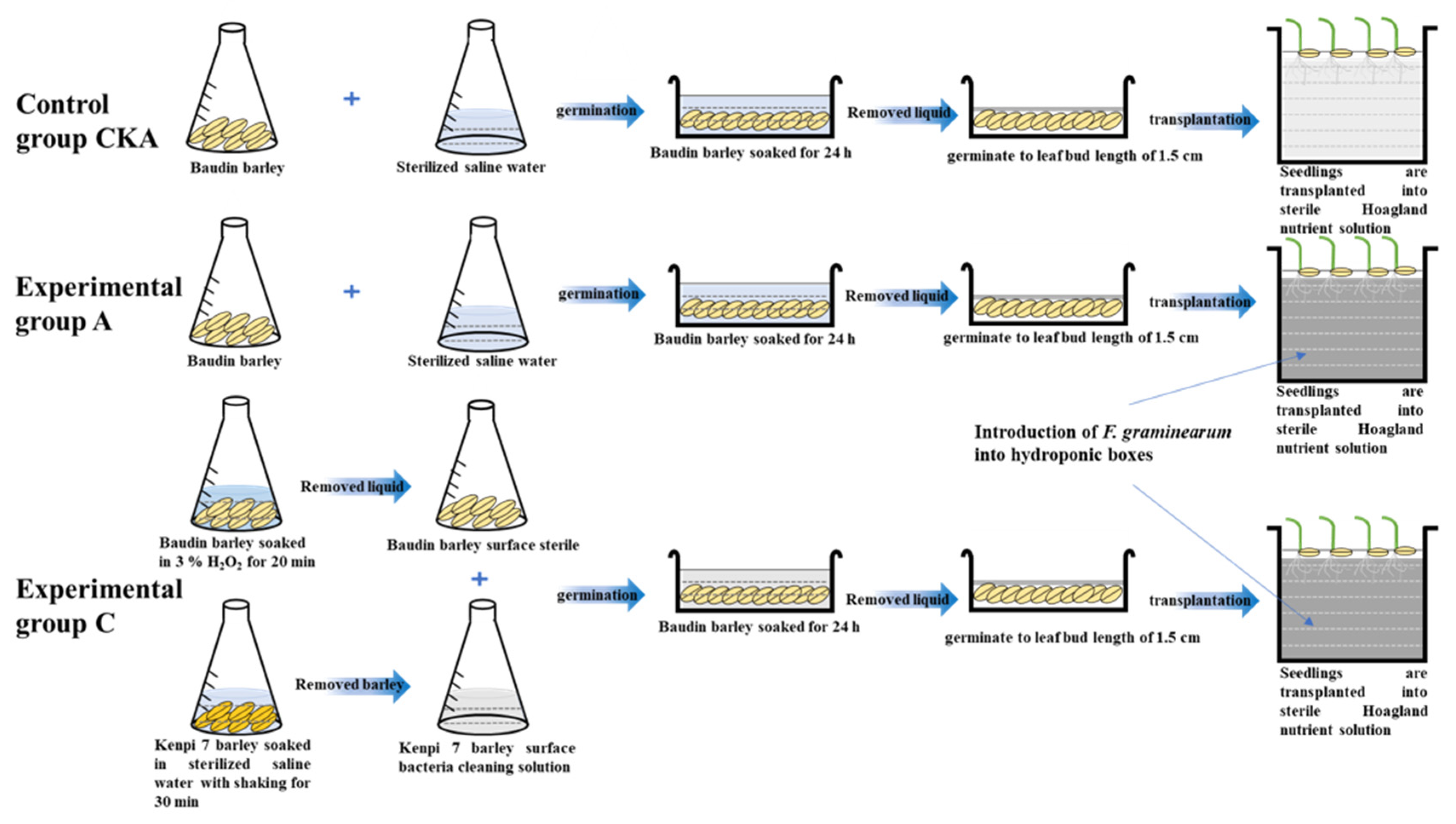
2.2. Preparation of Surface Microbiota Exchange
2.3. Barley Seedling Culture
2.4. Barley Rhizosphere Microbiota Sample Preparation
2.5. Total Community DNA Extraction and 16S rRNA Gene Amplicon Library Preparation
2.6. Effect of Exchange Rhizosphere Microbiota on the Growth of Barley Infected with Fusarium
2.7. Isolation of Fusarium Antagonistic Bacteria from the Surface of Barley Seeds
2.8. Screening of Fusarium Antagonistic Bacteria
2.9. Hydrolytic Enzyme Activity of Bacteria on Barley Surface
2.10. Identification of Effective Antibacterial Strains
2.11. Analysis of Antibacterial Components of Strain B1
2.12. In Vitro Inhibition of Strain B1 on F. graminearum
2.13. Statistical Analysis
3. Results
3.1. Effect of F. graminearum Infection on Barley Seedlings
3.2. Analysis of Bacterial Communities Diversity in Barley Rhizosphere
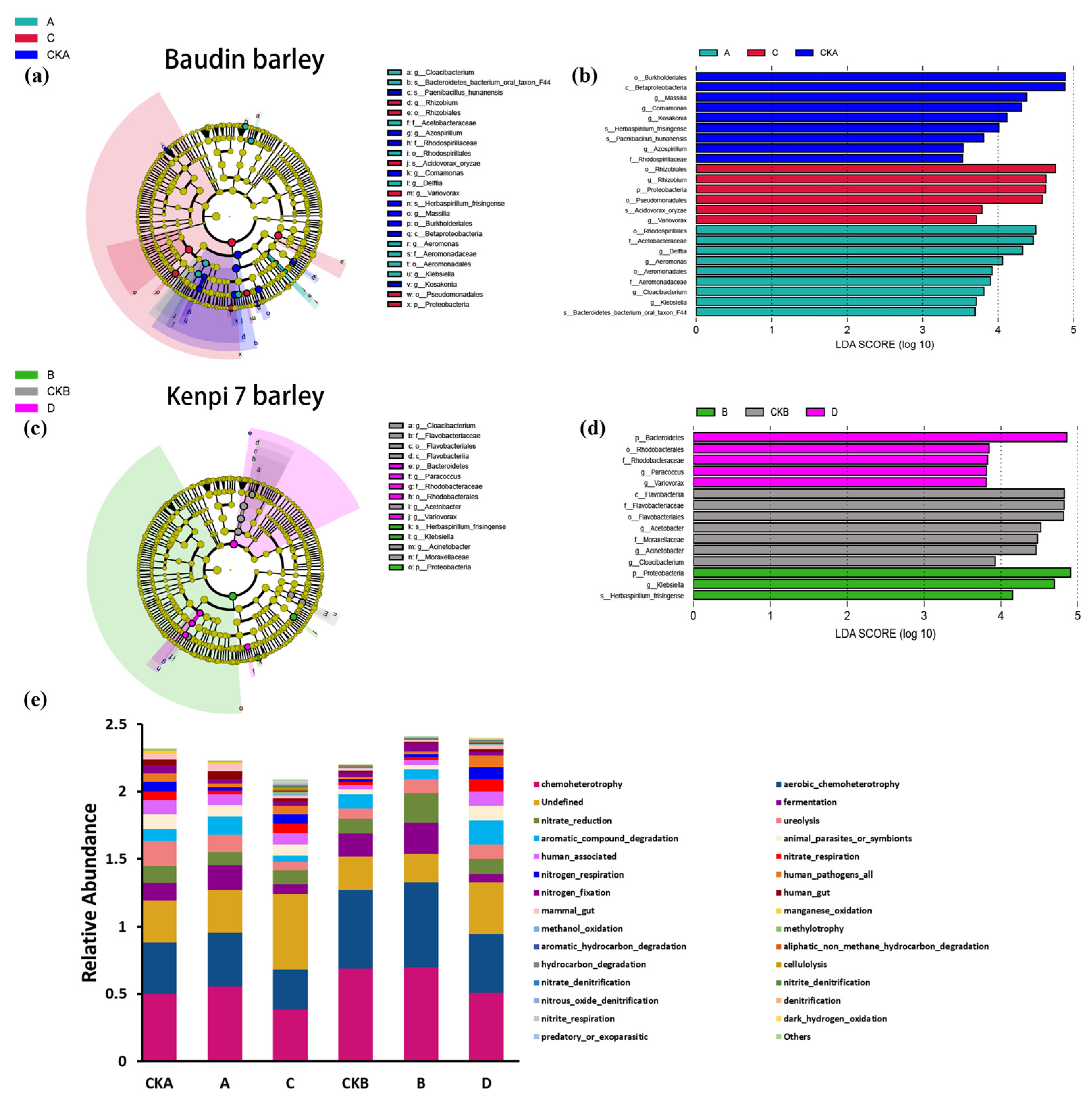
3.3. Bacterial Community Composition of Barley Rhizosphere
3.4. Analysis of Significantly Different Species and Potential Functions of Bacterial Communities in Barley Rhizosphere
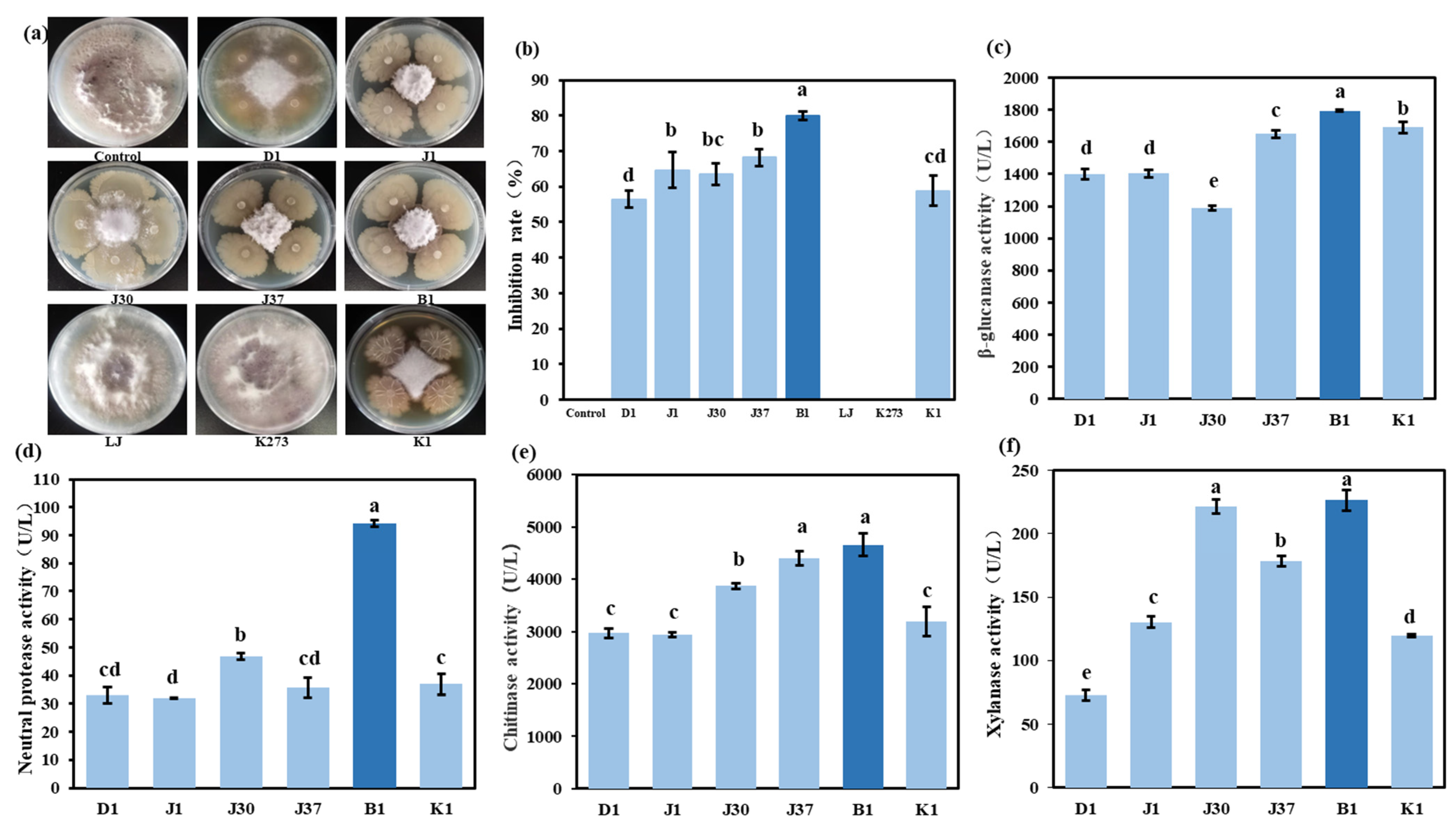
3.5. Isolation and Screening of Antagonistic Bacteria Against Fusarium
3.6. Identification of Highly Antagonistic Bacterial Strain B1
3.7. In Vitro Inhibitory Activity of Strain B1 Against F. graminearum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OUTs | Operational Taxonomic Units |
| PDA | Potato dextrose agar |
| LB | Luria–Bertani |
| TTC | Triphenyl tetrazolium chloride |
| PCoA | Principal coordinate analysis |
| CC | Cell content extract |
| SF | Sterile fermentation liquid |
| VOC | Volatile compound |
References
- Goswami, R.S.; Kistler, H.C. Pathogenicity and in planta mycotoxin accumulation among members of the Fusarium graminearum species complex on wheat and rice. Phytopathology 2005, 95, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Dawson, I.K.; Russell, J.; Powell, W.; Steffenson, B.; Thomas, W.T.B.; Waugh, R. Barley: A translational model for adaptation to climate change. New Phytol. 2015, 206, 913–931. [Google Scholar] [CrossRef]
