Fecal Microbiota Transplantation Using Donor Stool Obtained from Exercised Mice Suppresses Colonic Tumor Development Induced by Azoxymethane in High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. AOM-Induced Colorectal Cancer Model
2.3. Study Design
2.4. Fecal Sample Collection and FMT
2.5. Colonic Mucosa Sample Collection
2.6. Metabolic Marker Profiles of Mice
2.7. RT Quantitative PCR (qPCR)
2.8. Gut Microbiome Analysis
2.9. Fecal Metabolome Analysis
2.10. Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
3.1. Mouse Body Weight Change by Diet and FMT
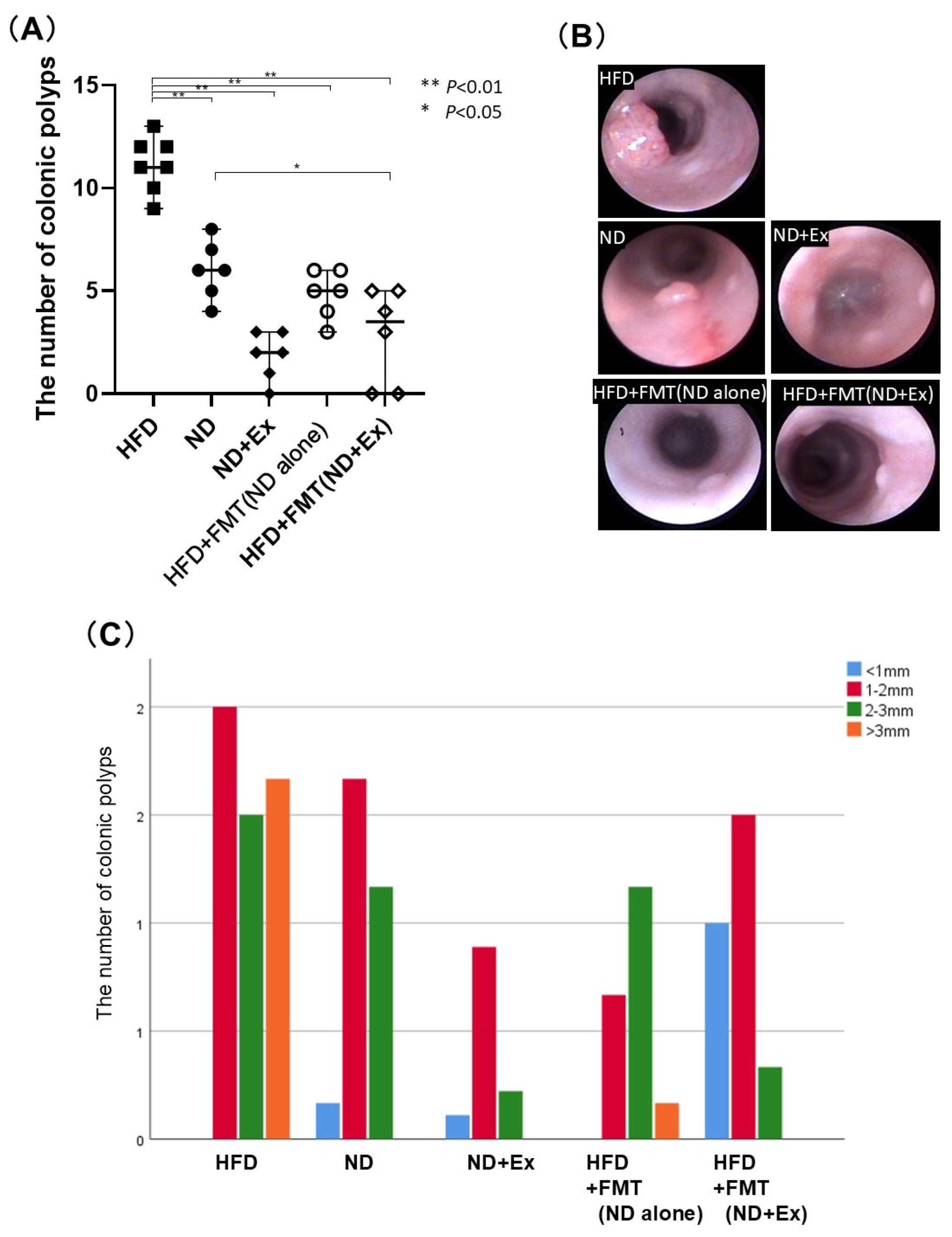
3.2. FMT’s Effect Against AOM-Induced Colorectal Tumor Count
3.3. Blood Glucose (BS) and Total Cholesterol Levels
3.4. SFA Analysis of Fecal Samples
3.5. Nonconjugated Bile Acid Analysis of Feces
3.6. Cytokine and Myokine Expressions in Colonic Tumors
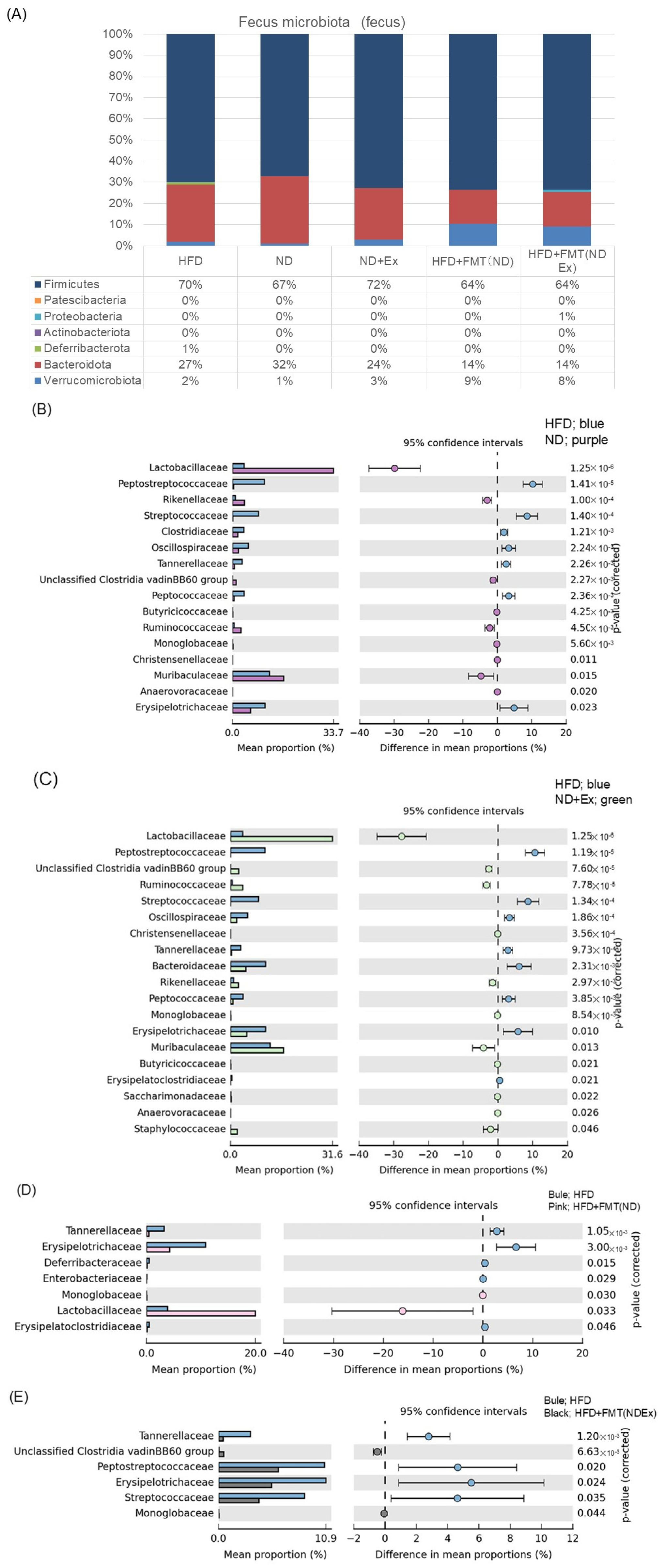
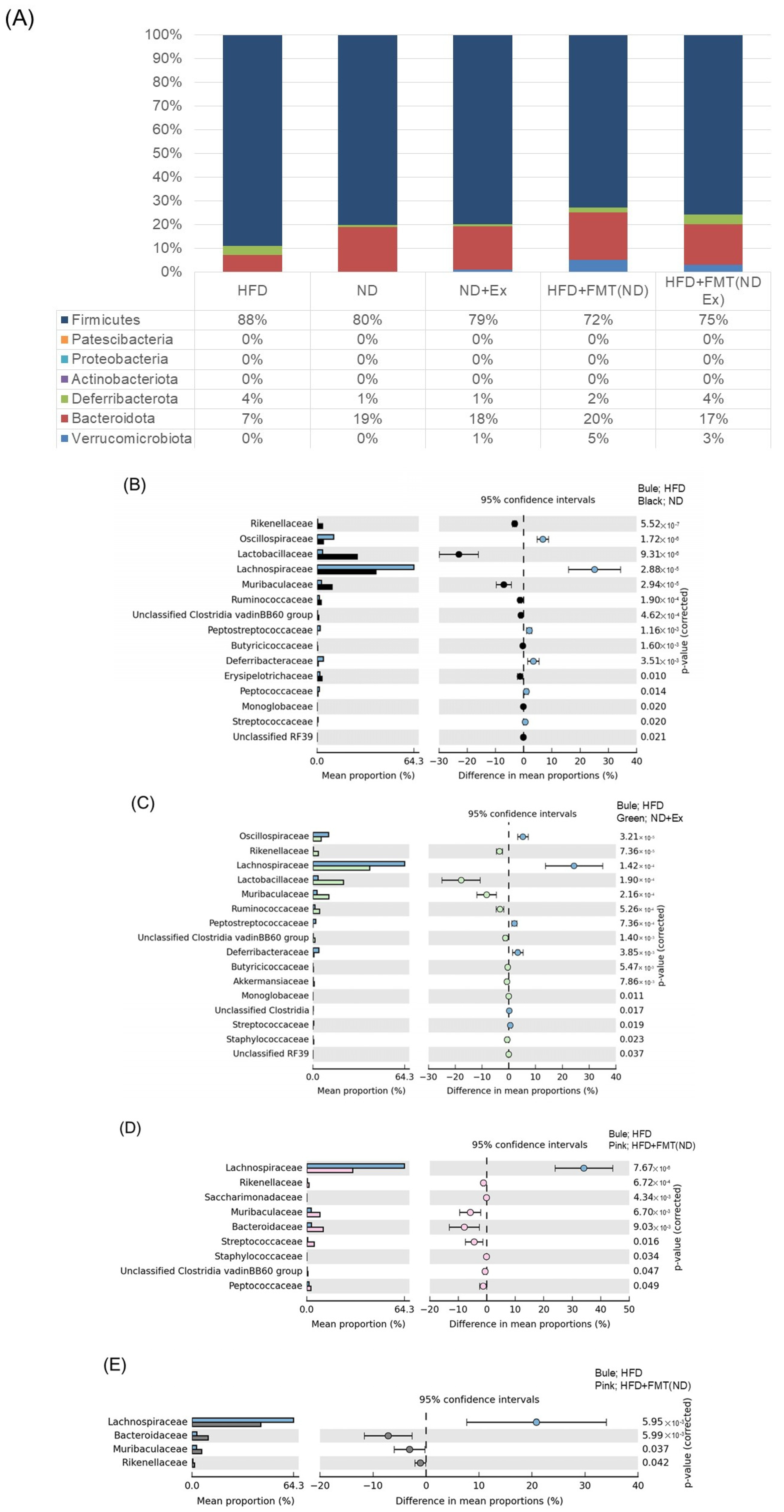
3.7. Changes in Fecal Microbiota
3.8. Changes in MAM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut Microbiota in Colorectal Cancer: Mechanisms of Action and Clinical Applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic Analysis of Faecal Microbiome as a Tool towards Targeted Non-Invasive Biomarkers for Colorectal Cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, G.; Li, X.; Zhou, H.; Sheng, J.; Wong, S.H.; Wu, W.K.K.; Ng, S.C.; Tsoi, H.; Dong, Y.; Zhang, N.; et al. Gut Mucosal Microbiome across Stages of Colorectal Carcinogenesis. Nat. Commun. 2015, 6, 8727. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut Microbial Metabolism Drives Transformation of Msh2-Deficient Colon Epithelial Cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef]
- Wong, C.C.; Yu, J. Gut Microbiota in Colorectal Cancer Development and Therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Clevers, H. Gut Microbiota in Colorectal Cancer: Associations, Mechanisms, and Clinical Approaches. Annu. Rev. Cancer Biol. 2022, 6, 65–84. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Van Citters, G.W.; Lin, H.C. Management of Small Intestinal Bacterial Overgrowth. Curr. Gastroenterol. Rep. 2005, 7, 317–320. [Google Scholar] [CrossRef]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal Microbiota Transplantation: Review and Update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef]
