Abstract
Cervical cancer ranks third in mortality and fourth in incidence among women worldwide as one of the leading causes of death from cancer in females. The main reason behind cervical carcinogenesis is long-term infection with high-risk human papillomavirus (HPV) genotypes, particularly HPV16 and HPV18. This review investigates HPV distribution across the world, along with cervical cancer molecular development mechanisms and current treatment strategies. Epidemiological data show that disease patterns vary significantly between different geographic regions because underdeveloped nations bear a higher disease burden. The molecular mechanisms of oncogenes E6 and E7 disrupt tumor suppressor pathways, while epigenetic modifications through DNA methylation and miRNA dysregulation promote malignant cell transformation. The reduction in HPV infection through prophylactic vaccination has shown promise, yet barriers related to accessibility and coverage still exist. The therapeutic technologies of gene expression inhibitors together with immunotherapies and epigenetic targeting agents show promise but require optimization to achieve specific targeting while minimizing off-target effects. A combined approach that integrates HPV vaccination with early diagnosis and molecular-specific therapies represents the most effective method to manage cervical cancer impact. The future care of patients will require increased translational research along with better immunization programs to drive prevention and therapeutic outcomes.
1. Introduction
1.1. Prevalence and the Impact of HPV on Cervical Cancer
Human papillomavirus (HPV) remains a critical global health concern due to its well-established etiological role in CC, which ranks as the fourth most frequently diagnosed cancer and the third leading cause of cancer-related mortality among women worldwide. According to GLOBOCAN 2022 data, an estimated 662,000 new cases of CC and approximately 350,000 deaths occurred globally that year. The burden of CC is disproportionately high in low- and middle-income countries (LMICs), which account for nearly 88% of new cases and 90% of CC-related deaths, primarily due to inadequate access to HPV vaccination, screening programs, and timely medical interventions. Persistent infection with high-risk HPV genotypes, particularly HPV 16 and 18, is responsible for nearly 70% of all CC cases worldwide. Given the substantial global burden of HPV-associated malignancies, a deeper understanding of its molecular pathogenesis, immune evasion strategies, and epigenetic alterations are essential for advancing prevention, early detection, and management strategies [1,2].
The epidemiology of human papillomavirus (HPV) infection demonstrates significant geographical variability, with notable differences between developed and emerging regions. In Western Europe, the prevalence of HPV infection is estimated to be 3.7%, with most European nations exhibiting low prevalence rates (below 30%), albeit there being certain exceptions. In contrast, HPV prevalence is markedly higher in emerging regions, reaching 42.2% compared to 22.6% in developed regions. Furthermore, independent of socioeconomic development, certain areas exhibit persistently elevated HPV prevalence, such as Eastern Europe (21.4%). Conversely, North Africa (9.2%) and Western Asia (2.2%) demonstrate lower infection rates [3,4,5,6]. In sub-Saharan Africa, HPV prevalence is estimated to be 24.4% [7], while in Ethiopia, approximately 17.3% of the population is infected with HPV, with high-risk strains comprising 15.8%. Notably, 54% of these CC cases occur in Asia, underscoring the substantial public health challenge posed by CC in the region [8,9,10,11].
Persistent high-risk HPV infection is responsible for nearly all cases of CC, with approximately 95% of cervical malignancies occurring in women who do not receive treatment for chronic HPV infection. Among immunocompromised individuals, particularly those with untreated HIV, disease progression may be significantly accelerated, reducing the transition time from persistent HPV infection to invasive cancer from the usual 15–20 years to as little as 5–10 years.
The global burden of high-risk HPV infection is subject to substantial epidemiological variation, influenced by factors such as geography, socioeconomic status, cultural practices, HPV genotype distribution, age, and host immune competence. Overall, high-risk HPV accounts for approximately 11.7% of infections worldwide, with prevalence exceeding 35.4% in some developing regions [12,13]. A more comprehensive understanding of the molecular interactions between HPV and host immune mechanisms is critical for elucidating the oncogenic potential of HPV and improving strategies for CC prevention and management.
A study encompassing diverse regions such as Europe, the Americas, and Africa demonstrated that women aged 45 years and older exhibit the second highest prevalence of HPV infection, with 32% of this demographic being infected with high-risk HPV types, namely HPV16 or HPV18, or a combination of both [14].
Worldwide, approximately 291 million women are estimated to harbor HPV DNA. A wealth of evidence supports the association between HPV and various risk factors, including multiple sexual partners [15], high parity [16], early pregnancy [17], concurrent sexually transmitted infections [18], the use of hormonal contraception [19], and tobacco smoking [20,21].
Globally, HPV is responsible for approximately 31% of all cancer cases, and it contributes to 13% of all infection-related cancers [22]. HPV types 16, 18, 26, 31, 33, 35, 39, 51, 52, 58, 59, 66, 68, 73, and 82 are considered carcinogenic to humans (Figure 1) [23]. In India, the substantial burden of cancer is attributed to HPV, with approximately 24% of all cancer cases in 2020 being linked to HPV infection, accounting for 7% of the global cancer burden [24].