- Dahl, B.; Wilson, W.W. Risk premiums due to Fusarium Head Blight (FHB) in wheat and barley. Agric. Syst. 2018, 162, 145–153. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogennomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Nichea, M.J.; Monge, M.D.P.; Zachetti, V.G.L.; Chiacchiera, S.M.; Ramirez, M.L. Effect of fungicides commonly used for Fusarium head blight management on growth and fumonisin production by Fusarium proliferatum. Rev. Argent. Microbiol. 2021, 53, 64–74. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Q.; Yuan, K.; Li, Y.; Shi, M.; Miao, J.; Liu, X. Monitoring and characterization of prochloraz resistance in Fusarium fujikuroi in China. Pestic. Biochem. Physiol. 2022, 187, 105189. [Google Scholar] [CrossRef]
- Mülner, P.; Schwarz, E.; Dietel, K.; Junge, H.; Herfort, S.; Weydmann, M.; Lasch, P.; Cernava, T.; Berg, G.; Vater, J. Profiling for bioactive peptides and volatiles of plant growth promoting strains of the Bacillus subtilis complex of industrial relevance. Front. Microbiol. 2020, 11, 1432. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Xing, S.H.; Chen, C.R.; Zhou, B.Q.; Zhang, H.; Nang, Z.M.; Xu, Z.H. Soil soluble organic nitrogen and active microbial characteristics under adjacent coniferous and broadleaf plantation forests. J. Soils Sediments 2010, 10, 748–757. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; El-Mageed, T.A.A.; Negm, S.H. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Berg, G.; Raaijmakers, J.M. Saving seed microbiomes. ISME J. 2018, 12, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Abdi, R.; Cao, W.; Zogheib, A.; Pukazhendhi, K.M.K.; Espinal-Ruiz, M.; Gammage, S.; Warriner, K.; Joye, I.J. Surface disinfection of wheat kernels using gas phase hydroxyl-radical processes: Effect on germination characteristics, microbial load, and functional properties. J. Food Sci. 2024, 89, 1154–1166. [Google Scholar] [CrossRef]