- Neufert, C.; Becker, C.; Neurath, M.F. An Inducible Mouse Model of Colon Carcinogenesis for the Analysis of Sporadic and Inflammation-Driven Tumor Progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.-F. High Fat Diet-Induced Obesity Increases the Formation of Colon Polyps Induced by Azoxymethane in Mice. Ann. Transl. Med. 2015, 3, 79. [Google Scholar] [PubMed]
- Morris, J.S.; Bradbury, K.E.; Cross, A.J.; Gunter, M.J.; Murphy, N. Physical Activity, Sedentary Behaviour and Colorectal Cancer Risk in the UK Biobank. Br. J. Cancer 2018, 118, 920–929. [Google Scholar] [CrossRef]
- Tanaka, T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflamm. 2012, 2012, 658786. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-L.; Tseng, C.-H.; Ho, H.J.; Cheung, C.K.Y.; Lin, J.-Y.; Chen, Y.-J.; Cheng, F.-C.; Hsu, Y.-C.; Lin, J.-T.; El-Omar, E.M.; et al. Fecal Microbiota Transplantation Confers Beneficial Metabolic Effects of Diet and Exercise on Diet-Induced Obese Mice. Sci. Rep. 2018, 8, 15625. [Google Scholar] [CrossRef]
- Basterfield, L.; Mathers, J.C. Intestinal Tumours, Colonic Butyrate and Sleep in Exercised Min Mice. Br. J. Nutr. 2010, 104, 355–363. [Google Scholar] [CrossRef]
- Bokoliya, S.C.; Dorsett, Y.; Panier, H.; Zhou, Y. Procedures for Fecal Microbiota Transplantation in Murine Microbiome Studies. Front. Cell. Infect. Microbiol. 2021, 11, 711055. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma Lucidum Reduces Obesity in Mice by Modulating the Composition of the Gut Microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Reikvam, D.H.; Erofeev, A.; Sandvik, A.; Grcic, V.; Jahnsen, F.L.; Gaustad, P.; McCoy, K.D.; Macpherson, A.J.; Meza-Zepeda, L.A.; Johansen, F.-E. Depletion of Murine Intestinal Microbiota: Effects on Gut Mucosa and Epithelial Gene Expression. PLoS ONE 2011, 6, e17996. [Google Scholar] [CrossRef]