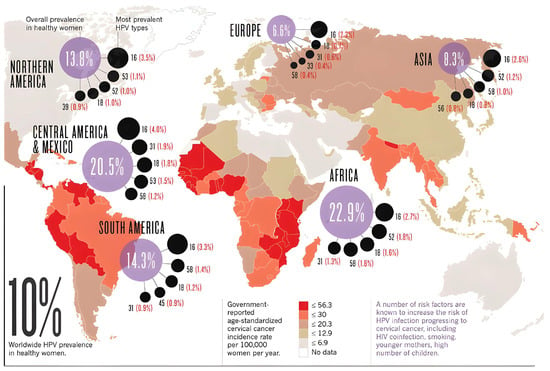
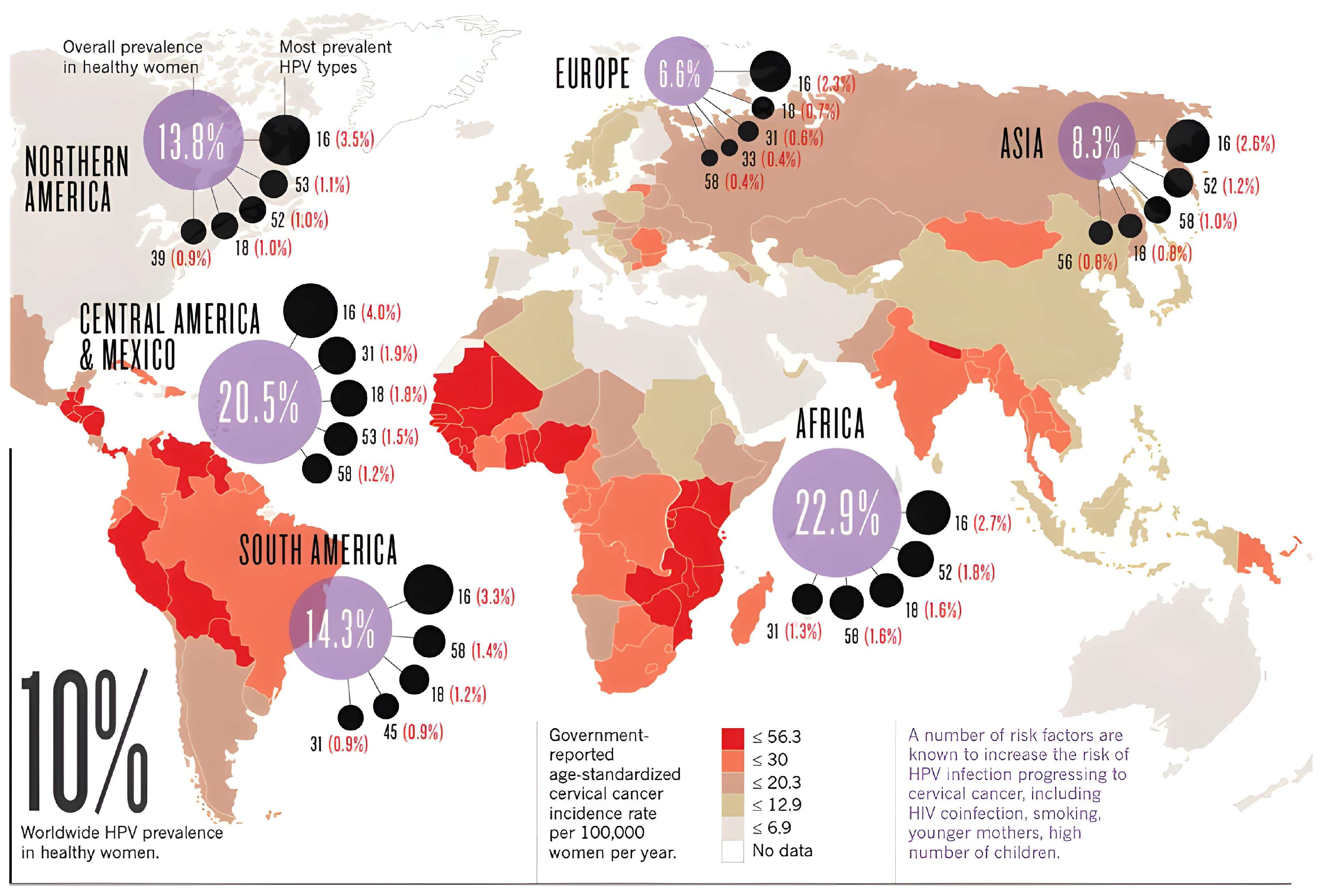
Figure 1.
World map with the prevalence of the predominant high-risk HPV subtypes by continent or region in healthy women (Adapted from: https://www.cancer.gov/about-nci/organization/cgh/blog/2018/world-report, accessed on 1 February 2025).
1.2. HPV Classification
More than 40 HPV genotypes exhibit tropism for the anogenital epithelium [25,26]. According to the IARC Monograph 100B [2012] and subsequent updates, these are classified as follows:
- Group 1 (Carcinogenic): HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 [27,28].
- Mechanism: E6/E7-mediated p53/Rb degradation, genomic instability.
- Group 2A (Probably carcinogenic): HPV 68 (previously listed here; now reclassified to Group 1), with no other genital HPV types currently assigned to Group 2A by IARC [27,29]. Some studies propose HPV 26, 53, or 66 for Group 2A, but IARC retains them in Group 2B due to insufficient evidence [26,28].
- Group 2B (Possibly carcinogenic): HPV 26, 53, 66, 67, 70, 73, and 82 [28,30].
- Group 3 (Not classifiable/Low risk): HPV 6, 11, 40, 42, 43, 44, 54, 61, 72, 81, and 89 [28,30].
Current IARC Monographs list no genital HPV types as Group 2A. Non-genital types (e.g., HPV 5, 8 for skin cancer) may be included here.
However, standardized classification remains elusive for many low-prevalence HPV genotypes due to insufficient oncogenicity data [25,31]. Globally, HPV-16 and HPV-18 account for approximately 70% of CC cases, with molecular studies confirming their dominant etiological role through E6/E7 oncoprotein-mediated carcinogenesis [25,32].
Notably, meta-analyses demonstrate modest but consistent geographic variation in HPV-16/18 attribution, accounting for 72–77% of cervical cancers in high-income countries compared to 65–72% in low- and middle-income countries [27,33]. This disparity underscores the need for refined classification criteria incorporating the following: (1) population-specific genotype distributions, (2) molecular markers of oncogenic progression (e.g., viral integration status, E6/E7 expression levels), and (3) longitudinal persistence data [26]. Such multidimensional stratification would improve risk prediction beyond the current dichotomous (high/low-risk) systems.