- Bziuk, N.; Maccario, L.; Sørensen, S.J.; Schikora, A.; Smalla, K. Barley Rhizosphere Microbiome Transplantation–A Strategy to Decrease Susceptibility of Barley Grown in Soils with Low Microbial Diversity to Powdery Mildew. Front. Microbiol. 2022, 13, 830905. [Google Scholar] [CrossRef]
- Luan, J.; Wei, X.; Li, Z.; Tang, W.; Yang, F.; Yu, Z.; Li, X. Inhibition of chitosan with different molecular weights on barley-borne Fusarium graminearum during barley malting process for improving malt quality. Foods 2022, 11, 3058. [Google Scholar] [CrossRef]
- Rani, H.; Bhardwaj, R.D. Quality attributes for barley malt: “The backbone of beer”. J. Food Sci. 2021, 86, 3322–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wei, M.; Sun, Q.; Shen, T.; Xie, M.; Liu, D. Nitrogen Fertilization Alleviates Microplastic Effects on Soil Protist Communities and Rape (Brassica napus L.) Growth. Microorganisms 2025, 13, 657. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F. Plant growth regulators modulate photosynthetic efficiency, antioxidant system, root cell viability and nutrient acquisition to promote growth, yield and quality of Indian mustard. Acta Physiol. Plantarum. 2022, 44, 132. [Google Scholar] [CrossRef]
- Mosimann, C.; Oberhänsli, T.; Ziegler, D.; Nassal, D.; Kandeler, E.; Boller, T.; Mäder, P.; Thonar, C. Tracing of two Pseudomonas strains in the root and rhizoplane of maize, as related to their plant growth-promoting effect in contrasting soils. Front. Microbiol. 2017, 7, 2150. [Google Scholar] [CrossRef]
- Sakdapetsiri, C.; Fukuta, Y.; Aramsirirujiwet, Y.; Shirasaka, N.; Kitpreechavanich, V. Antagonistic activity of endo-β-1, 3-glucanase from a novel isolate, Streptomyces sp. 9X166, against black rot in orchids. J. Basic Microbiol. 2016, 56, 469–479. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Bai, C.; Zhang, Y.; Wang, Z. A chitinase with antifungal activity from naked oat (Avena chinensis) seeds. J. Food Biochem. 2019, 43, e12713. [Google Scholar] [CrossRef]