- McCafferty, J.; Mühlbauer, M.; Gharaibeh, R.Z.; Arthur, J.C.; Perez-Chanona, E.; Sha, W.; Jobin, C.; Fodor, A.A. Stochastic Changes over Time and Not Founder Effects Drive Cage Effects in Microbial Community Assembly in a Mouse Model. ISME J. 2013, 7, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E., 4th; Taylor, C.M.; Welsh, D.A.; Berthoud, H.-R. Obese-Type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and Management of Complicated Intra-Abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; Li, J.; An, Y.; Wang, M.; Zhong, G. The Differences between Luminal Microbiota and Mucosal Microbiota in Mice. J. Microbiol. Biotechnol. 2020, 30, 287–295. [Google Scholar] [CrossRef]
- Vidal-Lletjós, S.; Andriamihaja, M.; Blais, A.; Grauso, M.; Lepage, P.; Davila, A.-M.; Viel, R.; Gaudichon, C.; Leclerc, M.; Blachier, F.; et al. Dietary Protein Intake Level Modulates Mucosal Healing and Mucosa-Adherent Microbiota in Mouse Model of Colitis. Nutrients 2019, 11, 514. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; van de Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic Methods of Gut Microbiota Modification in Colorectal Cancer Management—Fecal Microbiota Transplantation, Prebiotics, Probiotics, and Synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.-X.; Han, X.; Chen, B.-X.; Zhang, X.-H.; Gao, S.; Xu, D.-Q.; Wang, Y.; Gao, Z.-K.; Yu, L.; et al. Fecal Microbiota Transplantation Inhibits Colorectal Cancer Progression: Reversing Intestinal Microbial Dysbiosis to Enhance Anti-Cancer Immune Responses. Front. Microbiol. 2023, 14, 1126808. [Google Scholar] [CrossRef]
- Wang, L.; Yu, K.-C.; Hou, Y.-Q.; Guo, M.; Yao, F.; Chen, Z.-X. Gut Microbiome in Tumorigenesis and Therapy of Colorectal Cancer. J. Cell. Physiol. 2023, 238, 94–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, Y.; Cen, R.; Hou, X.; Yu, H.; Sun, J.; Zhou, L.; Ji, Q.; Zhao, L.; Wang, Y.; et al. San-Wu-Huang-Qin Decoction Attenuates Tumorigenesis and Mucosal Barrier Impairment in the AOM/DSS Model by Targeting Gut Microbiome. Phytomedicine 2022, 98, 153966. