Microbial Community Responses and Nitrogen Cycling in the Nitrogen-Polluted Urban Shi River Revealed by Metagenomics
Abstract
1. Introduction
2. Materials and Methods
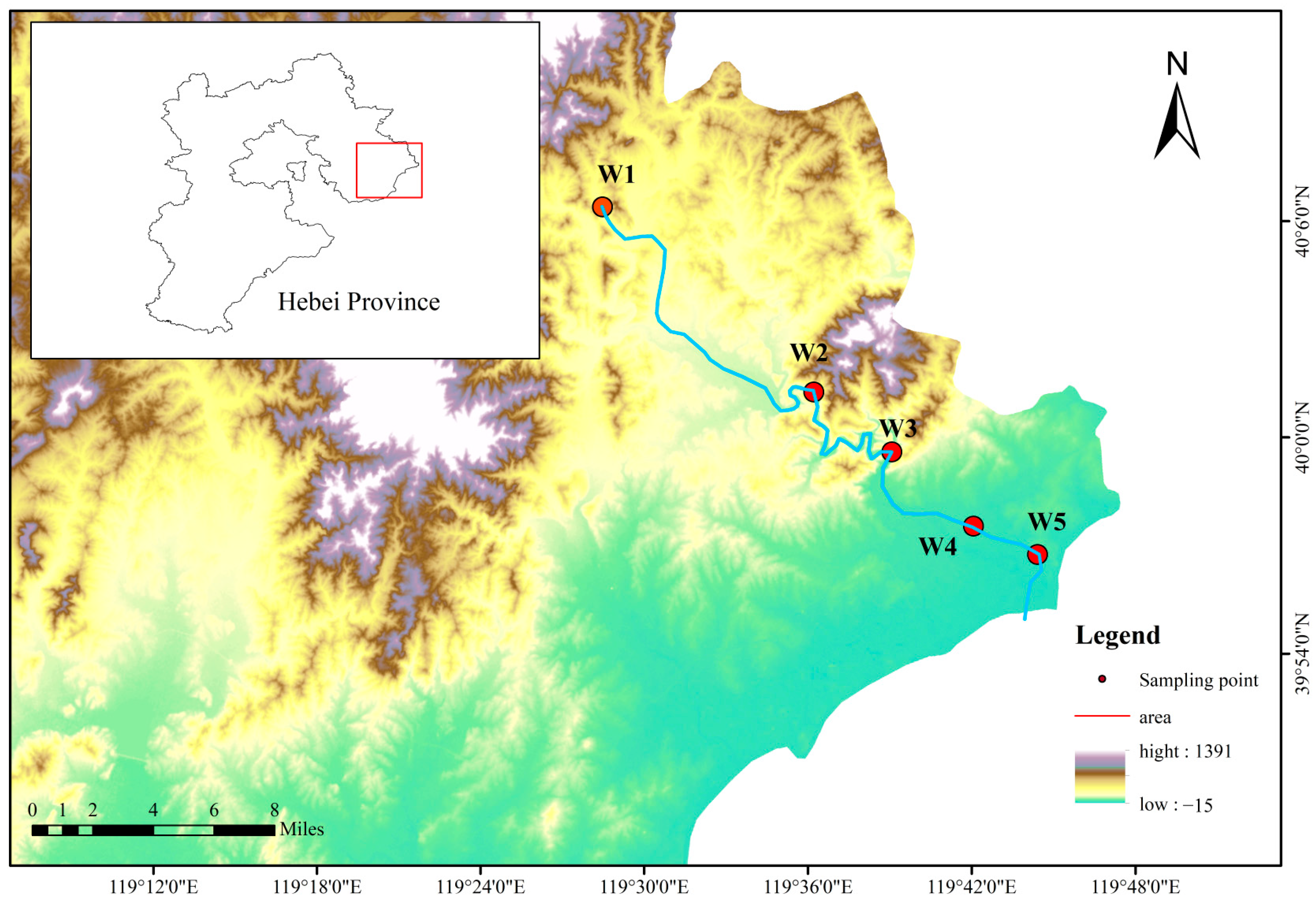
2.1. Study Area
2.2. Sample Collection and Analysis
2.2.1. Testing of Water Quality Physical and Chemical Indicators
2.2.2. DNA Extraction
2.2.3. Methods of Analysis
2.3. Biodiversity Analysis
2.4. Correlation Network Analysis
2.5. Analysis of Species Contribution to Function
3. Results
3.1. Shi River Water Quality Characteristics Analysis
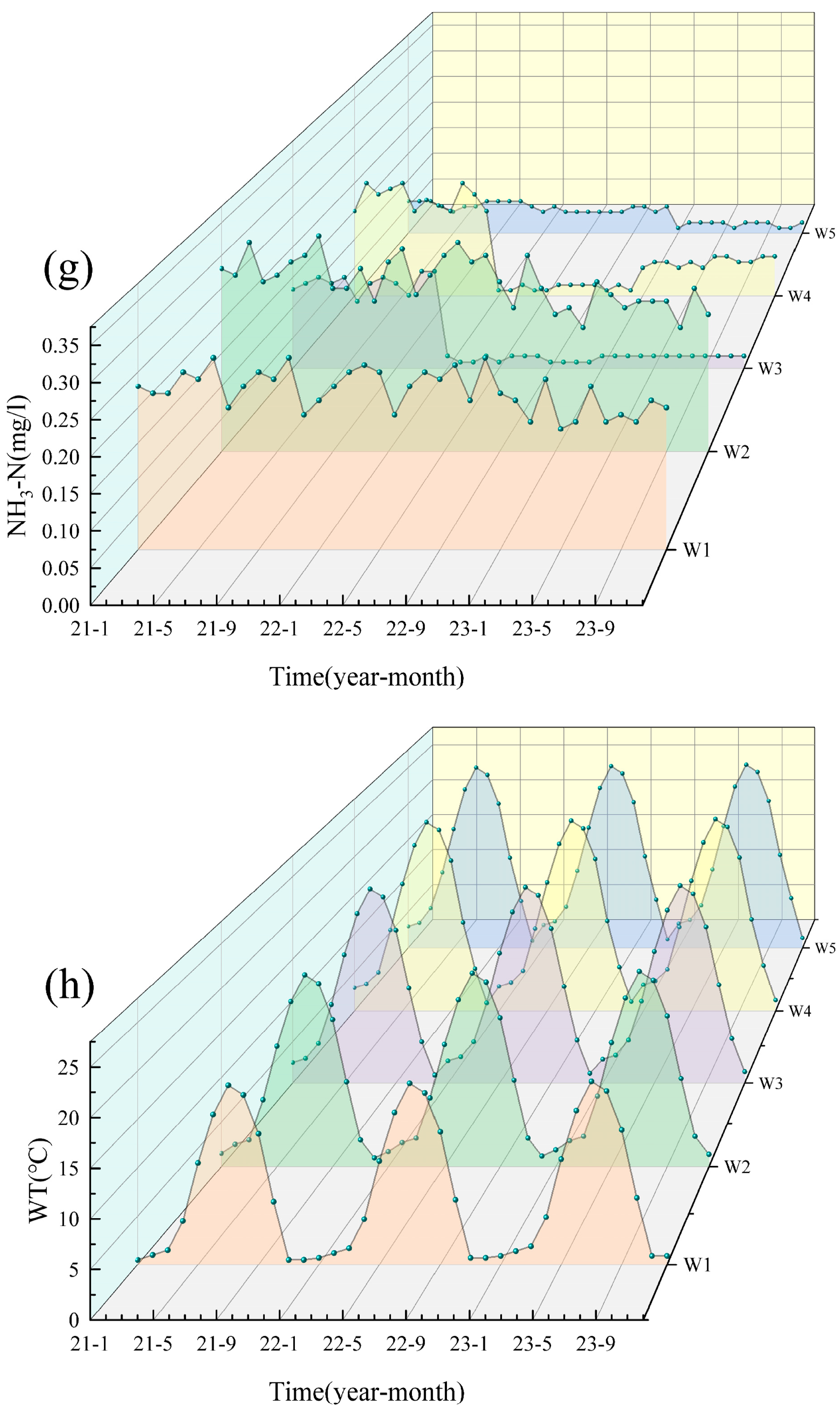
3.1.1. 2021–2023 Water Quality Analysis
3.1.2. Nitrogen Pollution Analysis
3.2. Shi River Biodiversity Analysis
3.2.1. Alpha Diversity Analysis
3.2.2. Bacterial Community Structure Analysis
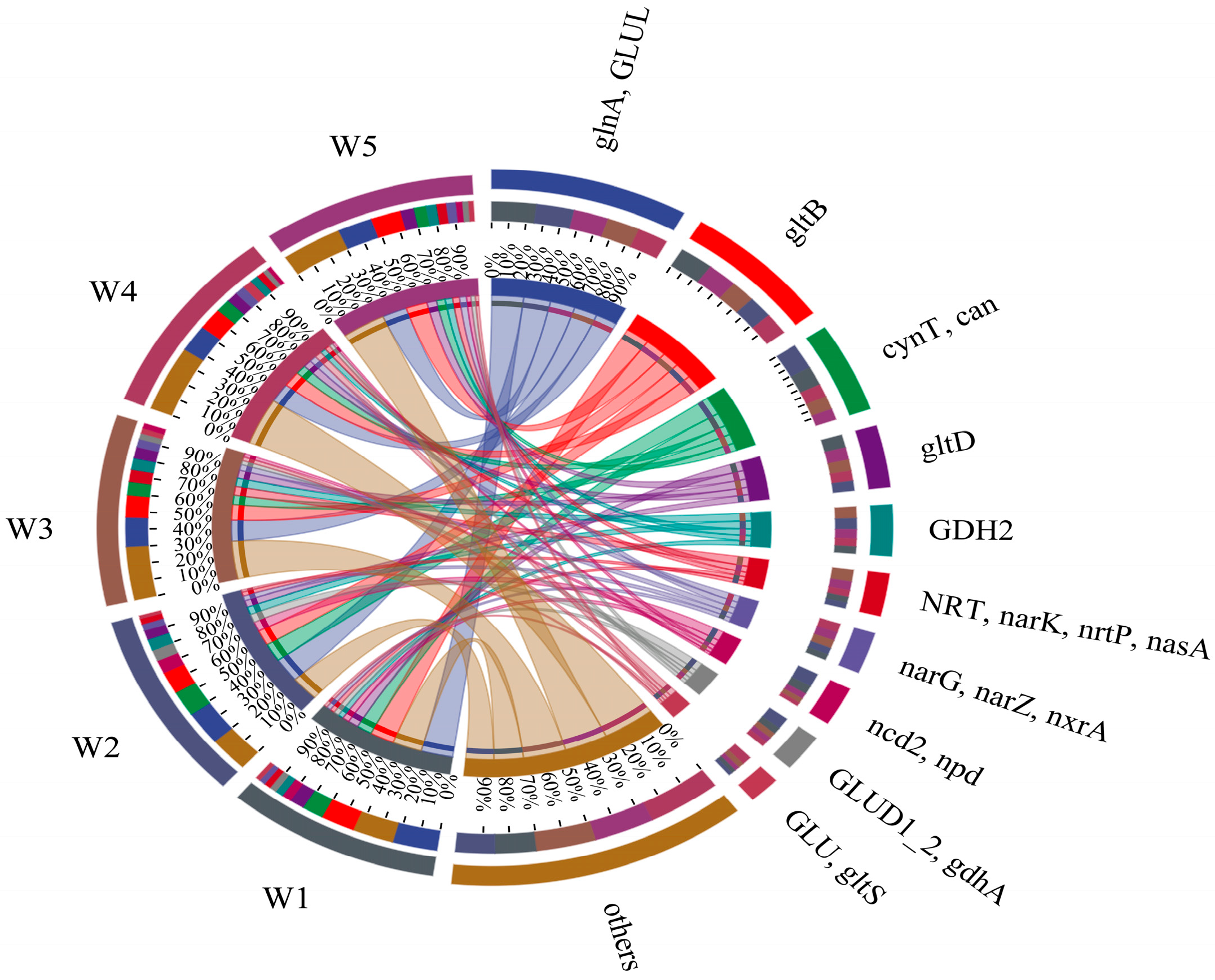
3.2.3. Bacteria Nitrogen Metabolism Functional Gene Analysis