- He, J.; Tang, F.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Yu, F. Design, expression and functional characterization of a thermostable xylanase from Trichoderma reesei. PLoS ONE 2019, 14, e0210548. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Lei, T.; Sohail, M.A.; Wang, J.; Wang, H. Synergistic effects of Bacillus amyloliquefaciens SDTB009 and difenoconazole on Fusarium wilt of tomato. Plant Dis. 2022, 106, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Alkan, N.; Elad, Y.; Sela, N.; Graber, E.R.; Frenkel, O. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 2020, 10, 13934. [Google Scholar] [CrossRef] [PubMed]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Agha, S.I.; Ullah, M.; Khan, A.; Jahan, N.; Ullah, S.M.; Tabassum, B.; Parveen, S.; Rehmat, Z.; Hussain, A.; Ahmed, S.; et al. Biocontrol rhizobacteria enhances growth and yield of wheat (Triticum aestivum) under field conditions against Fusarium oxysporum. Bioengineered 2023, 14, 2260923. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lima, D.; Spadari, C.d.C.; Barroso, V.d.M.; Carvalho, J.C.S.; de Almeida, L.C.; Alcalde, F.S.C.; Ferreira, M.J.P.; Sannomiya, M.; Ishida, K. Lipopeptides from an isolate of Bacillus subtilis complex have inhibitory and antibiofilm effects on Fusarium solani. Appl. Microbiol. Biotechnol. 2023, 107, 6103–6120. [Google Scholar] [CrossRef]
- Bhusal, B.; Mmbaga, M.T. Biological control of Phytophthora blight and growth promotion in sweet pepper by Bacillus species. Biol. Control 2020, 150, 104373. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Hou, S.; Thiergart, T.; Vannier, N.; Mesny, F.; Ziegler, J.; Pickel, B.; Hacquard, S. A microbiota–root–shoot circuit favours Arabidopsis growth over defence under suboptimal light. Nat. Plants 2021, 7, 1078–1092. [Google Scholar] [CrossRef]
- Zhou, S.-M.; Zhang, M.; Zhang, K.-K.; Yang, X.-W.; He, D.-X.; Yin, J.; Wang, C.-Y. Effects of reduced nitrogen and suitable soil moisture on wheat (Triticum aestivum L.) rhizosphere soil microbiological, biochemical properties and yield in the Huanghuai Plain, China. J. Integr. Agric. 2020, 19, 234–250. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Chanda, B.; Xia, Y.E.; Mandal, M.K.; Yu, K.; Sekine, K.-T.; Gao, Q.-M.; Selote, D.; Hu, Y.; Stromberg, A.; Navarre, D.; et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 2011, 43, 421–427. [Google Scholar] [CrossRef]