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; Liu, Z. Antitumor Effects of Fecal Microbiota Transplantation: Implications for Microbiome Modulation in Cancer Treatment. Front. Immunol. 2022, 13, 949490. [Google Scholar] [CrossRef]
- Robinson, T.O.; Schluns, K.S. The Potential and Promise of IL-15 in Immuno-Oncogenic Therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bahri, R.; Pateras, I.S.; D’Orlando, O.; Goyeneche-Patino, D.A.; Campbell, M.; Polansky, J.K.; Sandig, H.; Papaioannou, M.; Evangelou, K.; Foukas, P.G.; et al. IL-15 Suppresses Colitis-Associated Colon Carcinogenesis by Inducing Antitumor Immunity. Oncoimmunology 2015, 4, e1002721. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Caligiuri, M.A.; Yu, J. Harnessing IL-15 Signaling to Potentiate NK Cell-Mediated Cancer Immunotherapy. Trends Immunol. 2022, 43, 833–847. [Google Scholar] [CrossRef]
- Ong, C.Y.; Abdalkareem, E.A.; Khoo, B.Y. Functional Roles of Cytokines in Infectious Disease Associated Colorectal Carcinogenesis. Mol. Biol. Rep. 2022, 49, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, C.; Zhang, Y.; Lei, G.; Xu, K.; Zhao, N.; Lu, J.; Meng, F.; Yu, L.; Yan, J.; et al. Integrated Gut Virome and Bacteriome Dynamics in COVID-19 Patients. Gut Microbes 2021, 13, 1887722. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Ferriz-Jordán, M.; García-Hernández, J.; Heredia, A.; Andrés, A. Effect of Lactobacillaceae Probiotics on Colonic Microbiota and Metabolite Production in Cystic Fibrosis: A Comparative In Vitro Study. Nutrients 2023, 15, 3846. [Google Scholar] [CrossRef]
- Kumar, R.; Maynard, C.L.; Eipers, P.; Goldsmith, K.T.; Ptacek, T.; Grubbs, J.A.; Dixon, P.; Howard, D.; Crossman, D.K.; Crowley, M.R.; et al. Colonization Potential to Reconstitute a Microbe Community in Patients Detected Early after Fecal Microbe Transplant for Recurrent C. difficile. BMC Microbiol. 2016, 16, 5. [Google Scholar] [CrossRef]