3.3. Shi River Microbial Community Response to Nitrogen Pollution
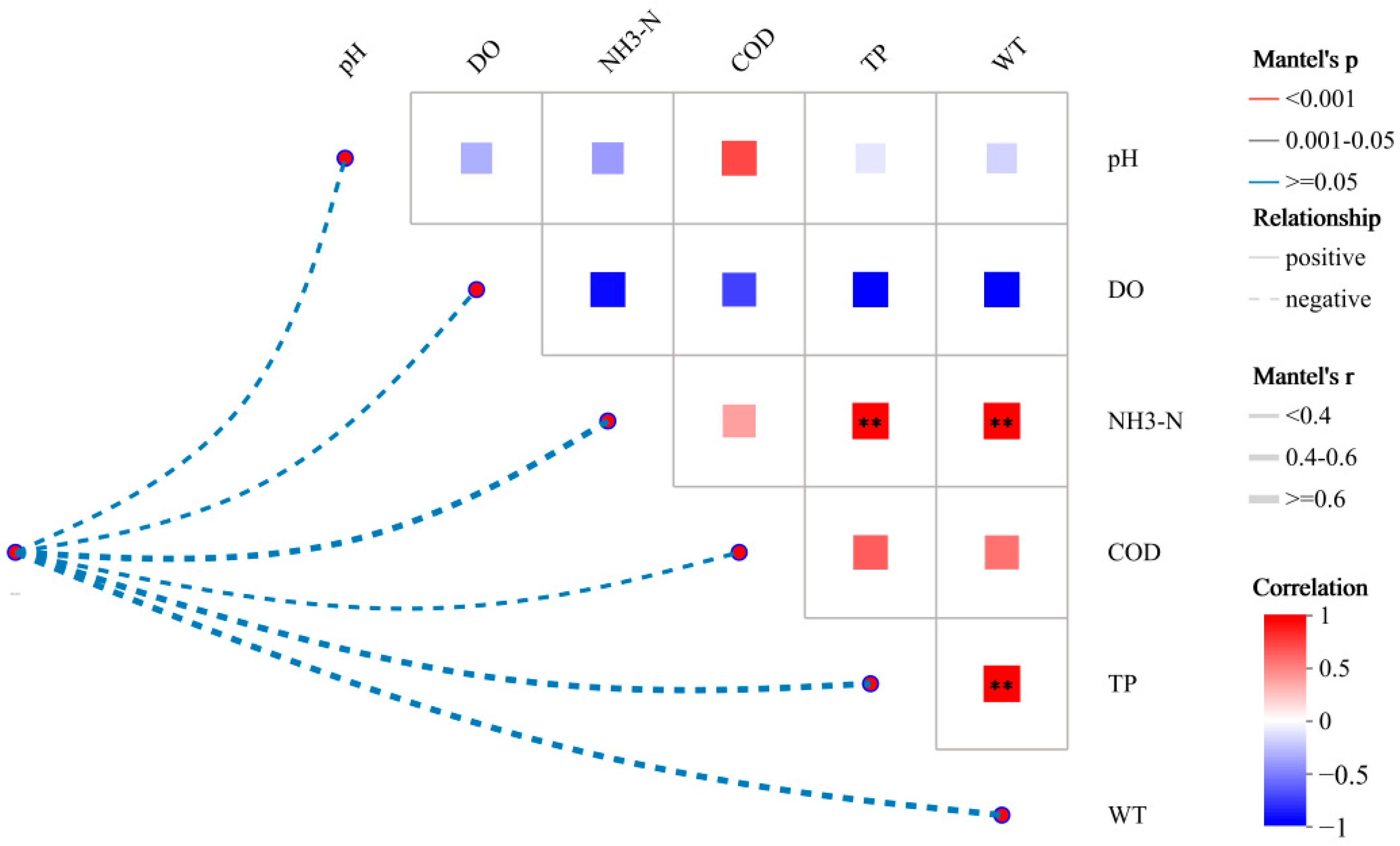
3.3.1. Effects of Water Quality Factors on Bacterial Communities
3.3.2. Nitrogen Metabolism Functional Genes
3.3.3. Bacterial Symbiotic Pattern Analysis
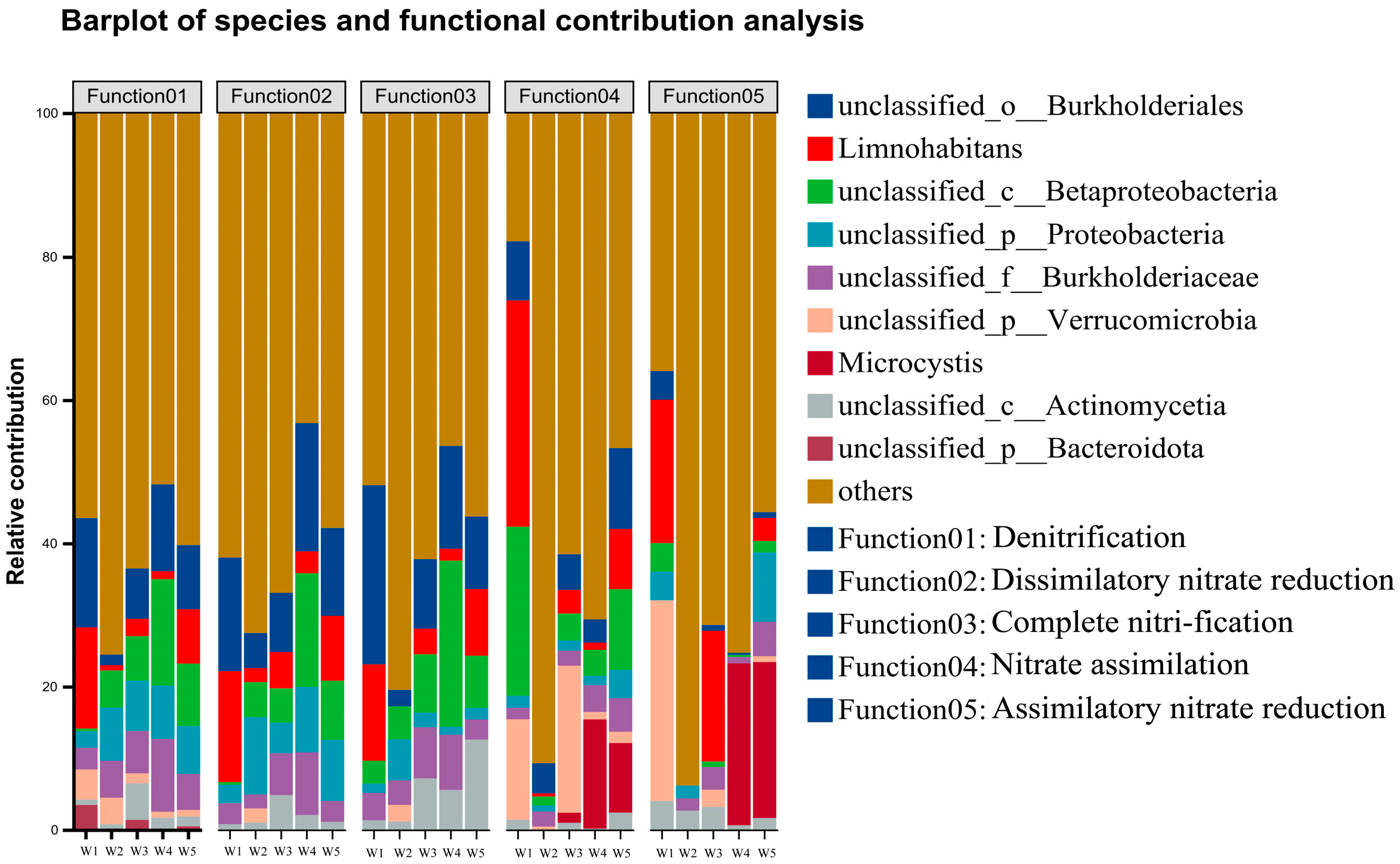
3.3.4. Functional Gene Analysis of Shi River Bacteria
4. Discussion
4.1. Nitrogen Pollution Drivers and Spatial Patterns
4.2. Spatial Patterns of Microbial Alpha Diversity
4.3. Microbial Community Structure and Adaptation
4.4. Functional Genes and Responses to Nitrogen Availability
4.5. Functional Genes and Environmental Drivers of Nitrogen Cycling
4.6. Microbial Interactions and Their Role in Nitrogen Metabolism
4.7. Future Research Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soni, H.; Yadav, R.K.; Patra, S.K. Global impact of urbanization on ecosystems: A comprehensive bibliometric analysis. Nat. Hazards Res. 2024, 5, 21–35. [Google Scholar] [CrossRef]