- Dusek, M.; Belakova, S.; Piacentini, K.C.; Jandovská, V. Fate and behavior of field-applied pesticides during malting and mashing processes. J. Agric. Food Chem. 2021, 69, 8649–8659. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Co-occurrence of nivalenol, deoxynivalenol and deoxynivalenol-3-glucoside in beer samples. Food Control 2018, 92, 319–324. [Google Scholar] [CrossRef]
- Hakme, E.; Lozano, A.; Ferrer, C.; Díaz-Galiano, F.J.; Fernández-Alba, A.R. Analysis of pesticide residues in olive oil and other vegetable oils. TrAC Trends Anal. Chem. 2018, 100, 167–179. [Google Scholar] [CrossRef]
- Hanif, A.; Zhang, F.; Li, P.P.; Li, C.C.; Xu, Y.J.; Zubair, M.; Zhang, M.X.; Jia, D.; Zhao, X.; Liang, J.; et al. Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins 2019, 11, 295. [Google Scholar] [CrossRef]
- Ren, L.; Yuan, Z.Q.; Xie, T.Y.; Wu, D.R.; Kang, Q.J.; Li, J.M.; Li, J. Extraction and characterization of cyclic lipopeptides with antifungal and antioxidant activities from Bacillus amyloliquefaciens. J. Appl. Microbiol. 2022, 133, 3573–3584. [Google Scholar] [CrossRef]
- Jiao, R.; Munir, S.; He, P.F.; Yang, H.W.; Wu, Y.X.; Wang, J.; He, P.; Cai, Y.; Wang, G.; He, Y. Biocontrol potential of the endophytic Bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biol. Control 2020, 143, 104160. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; He, K.; Song, X.; Yu, J.; Guo, Z.; Xu, M. Isolation and Identification of Bacillus subtilis LY-1 and Its Antifungal and Growth-Promoting Effects. Plants 2023, 12, 4158. [Google Scholar] [CrossRef]





| Varieties | Group | Root Length (cm) | Plant Height (cm) | Fresh Mass (g) | Dry Mass (g) | Root Activity (mg/g/h) |
|---|---|---|---|---|---|---|
| Baudin barley | CKA 1 | 4.73 ± 0.79 a 2 | 15.95 ± 1.86 a | 0.31 ± 0.05 a | 0.0338 ± 0.0023 a | 5.27 ± 0.14 a |
| A | 4.38 ± 0.70 a | 14.81 ± 1.19 b | 0.25 ± 0.03 b | 0.0325 ± 0.0013 a | 4.72 ± 0.07 b | |