Based on their association with cervical cancer and precursor lesions, HPVs can also be grouped into high-risk and low-risk HPV types. Low-risk HPV types include types 6, 11, 42, 43, and 44. High-risk HPV types include types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70. Included in the high-risk group are some HPV types that are less frequently found in cancers, but are often found in squamous intraepithelial lesions [SILs]. Some authors refer to these HPV types as intermediate risk. Low-risk subtypes are also occasionally found in cervical carcinomas [34]. The current diagnostic paradigms for cervical HPV infection and carcinogenesis rely on viral DNA detection in cervical scrapes or biopsy specimens via PCR-based assays (e.g., Cobas® HPV Test, Linear Array) [31].
The molecular evolution of HPVs reveals an intricate diversification shaped by genomic drift, host adaptation, and lineage-specific selective pressures. Early comparative phylogenetic analyses across 48 HPV types and 28 subtypes highlighted profound evolutionary divergence among HPV clades, with lineage-specific genetic signatures being evident even within closely related types. More recent genome-wide analyses have reinforced this complexity, using molecular evolutionary genetics frameworks to elucidate subtype-specific microRNA repertoires and revealing that viral genomic regions involved in oncogenesis exhibit accelerated evolution relative to structural genes [35].
Phylogenetic reconstructions focusing on HPV16-related types, including HPV31, HPV33, and HPV58, have demonstrated that subtype diversification often parallels geographic and ethnic population structures, suggesting the co-migration of viral lineages with human hosts [36]. Analyses of Alpha papillomavirus 7 species (HPV18, HPV45, and HPV59, among others) revealed an ancient phylogenetic root in Africa, followed by a global radiation associated with human dispersal events [36].
Notably, phylogenetic trees constructed for HPV18 isolates revealed distinct African and European lineages, reflecting both ancient divergence and recent epidemiological dynamics [37]. Comparative studies on variants of HPV types 18, 45, and 97 further illustrate that intratype genomic variations are not stochastic, but reflect distinct evolutionary histories shaped by founder effects and host–virus interactions [36].
Thus, phylogenetic frameworks provide a robust lens for interpreting the molecular evolution of HPV, revealing that HPV subtypes and variants represent natural, ancient viral taxa with deep evolutionary roots, intertwined with human demographic expansions [38].
1.3. Significance of HPV as a Public Health Concern, Particularly Its Association with CC
Human papillomavirus [HPV] is established as the necessary cause of CC, with persistent infection implicated in 99.7% of high-grade precarcinomas. While approximately 70% of sexually active women acquire genital HPV infections, 90% undergo spontaneous clearance within 1–2 years due to robust cell-mediated immunity, posing no long-term risk [39,40].
Many nations now adopt HPV genotyping as the primary screening modality, superseding conventional cytology (Papanicolaou testing) due to superior sensitivity for detecting precancerous lesions [10]. However, the low specificity (~85%) of HPV DNA testing can generate unnecessary colposcopies [10].
To mitigate overdiagnosis, secondary triage methods are imperative, including the following:
- Cytology co-testing (e.g., Pap smear for HPV-positive cases);
- p16/Ki-67 dual staining (improves specificity for CIN2+);
- Methylation markers (e.g., FAM19A4/miR124-2) for progressive lesions.
1.4. Pathogenesis of HPV
The transition from a normal epithelium to invasive cervical carcinoma is a long and multi-step process, involving viral integration, the aberrant regulation of the host cell cycle, immune avoidance, and metabolic rewiring.
HPV is a small, spherical, non-enveloped, double-stranded DNA virus that belongs to the Papaviridae family. Its diameter ranges from around 50 to 55 nanometers. A total of 72 capsomers are contained within this structure, which is an icosahedral capsid, as stated by Baker et al. [41,42].
According to Stanley et al., the genome of HPV is circular and is separated into three functional parts [43]. These segments are the non-coding long control region [LCR], the early (E) region, and the late (L) region. Favre and Torrisi et al. found that the late region is responsible for the production of the structural proteins L1 and L2 [41,44]. It is the LCR that controls the expression of viral genes and the replication of DNA. The early region is responsible for the encoding of proteins (E1, E2, E4, E5, E6, and E7) that play a role in the replication of viruses and the occurrence of carcinogenic events [45,46,47,48].
2. Early Proteins and Viral Integration Process
2.1. E1 and E2 Proteins: Initiation of HPV Replication and Viral Integration
Viral receptors on host cells let infection start in the basal layer of the squamous epithelium. The viral genome is originally housed in the nucleus in an episomal form, which allows the virus to replicate inside the host cell like a plasmid. The strict control of infection across the early phases is attributed to the E1 and E2 proteins [49,50,51].
E1 protein, which is essential for the beginning of viral DNA replication, shares a significant degree of similarity across different HPV types. It functions as a helicase and is essential for viral replication, since it unwinds the DNA of the virus. Upon recruitment to the origin of replication, E1 initiates the formation of a complex with E2, the principal regulatory protein of HPV replication [52,53,54,55,56,57,58,59,60].
E2 is a multifunctional protein, as it is needed both for the transcriptional regulation of the viral genes, as well as for the integrity of the viral genome during replication. By binding to the E2 binding sites (E2BS), E2 recruits E1 and forms the E1–E2 complex. It is possible to start the replication process once this complex has finished unwinding the DNA. During the integration of the viral genome into the host’s genome, the E2 gene is often disrupted, leading to the loss of its regulatory functions and the unregulated activation of the E6 and E7 oncoproteins [52,55,56,57,58,59,60,61,62,63,64,65].
The disruption of E2 in the viral life cycle is a signature of HPV integration and cancer. In fact, 83% of HPV-positive cases have the viral genome integrated into the host itself. The biology of this process has been studied in depth. The HPV genome undergoes partial replication via E1 and E2, which can trigger DNA damage response (DDR) pathways and cause genome damage [66,67]. Upon the uncontrolled nuclear expression of E1, DNA damage leads to the activation of kinases including the recruitment of the ATR and ATM kinases, which promote double-stranded DNA breaks (DSBs) and enable the integration of HPV. In addition, E2 also targets nucleases to its binding sites in the long control region (LCR), producing DSBs which may account for the shortened HPV genomes found in CC [68,69].