- Batta, A.K.; Salen, G.; Holubec, H.; Brasitus, T.A.; Alberts, D.; Earnest, D.L. Enrichment of the More Hydrophilic Bile Acid Ursodeoxycholic Acid in the Fecal Water-Soluble Fraction after Feeding to Rats with Colon Polyps. Cancer Res. 1998, 58, 1684–1687. [Google Scholar] [PubMed]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome Remodelling Leads to Inhibition of Intestinal Farnesoid X Receptor Signalling and Decreased Obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef]
- Bustamante, J.-M.; Dawson, T.; Loeffler, C.; Marfori, Z.; Marchesi, J.R.; Mullish, B.H.; Thompson, C.C.; Crandall, K.A.; Rahnavard, A.; Allegretti, J.R.; et al. Impact of Fecal Microbiota Transplantation on Gut Bacterial Bile Acid Metabolism in Humans. Nutrients 2022, 14, 5200. [Google Scholar] [CrossRef] [PubMed]
- Markandey, M.; Bajaj, A.; Verma, M.; Virmani, S.; Singh, M.K.; Gaur, P.; Das, P.; Srikanth, C.V.; Makharia, G.; Kedia, S.; et al. Fecal Microbiota Transplantation Refurbishes the Crypt-Associated Microbiota in Ulcerative Colitis. iScience 2023, 26, 106738. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Benech, N.; Valles-Colomer, M.; Segata, N.; Gasbarrini, A.; Cammarota, G.; Sokol, H.; Ianiro, G. Key Determinants of Success in Fecal Microbiota Transplantation: From Microbiome to Clinic. Cell Host Microbe 2023, 31, 712–733. [Google Scholar] [CrossRef]
- Almeida, C.; Oliveira, R.; Baylina, P.; Fernandes, R.; Teixeira, F.G.; Barata, P. Current Trends and Challenges of Fecal Microbiota Transplantation—An Easy Method That Works for All? Biomedicines 2022, 10, 2742. [Google Scholar] [CrossRef]
- Yo, S.; Matsumoto, H.; Gu, T.; Sasahira, M.; Oosawa, M.; Handa, O.; Umegaki, E.; Shiotani, A. Exercise Affects Mucosa-Associated Microbiota and Colonic Tumor Formation Induced by Azoxymethane in High-Fat-Diet-Induced Obese Mice. Microorganisms 2024, 12, 957. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimaraes, R.