- Schulte-Uebbing, L.F.; Beusen, A.H.W.; Bouwman, A.F. From planetary to regional boundaries for agricultural nitrogen pollution. Nature 2022, 610, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Sanaullah, M.; Ullah, A. Nitrogen pollution originating from wastewater and agriculture: Advances in treatment and management. Rev. Environ. Contam. 2022, 260, 9. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.X.; Seabloom, E.W.; Sardans, J.; Peñuelas, J.; Zhang, H.Y.; Wei, C.Z.; Han, X.G. Multiple nutrient additions homogenize multidimensional plant stoichiometry in a meadow steppe. Glob. Change Biol. 2025, 31, e70123. [Google Scholar] [CrossRef]
- Li, G.; Fang, C.; Li, Y.; Wang, Z.; Sun, S.; He, S.; Qi, W.; Bao, C.; Ma, H.; Fan, Y.; et al. Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 1628. [Google Scholar] [CrossRef]
- Nguyen Tua, A.; Le Duy, C.; Nguyen Thi, N.; Schmalz, B.r.i.; Tran Le, L. Influences of key factors on river water quality in urban and rural areas: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Yongo, E.; Mutethya, E.; Zhang, P.; Lek, S.; Fu, Q.; Guo, Z. Comparing the performance of the water quality index and phytoplankton index of biotic integrity in assessing the ecological status of three urban rivers in Haikou City, China. Ecol. Indic. 2023, 157, 111286. [Google Scholar] [CrossRef]
- Yu, L.; Liu, S.; Jiang, L.; Wang, X.; Xiao, L. Insight into the Nitrogen Accumulation in Urban Center River from Functional Genes and Bacterial Community. PLoS ONE 2020, 15, e0238531. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Zhang, X.; Pan, C.; Zhang, S.; Zheng, B. Water Quality and Microbial Community in the Context of Ecological Restoration: A Case Study of the Yongding River, Beijing, China. Int. J. Environ. Res. Public Health 2022, 19, 13056. [Google Scholar] [CrossRef]
- Karimi, B.; Maron, P.A.; Chemidlin-Prevost Boure, N.; Bernard, L.; Dufour, A.; Dequiedt, S.; Ranjard, L. Microbial Diversity and Ecological Networks as Indicators of Environmental Quality. Environ. Chem. Lett. 2017, 15, 265–281. [Google Scholar] [CrossRef]
- Ahmad, N.B.; Jaafaru, M.S.; Isa, Z.; Abdulhamid, Y.; Kakudi, R.A.; Ugya, A.Y.; Meguellati, K. High pollution loads engineer oxygen dynamics, ecological niches, and pathogenicity shifts in freshwater environments. J. Hazard. Mater. Adv. 2024, 14, 100425. [Google Scholar] [CrossRef]
- HJ 494—2009; Water Quality—Technical Guidance on Water Quality Sampling. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2009.