A different theory involves the hyperactivity of apolipoprotein B mRNA-editing enzyme catalytic polypeptides (APOBEC), which can cause hypermutation, disrupt E2, and facilitate the integration of HPV DNA into the host genome [69,70,71]. More studies indicate that E1 causes genomic instability locally because of DNA overamplification, and that E2 mediates E6-induced apoptosis; these findings suggest strategies that HPV uses to hijack host cellular events and facilitate its life cycle.
The degradation of E2 leads to the overexpression of E6 and E7, which obliterates tumor suppressor proteins like p53 and retinoblastoma (Rb) [72,73]. The disruption of the cell cycle and the inactivation of apoptotic mechanisms lead to a cancerous transformation of these infected cells [73,74].
2.2. E4 Protein: Role in Cytopathic Effects and Viral Release
The E4 protein is a product of the alternative splicing of the E2 gene and is an important player in late viral infections. E4, which is mainly expressed in the upper layers of the epithelium, plays a role in the amplification of the viral genome, as well as in the disintegration of host cellular structures. Interaction with host cell keratin filaments is one of the major functions of E4. E4 is tethered to keratin via an N-terminal leucine cluster motif, which also destabilizes the keratin cytoskeleton and enables the budding of new virions from the infected cells [75,76,77,78,79,80,81,82].
In addition, E4 is involved in cytoplasmic granule formation, which is implicated in the cytopathic effects of HPV-infected cells. In the most straightforward terms, this impact varies from generating cell hyperplasia to having an impact on an intracellular and extracellular level, which is known to be involved in virus pathogenicity. Moreover, E4 is subject to various forms of post-translational modification like phosphorylation, which is known to increase its stability and ability to cohesively bind keratin. The S phase-dependent phosphorylation of E4 by cyclin-dependent kinases (CDKs) in turn helps to modulate the host cell cytoskeletal network to facilitate the release of nascent virions while also playing a role in controlling viral replication itself [51,69,83,84].
2.3. E5 Protein: Modulation of Immune Response and Cell Growth
The E5 is a small hydrophobic transmembrane viroporin involved in viral replication and modulating signaling pathways. One of its major mechanisms is the activation of the epidermal growth factor receptor (EGFR), resulting in downstream signaling through the PI3K-AKT and MAPK pathways, leading to cell growth and survival. This dysregulation causes the uncontrolled proliferation of infected cells. E5 is also important in immune evasion, helping HPV to evade host defense mechanisms. It inhibits interferon (IFN) signaling by binding to a stimulator of interferon genes (STING), which is associated with the decreased activation of the JAK/STAT pathway [51,84,85,86,87].
E5 also downregulates the CDK inhibitors p27Kip−1 and p21Waf−1, enabling infected cells to avoid cell cycle arrest and divide further. Additionally, it inhibits keratinocyte differentiation by downregulating keratinocyte growth factor receptor (KGFR), and at the same time, increases EGFR activity, leading to delayed differentiation and the maintenance of keratinocyte proliferation [88,89,90].
E5 inhibits molecules of the major histocompatibility complex class I (MHC I) to evade immune system identification, hence diminishing the functionality of T cells and natural killer (NK) cells. To evade detection by the immune system, HPV inhibits the synthesis of viral antigens on the surfaces of infected cells [91].
In addition, E5 also has been shown to upregulate the expression of VEGF, thereby stimulating angiogenesis, which supplies the growing tumor.
2.4. E6 Oncoprotein: Regulator of Tumor Suppression and DNA Repair
The E6 and E7 oncoproteins are the most largely studied HPV proteins associated with cancer. These proteins serve as the main drivers of HPV carcinogenesis.
E6 is a potent oncoprotein, promoting carcinogenesis via the suppression pathways, primarily through the degradation of p53. E6 binding to E6AP (an E3 ubiquitin ligase) causes p53 degradation, eliminating cell cycle inhibition at the G1 checkpoint and allowing infected cells to replicate uncontrollably [92]. It can also induce an upregulation of OCT-4 that not only inhibits the activity of p53 but also promotes stem cell-like characteristics in infected cells [93,94,95].
In addition to inhibiting apoptosis, E6 is also involved in manipulating DNA damage repair mechanisms, promoting increased genomic instability. It perturbs essential proteins involved in DNA damage responses, including CHEK2, CLK2/3, ERCC3, MNAT1, PER1, RMI1, RPA1, UVSSA, and XRCC6, which facilitates viral amplification but damages the host genome. E6 enhances telomerase activity by degrading NFX1-91, a known telomerase inhibitor, subsequently activating hTERT. This process allows cells to bypass senescence and achieve immortality [92,96].
E6 further affects key signaling cascades involved in cell survival and proliferation, including PI3K/AKT and Wnt/β-catenin. E6 promotes the degradation of tumor suppressor proteins (hDLG, hSCRIB, MAGI-1, -2, -3, and NHERF-1) via its PDZ-binding motif, resulting in the uncontrolled activation of pro-survival and mitogenic cascades. Together, these molecular perturbations drive cellular transformation and tumor progression [61,97,98,99,100,101,102].
2.5. E7 Oncoprotein: A Driver of Cell Cycle and Genome Instability
E7 acts mainly by inactivating the tumor suppressor protein retinoblastoma (pRB), allowing E2F transcription factors to trigger the S-phase of the cell cycle. E7 does so by recruiting the cullin 2 (CUL2) ubiquitin ligase to degrade pRB. This enables HPV-infected cells to multiply uncontrollably [100,103,104].