| C | 2.96 ± 0.58 b | 12.43 ± 1.58 c | 0.20 ± 0.03 c | 0.0308 ± 0.0030 a | 3.77 ± 0.08 c | |
| Kenpi 7 barley | CKB | 4.52 ± 0.68 a | 15.39 ± 2.29 a | 0.28 ± 0.07 a | 0.0332 ± 0.0019 a | 4.88 ± 0.26 a |
| B | 3.70 ± 0.60 b | 13.40 ± 2.28 b | 0.23 ± 0.04 a | 0.0312 ± 0.0023 a | 4.18 ± 0.15 b | |
| D | 4.02 ± 0.44 b | 14.09 ± 1.48 b | 0.24 ± 0.04 a | 0.0322 ± 0.0028 a | 4.57 ± 0.16 ab |
| Test | Performance Characteristics | Test | Performance Characteristics |
|---|---|---|---|
| Gram stain | + 1 | Gelatin hydrolysis | − 2 |
| Spore forming | + | Starch hydrolysis | + |
| Indole production | + | Citrate | + |
| Voges–Proskauer | + | Methyl red | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Luan, J.; Shi, J.; Tang, W.; Li, X.; Yu, Z.; Yang, F. Alteration of the Rhizosphere Microbiota and Growth Performance of Barley Infected with Fusarium graminearum and Screening of an Antagonistic Bacterial Strain (Bacillus amyloliquefaciens). Microorganisms 2025, 13, 1010. https://doi.org/10.3390/microorganisms13051010
Fu Y, Luan J, Shi J, Tang W, Li X, Yu Z, Yang F. Alteration of the Rhizosphere Microbiota and Growth Performance of Barley Infected with Fusarium graminearum and Screening of an Antagonistic Bacterial Strain (Bacillus amyloliquefaciens). Microorganisms. 2025; 13(5):1010. https://doi.org/10.3390/microorganisms13051010
Chicago/Turabian StyleFu, Yang, Jing Luan, Jialei Shi, Wenzhu Tang, Xianzhen Li, Zhimin Yu, and Fan Yang. 2025. "Alteration of the Rhizosphere Microbiota and Growth Performance of Barley Infected with Fusarium graminearum and Screening of an Antagonistic Bacterial Strain (Bacillus amyloliquefaciens)" Microorganisms 13, no. 5: 1010. https://doi.org/10.3390/microorganisms13051010
APA StyleFu, Y., Luan, J., Shi, J., Tang, W., Li, X., Yu, Z., & Yang, F. (2025). Alteration of the Rhizosphere Microbiota and Growth Performance of Barley Infected with Fusarium graminearum and Screening of an Antagonistic Bacterial Strain (Bacillus amyloliquefaciens). Microorganisms, 13(5), 1010. https://doi.org/10.3390/microorganisms13051010