- GB/T 6920-1986; Water Quality—Determination of pH Value—Glass Electrode Method. National Standardization Administration of China: Beijing, China, 1986.
- GB 11893-89; Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. National Standardization Administration of China: Beijing, China, 1989.
- HJ 636-2012; Water Quality—Determination of Total Nitrogen—Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2012.
- GB 7489-87; Water Quality—Determination of Dissolved Oxygen—Iodometric Method. National Standardization Administration of China: Beijing, China, 1987.
- HJ/T 84-2001; Water Quality—Determination of Nitrate Nitrogen—Ultraviolet Spectrophotometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2001.
- HJ 536-2009; Water Quality—Determination of Ammonia Nitrogen—Salicylic Acid Spectrophotometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2009.
- GB/T 32208-2015; Water Quality—Determination of Chemical Oxygen Demand—Potassium Dichromate Method. National Standardization Administration of China: Beijing, China, 2015.
- Dong, Y.; Wu, S.; Deng, Y.; Wang, S.; Fan, H.; Li, X.; Bai, Z.; Zhuang, X. Distinct functions and assembly mechanisms of soil abundant and rare bacterial taxa under increasing pyrene stresses. Front. Microbiol. 2021, 12, 689762. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Strokal, M.; Bai, Z.; Franssen, W.; Hofstra, N.; Koelmans, A.A.; Ludwig, F.; Ma, L.; van Puijenbroek, P.; Spanier, J.E.; Vermeulen, L.C.; et al. Urbanization: An increasing source of multiple pollutants to rivers in the 21st century. npj Urban Sustain. 2021, 1, 24. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chia, B.; Wang, D. Upstream-downstream water quality comparisons of restored channelized streams. Ecol. Eng. 2021, 170, 106367. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Sun, R.; Hu, S.; Qiao, Z.; Wang, S.; Mi, X. NH4+-N/NO3−-N ratio controlling nitrogen transformation accompanied with NO2−-N accumulation in the oxic-anoxic transition zone. Environ. Res. 2020, 189, 109962. [Google Scholar] [CrossRef]
- Chang, R.; Wang, Y.; Liu, H.; Wang, Z.; Ma, L.; Zhang, J.; Li, J.; Yan, Z.; Zhang, Y.; Li, D. Optimizing Non-Point Source Pollution Management: Evaluating Cost-Effective Strategies in a Small Watershed within the Three Gorges Reservoir Area, China. Land 2024, 13, 742. [Google Scholar] [CrossRef]
- Mulholland, P.; Helton, A.; Poole, G. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 2008, 452, 202–205. [Google Scholar] [CrossRef]
- Wu, H.; Xing, Z.; Zhan, G. Dissolved oxygen drives heterotrophic microorganism succession to regulate low carbon source wastewater treatment enhanced by slurry. J. Environ. Manag. 2024, 366, 121804. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hohn, M.H.; Hellerman, J. The taxonomy and structure of diatom populations from three Eastern North American rivers using three sampling methods. Trans. Am. Microsc. Soc. 1963, 82, 250–329. [Google Scholar] [CrossRef]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China—A review of the past decade. Fish Shellfish. Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Allen, A.; Badger, J. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, M.; Qu, C.; Li, J.; Hua, R.; Zhao, S.; Zhang, B.; Zhang, L. Metagenomic Insight into the Soil Microbial Functions Across Land Uses. J. Soils Sediments 2024, 24, 3684–3693. [Google Scholar] [CrossRef]
- Kermorvant, C.; Liquet, B.; Litt, G.; Mengersen, K.; Peterson, E.E.; Hyndman, R.J.; Jones, J.B., Jr.; Leigh, C. Understanding links between water-quality variables and nitrate concentration in freshwater streams using high frequency sensor data. PLoS ONE 2023, 18, e0287640. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. National Standardization Administration of China: Beijing, China, 2002.