It also upregulates the expression of the eIF4E translation factor, which promotes the protein synthesis needed for viral replication. Moreover, E7 directly regulates the G2/M checkpoint via histone H1 kinase activity modulation, creating an optimal environment for viral genome replication on the cell cycle front, while promoting cellular genome instability [99,100,102].
In addition, E7 takes advantage of the DNA damage response that is begun by RN1698 E3 ubiquitin ligase in order to assist the virus in replicating (this ultimately poses a danger to the integrity of the host genome). Furthermore, E7 is responsible for activating the PI3K/AKT/SGK signaling cascade, which is responsible for facilitating cell migration, survival, and the transition from epithelial to mesenchymal cells. It is evident from this that E7 plays a significant part in the pathway that leads to the formation of tumors that are connected to HPV [105,106,107,108].
Aside from their role in the cell cycle and apoptosis, the E6 and E7 proteins contribute to other hallmarks of cancer, including genomic instability, immune evasion, and cell invasion. E6 leads to genomic instability through mutagenesis and the inhibition of DNA repair, and E7 promotes invasion by stimulating extracellular matrix degradation. These proteins act together to drive cells infected with HPV down the path from normal cells to malignant transformation, and ultimately to HPV-associated cancers (Figure 2) [51,109,110].
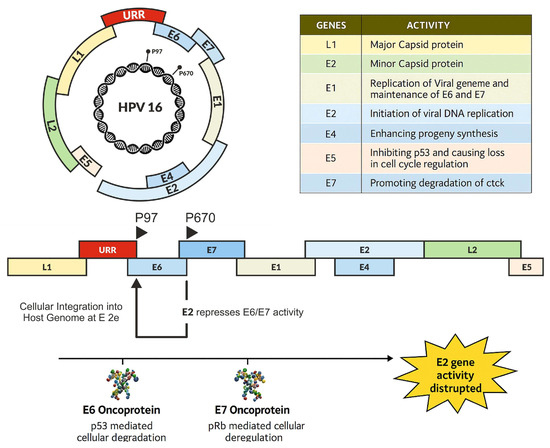
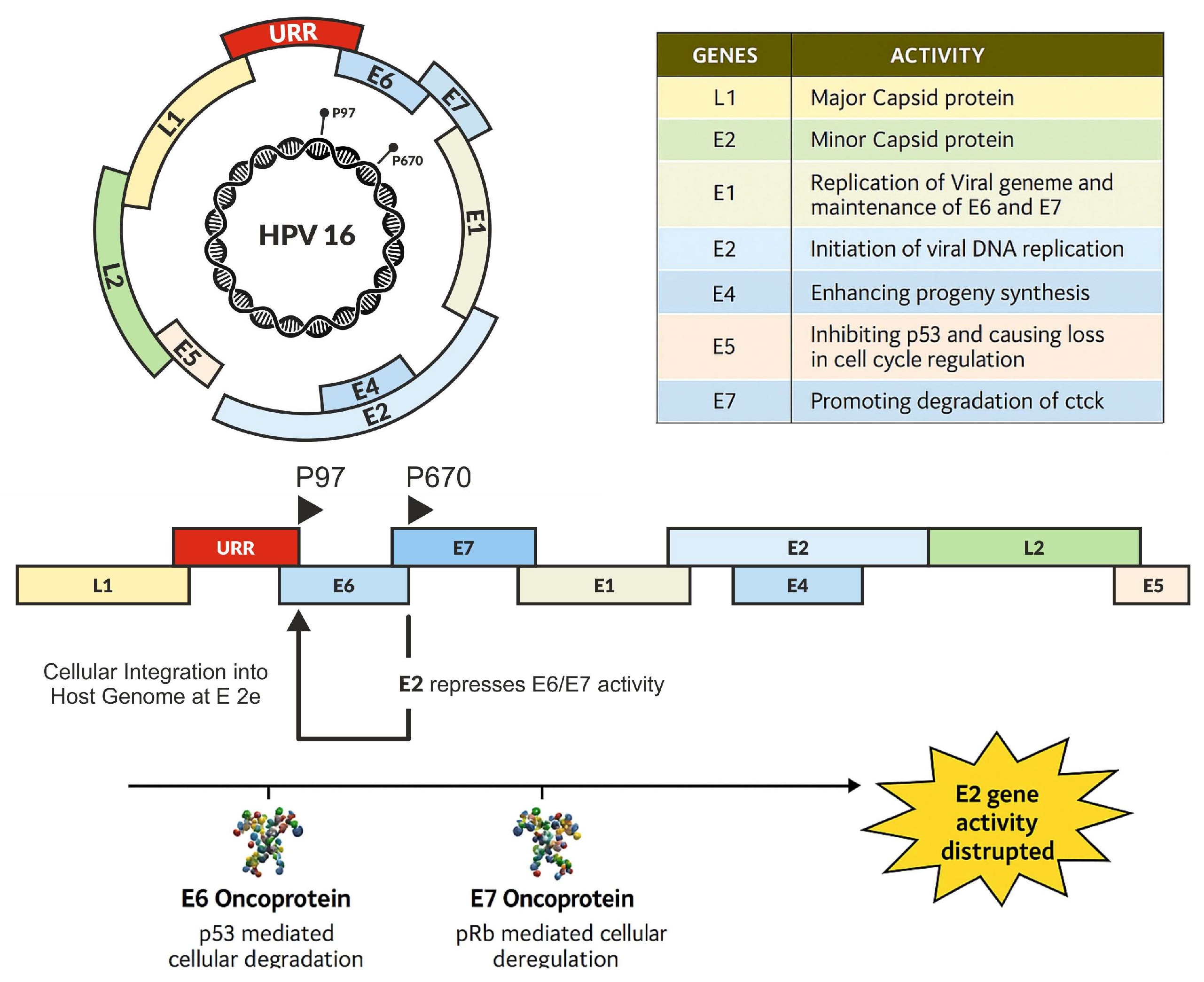
Figure 2.