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its related metabolic dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as Deep Modulators of Gut Microbiota: Between Good and Evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Amorim, N.; McGovern, E.; Raposo, A.; Khatiwada, S.; Shen, S.; Koentgen, S.; Hold, G.; Behary, J.; El-Omar, E.; Zekry, A. Refining a Protocol for Faecal Microbiota Engraftment in Animal Models After Successful Antibiotic-Induced Gut Decontamination. Front. Med. 2022, 9, 770017. [Google Scholar] [CrossRef]






| Target Gene | Sequence | Length | |
|---|---|---|---|
| IL-6 | FP | CGGCCTTCCCTACTTCACAAGTCCG | 66 |
| RP | CAGGTCTGTTGGGAGTGGTATCC | ||
| TNF-alpha | FP | CAACCATCAAGGACTCAAATGG | 74 |
| RP | CCTTTGCAGAACTCAGGAATGGACATTCG | ||
| IL-15 | FP | CATCCATCTCGTGCTACTTGTG | 112 |
| RP | GCCTCTGTTTTAGGGAGACCT | ||
| SPARC | FP | CCACACGTTTCTTTGAGACC | 95 |
| RP | GATGTCCTGCTCCTTGATGC | ||
| Oncostatin M | FP | GTGGCTGCTCCAACTCTTCC | 81 |
| RP | AGAGTGATTCTGTGTTCCCCGT | ||
| Irisin | FP | GAGCCCAATAACAACAAGG | 242 |
| RP | GAGGATAATAAGCCCGATG | ||
| Actb | FP | CACTGTCGAGTCGCGTCC | 102 |
| RP | CGCAGCGATATCGTCATCCA |
| Phylum | Class | Order | Family | p-Value | Effect Size |
|---|---|---|---|---|---|
| Firmicutes | Clostridia | Peptostreptococcales-Tissierellales | Peptostreptococcaceae | 3.22 × 10−11 | 0.784391462 |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | 3.94 × 10−9 | 0.71478921 |
| Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | 1.28 × 10−8 | 0.694533816 |
| Bacteroidota | Bacteroidia | Bacteroidales | Tannerellaceae | 6.54 × 10−7 | 0.615028199 |
| Bacteroidota | Bacteroidia | Bacteroidales | Rikenellaceae | 1.28 × 10−6 | 0.599399448 |
| Firmicutes | Clostridia | Oscillospirales | Ruminococcaceae | 1.58 × 10−6 | 0.594330797 |
| Firmicutes | Clostridia | Peptococcales | Peptococcaceae | 4.08 × 10−6 | 0.570917473 |
| Firmicutes | Clostridia | Christensenellales | Christensenellaceae | 1.67 × 10−5 | 0.533237021 |
| Phylum | Class | Order | Family | p-Value | Effect Size |
|---|---|---|---|---|---|
| Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | 1.63 × 10−10 | 0.753614 |