- Wang, S.; Rao, P.; Yang, D.; Tang, L. A Combination Model for Quantifying Non-Point Source Pollution Based on Land Use Type in a Typical Urbanized Area. Water 2020, 12, 729. [Google Scholar] [CrossRef]
- Liu, J.; Yan, T.; Yang, Y. Source identification and impact of landscape pattern on riverine nitrogen pollution in a typical urbanized watershed, Beijing, China. Sci. Total Environ. 2018, 636, 1129–1140. [Google Scholar] [CrossRef]
- Cui, M.; Guo, Q.; Wei, R. Temporal-spatial dynamics of anthropogenic nitrogen inputs and hotspots in a large river basin. Sci. Total Environ. 2021, 776, 145741. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Q.; Zou, L.; Xia, J.; Qiao, Y.; Bai, F. Spatiotemporal variation characteristics and source identification of water pollution: Insights from urban water system. Ecol. Indic. 2022, 132, 108061. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; van Donk, E.; Hansson, L.A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Change Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Chen, Y.J.; Leung, P.M.; Cook, P.L.M.; Hutchins, R.H.S.; Orphan, V.J.; Tyson, G.W. Hydrodynamic disturbance controls microbial community assembly and biogeochemical processes in coastal sediments. ISME J. 2022, 16, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, M.; Zhu, Z.; Lu, L.; Ding, J.; Zhou, Q.; Gao, X.; Tang, N.; Li, S.; Li, X.; et al. The role of microorganisms in phosphorus cycling at river-lake confluences: Insights from a study on microbial community dynamics. Water Res. 2025, 268, 122556. [Google Scholar] [CrossRef]
- Li, P.; Chen, T.; An, M.; Zhang, Y.; Li, Y.; Li, Y.; Wang, J. Effects of Different Types of Human Disturbance on Total and Nitrogen-Transforming Bacteria in Haihe River. Life 2022, 12, 2081. [Google Scholar] [CrossRef]
- Merrick, M.J.; Edwards, R.A. Nitrogen control in bacteria. Microbiol. Rev. 1995, 59, 604–622. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Johnson, M.F.; Albertson, L.K.; Algar, A.C.; Dugdale, S.J.; Edwards, P.; England, J.; Wood, P.J. Rising water temperature in rivers: Ecological impacts and future resilience. WIREs Water 2024, 11, e1696. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Zhu, Y.; Dai, H.; Yuan, S. The competition between heterotrophic denitrification and DNRA pathways in hyporheic zone and its impact on the fate of nitrate. J. Hydrol. 2023, 626, 130175. [Google Scholar] [CrossRef]
- Smith, C.J.; Nedwell, D.B.; Dong, L.F.; Osborn, A.M. Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl. Environ. Microbiol. 2007, 73, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chandran, K.; Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Z.; Zhang, H.; Klausen, L.H.; Moonhee, R.; Kang, S. Emerging high-ammonia-nitrogen wastewater remediation by biological treatment and photocatalysis techniques. Sci. Total Environ. 2023, 875, 162603. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.B.L.; Thoma, R.; Callbeck, C.M.; Niederdorfer, R.; Schubert, C.J.; Müller, B.; Lever, M.A.; Bürgmann, H. Microbial nitrogen transformation potential in sediments of two contrasting lakes is spatially structured but seasonally stable. mSphere 2022, 7, e01013-21. [Google Scholar] [CrossRef]
- Deng, D.; Yang, Z.; Yang, Y.; Wan, W.; Liu, W.; Xiong, X. Metagenomic insights into nitrogen-cycling microbial communities and their relationships with nitrogen removal potential in the Yangtze River. Water Res. 2024, 265, 122229. [Google Scholar] [CrossRef]
- Liu, X.; Hu, S.; Sun, R.; Wu, Y.; Qiao, Z.; Wang, S.; Zhang, Z.; Cui, C. Dissolved oxygen disturbs nitrate transformation by modifying microbial community, co-occurrence networks, and functional genes during aerobic-anoxic transition. Sci. Total Environ. 2021, 790, 148245. [Google Scholar] [CrossRef]
- Gao, D.; Liu, C.; Li, X.; Zheng, Y.; Dong, H.; Liang, X.; Niu, Y.; Yin, G.; Liu, M.; Hou, L. High importance of coupled nitrification-denitrification for nitrogen removal in a large periodically low-oxygen estuary. Sci. Total Environ. 2022, 846, 157516. [Google Scholar] [CrossRef]