The mechanisms of oncoproteins in carcinogenesis.
2.6. Late Proteins—L1 and L2
The structural proteins of the viral capsid are encoded by the late (L) region of the genome, and consist of two components: L1, the major capsid protein, and L2, the minor capsid protein. [90,111].
The principal capsid protein, L1, is structured in 72 pentameric subunits termed capsomeres, each consisting of five L1 monomers, culminating in a total of 360 L1 molecules per mature virion. The minor capsid protein, L2, exists in a reduced quantity, with roughly one molecule per capsomere—amounting to approximately 72 L2 molecules per capsid [112]. L1, a protein of approximately 55 kDa, is crucial for capsid assembly, serves as the principal structural element of the virion, and enables viral attachment by binding with heparan sulfate proteoglycan receptors on keratinocyte surfaces. Following receptor activation, the viral capsid undergoes conformational changes, which are essential for the subsequent entry processes. Proteolytic cleavage of L1 and L2 by host cell enzymes transpires before or during cellular entrance, facilitating the effective uncoating and internalization of the viral genome [111].
The regulation of viral replication and transcription is mediated by a non-coding segment known as the Long Control Region (LCR), which makes up about 10% of the human papillomavirus (HPV) genome. It is found between the open reading frames L2 and E6. The LCR contains several conserved regulatory and tissue-specific elements arranged into three separate domains: the distal domain, the keratinocyte-specific (KE) domain, and the proximal domain [113]. Many transcription factor binding sites in the KE domain control RNA polymerase II-driven transcription from both early and late promoters, hence enhancing their value. Among others, they comprise binding motifs for transcription factors AP-1, NF-1, TEF-1, OCT-1, YY-1, BRN-3a, NF-IL6, KRF-1, NF-κB, FOXA1, and GATA3 [90]. Three E2 binding sites found in the proximal domain are vital for the control of viral transcription and replication. Binding sites for basal transcriptional machinery abound in this region as well: Sp1, the TATA box, and the early promoter. Moreover, this proximal area contains the origin of replication (ori), necessary for viral DNA replication [90].
2.7. HPV Signaling Pathway
Important for the development of cancer, the HPV oncoproteins E5, E6, and E7 modulate several cellular signaling pathways. These pathways show interesting targets for the development of new therapeutic approaches since they are central in malignant transformation. While E6 disturbs several important molecular pathways, including the PI3K/AKT, Wnt, and Notch pathways, the HPV E5 oncoprotein activates the MAPK-ERK signaling pathway [97]. The E7 oncoprotein also tunes the PI3K/AKT axis. Particularly important in malignant transformation, the PI3K/AKT pathway drives cell proliferation, metastases, and drug resistance.
2.8. MAPK-ERK Pathway
The E5 oncoprotein has been previously described to activate the MAPK-ERK pathway [97]. Involved in cell proliferation and differentiation, this pathway is critical, as MAPK-ERK activation leads to enhanced expression of oncogenes like c-Fos for cancer cell abiding and metastasis. Moreover, SRC proto-oncogene and phosphorylated ERK1/2 also modulate ERK signal transduction to play a role in tumor progression [51,114].
There are several therapies currently undergoing clinical trials targeting the vascular endothelial growth factor (VEGF) and EGFR for CCs associated with HPV. It has been reported that HPV16 E6 oncoprotein induces hypoxia-inducible factor 1-alpha (HIF-lα) expression, as well as downstream target genes such as VEGF and interleukin-8 (IL-8) through the ERK1/2 signaling pathway [61,102]. Furthermore, high HER3 expression driven by E6/E7 correlates with poor CC prognosis [115]. HER3 mRNA and protein expression are positively correlated in HPV-mediated cervical carcinoma through PI3K signaling [116].
2.9. PI3K/AKT, Wnt, and Notch Pathways
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling system is important in promoting cell division, the maintenance of viability, and metabolic adaptation. The E6 oncoprotein mainly modifies the phosphatidylinositol 3-kinase and protein kinase B (PI3K/AKT) pathway, which then leads to cell proliferation and drug resistance [98,117]. The E6 protein can also inactivate tumor suppressor proteins such as p53 or PTEN [118,119], which leads to the dysregulation of the cell cycle. Abnormal activation suppresses apoptosis, accelerates cellular proliferation, and aids processes like the epithelial–mesenchymal transition (EMT), thus fostering malignant transformation.
The HPV E6 oncoprotein activates the Wnt/β-catenin signaling pathway, which is essential for epithelial cell immortalization, cell proliferation, and tissue homeostasis. Under typical circumstances, β-catenin levels are rigorously regulated by proteasomal degradation; however, E6-induced activation results in the stabilization and nuclear accumulation of β-catenin, hence facilitating the transcription of oncogenic target genes such as c-Myc and cyclin D1. Aberrant activation of the Wnt/β-catenin pathway has been associated with the onset and persistence of HPV-induced malignancies, leading to the disruption of the normal epithelial architecture and the development of malignant phenotypes. Furthermore, sustained Wnt/β-catenin signaling enhances the stem-like characteristics of cancer cells, contributing to tumor growth and resistance to therapy. The Wnt/β-catenin pathway, due to its pivotal involvement in cervical carcinogenesis, constitutes a prospective therapeutic target for cervical cancer treatment [120].
The Notch signaling pathway, recognized for its oncogenic role in leukemia and other cancers, paradoxically has tumor-suppressive activity in hr-HPV-induced cervical carcinogenesis [92,107,121]. E6 has an inhibitory role in the Notch signaling pathway, which promotes tumor progression via the loss of p53-mediated cellular control. Notch and p53 participate in a reciprocal regulatory loop, whereby Notch activation stabilizes p53, and its dysregulation fosters cancer. The Notch signaling pathway was shown to cooperate with the PI3K-AKT pathway to promote cellular progression, highlighting the intricate role of Notch signaling in HPV-associated carcinogenesis [51,92,119,121].