| Bacteroidota | Bacteroidia | Bacteroidales | Rikenellaceae | 6.18 × 10−9 | 0.697266 |
| Firmicutes | Clostridia | Oscillospirales | Oscillospiraceae | 5.30 × 10−8 | 0.657857 |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | 1.94 × 10−7 | 0.631545 |
| Firmicutes | Clostridia | Oscillospirales | Ruminococcaceae | 4.47 × 10−7 | 0.613466 |
| Firmicutes | Clostridia | Lachnospirales | Lachnospiraceae | 6.71 × 10−7 | 0.60433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, H.; Gu, T.; Yo, S.; Sasahira, M.; Monden, S.; Ninomiya, T.; Osawa, M.; Handa, O.; Umegaki, E.; Shiotani, A. Fecal Microbiota Transplantation Using Donor Stool Obtained from Exercised Mice Suppresses Colonic Tumor Development Induced by Azoxymethane in High-Fat Diet-Induced Obese Mice. Microorganisms 2025, 13, 1009. https://doi.org/10.3390/microorganisms13051009
Matsumoto H, Gu T, Yo S, Sasahira M, Monden S, Ninomiya T, Osawa M, Handa O, Umegaki E, Shiotani A. Fecal Microbiota Transplantation Using Donor Stool Obtained from Exercised Mice Suppresses Colonic Tumor Development Induced by Azoxymethane in High-Fat Diet-Induced Obese Mice. Microorganisms. 2025; 13(5):1009. https://doi.org/10.3390/microorganisms13051009
Chicago/Turabian StyleMatsumoto, Hiroshi, Tingting Gu, Shogen Yo, Momoyo Sasahira, Shuzo Monden, Takehiro Ninomiya, Motoyasu Osawa, Osamu Handa, Eiji Umegaki, and Akiko Shiotani. 2025. "Fecal Microbiota Transplantation Using Donor Stool Obtained from Exercised Mice Suppresses Colonic Tumor Development Induced by Azoxymethane in High-Fat Diet-Induced Obese Mice" Microorganisms 13, no. 5: 1009. https://doi.org/10.3390/microorganisms13051009
APA StyleMatsumoto, H., Gu, T., Yo, S., Sasahira, M., Monden, S., Ninomiya, T., Osawa, M., Handa, O., Umegaki, E., & Shiotani, A. (2025). Fecal Microbiota Transplantation Using Donor Stool Obtained from Exercised Mice Suppresses Colonic Tumor Development Induced by Azoxymethane in High-Fat Diet-Induced Obese Mice. Microorganisms, 13(5), 1009. https://doi.org/10.3390/microorganisms13051009