- Wei, H.; Wang, P.; Li, J.; Wang, Q.; Zhang, F.; Sun, D.; Gao, D.; Ding, Z.; Du, W.; Zhang, G.; et al. Nitrogen Mineralization/Immobilization Dynamics Across the River-Estuary-Sea Continuum: Effects of Organic Matter and Microorganisms. Mar. Pollut. Bull. 2024, 209, 117241. [Google Scholar] [CrossRef] [PubMed]
- Piña-Ochoa, E.; Álvarez-Cobelas, M. Denitrification in Aquatic Environments: A Cross-System Analysis. Biogeochemistry 2006, 81, 111–130. [Google Scholar] [CrossRef]
- Philippot, L.; Hallin, S. Finding the Missing Link Between Diversity and Activity Using Denitrifying Bacteria as a Model Functional Community. Curr. Opin. Microbiol. 2005, 8, 234–239. [Google Scholar] [CrossRef]
- Inomura, K.; Bragg, J.; Follows, M. A Quantitative Analysis of the Direct and Indirect Costs of Nitrogen Fixation: A Model Based on Azotobacter vinelandii. ISME J. 2017, 11, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, W.; Yuan, S.; Wang, K.; Wang, S.; Li, W.; Jiang, X.; Zhang, W.; Shan, B. Nitrogen Cycling and Functional Gene Diversity of Drinking Reservoir Area in Agricultural Districts: Implications for Nitrogen Transformation Processes. Ecol. Indic. 2025, 173, 113349. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Kang, S.; Ma, W.; Li, F.Y.; Zhang, Q.; Niu, J.; Ding, Y.; Han, F.; Sun, X. Functional Redundancy Instead of Species Redundancy Determines Community Stability in a Typical Steppe of Inner Mongolia. PLoS ONE 2015, 10, e0145605. [Google Scholar] [CrossRef]
- Huber, P.; Metz, S.; Unrein, F.; Mayora, G.; Sarmento, H.; Devercelli, M. Environmental Heterogeneity Determines the Ecological Processes that Govern Bacterial Metacommunity Assembly in a Floodplain River System. ISME J. 2020, 14, 2951–2966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Z.; Cai, K.; Wang, S.; Wright, A.L.; Jiang, X. Stability of Nitrogen-Cycling Microbial Communities and Impact on Microbial Nitrogen Function Under Different Land Use Practices. Appl. Soil Ecol. 2024, 204, 105729. [Google Scholar] [CrossRef]
- Smith, A.C.; Lacey, J.H. Tracing anthropogenic climate and environmental change using stable isotopes. Quat. Sci. Rev. 2024, 346, 109028. [Google Scholar] [CrossRef]
- Smith, N.C.; Matthews, J.M. Mechanisms of DNA-Binding Specificity and Functional Gene Regulation by Transcription Factors. Curr. Opin. Struct. Biol. 2016, 38, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, J.; Lian, J.; Song, Y.; Lu, C.; Li, H. Bio-Mixotrophic Perchlorate Reduction to Control Sulfate Production in a Step-Feed Sulfur-Based Reactor: A Study of Kinetics, ORP and Bacterial Community Structure. Chem. Eng. J. 2018, 269, 40–49. [Google Scholar] [CrossRef]
- Robinson, J.M.; Hodgson, R.; Krauss, S.L.; Liddicoat, C.; Malik, A.A.; Martin, B.C.; Mohr, J.J.; Moreno-Mateos, D.; Muñoz-Rojas, M.; Peddle, S.D.; et al. Opportunities and Challenges for Microbiomics in Ecosystem Restoration. Trends Ecol. Trends Ecol. Evol. 2023, 38, 1189–1202. [Google Scholar] [CrossRef]






| Sample | Ace | Chao | Shannon | Simpson | Shannon’s Evenness |
|---|---|---|---|---|---|
| W1 | 5776 | 5776 | 4.643351 | 0.041469 | 0.536093 |
| W2 | 5119 | 5119 | 5.032051 | 0.023646 | 0.589184 |
| W3 | 5702 | 5702 | 4.877654 | 0.0244 | 0.563984 |
| W4 | 5986 | 5986 | 5.170575 | 0.019274 | 0.594512 |
| W5 | 5881 | 5881 | 5.252409 | 0.014341 | 0.605152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, S.; Zhao, W. Microbial Community Responses and Nitrogen Cycling in the Nitrogen-Polluted Urban Shi River Revealed by Metagenomics. Microorganisms 2025, 13, 1007. https://doi.org/10.3390/microorganisms13051007
Wang R, Yang S, Zhao W. Microbial Community Responses and Nitrogen Cycling in the Nitrogen-Polluted Urban Shi River Revealed by Metagenomics. Microorganisms. 2025; 13(5):1007. https://doi.org/10.3390/microorganisms13051007
Chicago/Turabian StyleWang, Ran, Shang Yang, and Wei Zhao. 2025. "Microbial Community Responses and Nitrogen Cycling in the Nitrogen-Polluted Urban Shi River Revealed by Metagenomics" Microorganisms 13, no. 5: 1007. https://doi.org/10.3390/microorganisms13051007
APA StyleWang, R., Yang, S., & Zhao, W. (2025). Microbial Community Responses and Nitrogen Cycling in the Nitrogen-Polluted Urban Shi River Revealed by Metagenomics. Microorganisms, 13(5), 1007. https://doi.org/10.3390/microorganisms13051007