The E7 protein inactivates retinoblastoma protein (pRB) and causes uncontrolled cell proliferation and the progression of CC [61]. E7 also inhibits p27 through AKT, which promotes its retention in cytoplasmic domains, leading to an increase in cell cycle progression [51,92,122,123].
2.10. STAT3 and EMT Pathways in HPV-Induced Cancer
Signal transducer and activator of transcription 3 (STAT3) is one of the most important molecules involved in epithelial cancer progression. Numerous studies have indicated that the binding of STAT3 to the HPV16 LCR regulates the expression of viral oncogenes, thereby influencing cancer development [92,105,124]. HPV16 E6/E7 oncoproteins can also cause EMT through upregulated fibroblast growth factors (FGF)-2 and FGF-4, thereby promoting invasive cancer growth [125]. E-cadherin is a key epithelial differentiation marker and is known to be suppressed by E6/E7 and ErbB-2 during the epithelial–mesenchymal transition progression, which is further activated by central transcription factors such as SLUG, SNAIL, and TWIST [100,105].
2.11. Role of AP-1 and Notch Signaling Pathways in Cervical Carcinogenesis
The transcription factor Activator Protein-1 (AP-1) is essential for the expression of HPV oncogenes such as E6 and E7 and plays a pivotal role in the progression of HPV-associated cervical cancer [125]. Studies have identified two activator protein-1 (AP-1) binding sites within the LCR of HPV18, indicating the regulatory role of the AP-1 in viral oncogene expression. The key component of the AP-1 complex, c-Jun, is known to promote tumorigenesis, cell proliferation, invasion, migration, and angiogenesis. Moreover, c-Jun has been specifically associated with the enhanced expression of E6 and E7. In fact, mutations in AP-1 within the HPV upstream regulatory region (URR) can disrupt E6/E7 gene expression [126], emphasizing the importance of this transcription factor in CC progression. AP-1 alterations, such as the increased expression of c-Fos, facilitate tumorigenesis [69,118].
2.12. Strategies of Immune Evasion by HPV-Infected Cells
The ability of HPV to evade the host’s immune response is a key factor in the transition from chronic infection to CC. The occurrence of an HPV infection activates mechanisms of viral immune evasion, leading to a range of diseases, including chronic inflammation and the progression of cancer.
The detection and eradication of cells infected with HPV are frequently attributed to both innate and adaptive immune responses. On the other hand, HPV utilizes various mechanisms to escape immune recognition, impede antiviral defenses, and modify host immune responses, ultimately leading to tumor development and chronic infections.
2.13. Evasion of Antigen Presentation and Adaptive Immunity
The presentation of antigens to cytotoxic T lymphocytes (CTLs) via MHC class I molecules is a crucial process in the immunological elimination of HPV-infected cells. MHC class I downregulation by E5 and E7 HPV-derived proteins prevents the activation of antigen-specific CTLs. Moreover, HPV-infected keratinocytes prevent the migration of Langerhans cells by decreasing macrophage inflammatory protein-3α (MIP-3α) expression, leading to a decrease in antigen presentation. E6 further impairs immune recognition by preventing the differentiation of monocytes into dendritic cells [127,128].
2.14. Innate Immunity and Pattern Recognition Receptors (PRRs)
Keratinocytes, which are non-professional antigen-presenting cells (APCs) that activate cell-mediated immunity, are the first cells to initiate the innate immune response to an HPV infection. Since the multiplication of HPV in keratinocytes does not result in the lysis of the cells, it is possible for these cells to display viral peptides on MHC class I molecules for the purpose of immune system examination.
Even though keratinocytes often do not synthesize MHC class II molecules, there are a few cytokines that have the potential to trigger their production. One of these cytokines is interferon-gamma (IFN-γ), which plays a significant role in the activation of Th1 cells and has a significant impact on the regulation of immunity [129,130,131]. Among the pattern recognition receptors (PRRs) that are found in keratinocytes are Toll-like receptors (TLRs), which play an essential role in the identification of viral DNA. To be more specific, TLR9 is responsible for recognizing double-stranded HPV DNA and triggering the production of type I interferons (IFN), tumor necrosis factor-alpha [TNF-α], interleukin-18 (IL-18), and a variety of chemokines (CCL2, CCL20, CXCL9) [91,132,133,134,135]. This process ultimately leads to the activation of immune cells such as natural killer (NK), natural killer T cells (NKT), leukocyte cell tumor (LCT), macrophages, and dendritic cells (DCs). Macrophages and dendritic cells are examples of professional antigen-presenting cells. These cells phagocytose and deliver HPV antigens to T cells, which activates CD4+ helper T cells and CD8+ cytotoxic T cells, which in turn drives the generation of antibodies by B cells. PRRs are responsible for the identification of HPV by antigen-presenting cells (APCs). When these cells are activated, they release co-stimulatory chemicals and cytokines, which are essential for the generation of an appropriate immune response as well [136,137].
2.15. T Cell Responses and Adaptive Immunity
While innate immunity is critical for the early detection and control of HPV, long-term immunity is attributed to the adaptive immune system. CD4+ T helper (Th) cells and CD8+ cytotoxic T lymphocytes (CTLs) are typically elicited against HPV infections. These T cells recognize and destroy cells with HPV-infected cells [134,137,138].
- Th1 cells, which are CD4-positive T helper cells responsible for beginning cellular immune responses, generally facilitate viral clearance after HPV infection. HPV utilizes many mechanisms to avoid host immune responses, mostly facilitated by the viral oncoproteins E5, E6, and E7. Th2 cells are also involved in this process. Nonetheless, HPV infection induces a change in the differentiation of Th1 and Th2 cells towards a Th2 phenotype. This shift likely contributes to both the persistence of HPV infection and the development of cervical lesions. This modification results in a diminished immune response, perhaps accounting for both instances [137];
- CD8+ Cytotoxic T Cell (CTL): CTLs are important for directly killing HPV-infected cells. The E5 and E7 oncoproteins diminish the production of MHC class I molecules, obstructing antigen presentation and resulting in weak or missing HPV-specific CTL responses, commonly seen in persistent HPV infections [139]. In those who have genital warts or CIN, HPV-specific CD8+ T cells can be correlated with the regression of lesions and clearance of the virus. In cases of persistent infection, however, HPV-specific CTL responses are frequently weak or absent, and therefore contribute to an inability to clear the infection [139];
- Tregs function in immune suppression, and their higher numbers in the tumor microenvironment correlate with poor immune responses, as well as persistent HPV. E7 has been associated with the promotion of Treg growth, wherein Tregs secrete IL-10 and TGF-β, which downregulate effector T cell responses and enhance viral immune tolerance. E5 and E6 concurrently facilitate the recruitment of tumor-associated macrophages (TAMs), which release pro-tumor cytokines (including IL-10, VEGF, and TGF-β) and inhibit anti-tumor immune responses [140,141,142,143,144].
HPV establishes a highly effective immune evasion network by modulating Th1/Th2 differentiation, suppressing CTL activity, expanding Tregs, and recruiting TAMs, primarily through the actions of E5, E6, and E7 oncoproteins, thus enabling persistent infection and the progression of cervical cancer.
2.16. Activation and Mechanisms of Cytotoxicity of NK Cells
Natural killer (NK) cells are essential to eliminate HPV-mediated and malignant cells. Their activation is the result of a balance between inhibitory and activating receptors. MHC class I molecules typically bind inhibitory receptors such as KIR-L and NKG2A to downregulate NK cell activity. Indeed, to avoid T cell responses, HPV-infected tumor cells frequently downregulate MHC-I expression through the actions of viral oncoproteins like E5 and E7, thus releasing inhibitory signals and stimulating NK cells. NK cells can recognize stress or damage-associated receptors on CC cells, such as MICA/MICB and ULBPs [1,2,3,4,5,6], which engage with NKG2D, CD95 (which binds CD95L), and B7-H6 (which connects with NKp30). Fc receptors (CD16) facilitate antibody-dependent cytotoxicity (ADCC), while tumor-associated CD112 and CD155 interact with DNAM-1 to enhance NK cytotoxicity inside the tumor microenvironment. E6 and E7 not only downregulate MHC-I, but also modulate the expression of NKG2D ligands to evade NK cell surveillance, creating a complex interplay between viral immune evasion strategies and innate immune activation. [145,146,147,148,149,150].
2.17. Humoral Immunity and Antibody Responses
Both B cells and antibodies are responsible for the production of humoral immunity, which is the secondary component of the adaptive immune response to HPV. The virus is responsible for the production of antibodies that target capsid proteins (L1 and L2). By rendering the virus ineffective, these antibodies will prevent further infection from occurring. As a result of the fact that HPV is most prevalent in epithelial cell layers, which are areas where immune monitoring is restricted, antibody responses to HPV sometimes prove to be inadequate for eradicating preexisting infections [151,152].
2.18. Immunosuppressive Microenvironment and Host Genetic Factors
The host’s genetic characteristics influence both the sensitivity to chronic HPV infection and the likelihood of progression to cervical intraepithelial neoplasia (CIN), alongside viral immune evasion strategies.
Variants in HLA class I and II genes are associated with variations in immune responses to HPV. Increasing research on genetic susceptibility to cervical cancer has focused on the function of HLA II alleles in modulating immune responses to persistent hrHPV infection. Whereas Class II HLA molecules present HPV-derived peptides to CD4+ helper T cells, Class I HLA molecules present them to CD8+ cytotoxic T lymphocytes [109,141,153,154].
Many alleles have been found as either protective markers or risk factors for cervical neoplasia. Among those regularly linked to increased cervical cancer risk are HLA-DRB1*15 and DQB1*0602, as well as DRB1*0401, DRB1*0403, DQB1*0302, D QB1*0402, DQA101:02 and DQA102:01, and D QB1*0603. These alleles are believed to either promote a suboptimal immune response or fail to sufficiently present HPV antigens to CD4+ T helper cells, thus allowing viral persistence and the progression of cancer.
DQA105:01 was associated with significantly reduced odds of prevalent hrHPV infection, yet paradoxically, its presence was linked to an increased risk of persistent infection—a critical determinant of cervical cancer risk. Several haplotypes containing DQA105:01 also showed strong associations with persistent hrHPV infection. This suggests the dualistic role of this allele in modulating both initial immune resistance and long-term immune evasion by the virus, potentially through mechanisms that impair antigen presentation or cytotoxic T cell activation.
Some alleles, on the other hand, show protective actions. Reduced risk of cervical cancer has been linked to DRB1*1301, DQB1*0603, DQB1*0501, and DQA1*0301. These protective alleles probably improve antigen presentation’s efficiency, therefore encouraging viral clearance and a stronger anti-HPV immune response. Fascinatingly, DQB1*0603 has been shown in several studies to be both a risk and protective allele, suggesting possible variation in its functional relevance depending on environmental interactions or genetic background.
Several single-nucleotide polymorphisms (SNPs) in immune response genes, cell cycle regulators, and hormone pathways have been identified as important modulators.
The -1082 A > G polymorphism in the IL-10 gene promoter is associated with reduced immune effectiveness against HPV, thereby increasing cancer susceptibility [155].
Furthermore, a reduced ability to recognize and clear HPV infections has been linked to SNPs in TLR4 (Asp299Gly) and TLR9 (-1486T/C), which promote viral persistence [156].
Among the most investigated polymorphisms are those in the TP53 tumor suppressor gene, especially the Arg72Pro SNP. This variant influences p53’s sensitivity to degradation, driven by HPV E6. The HPV E6 oncoprotein breaks down the Arginine variant (Arg72) more effectively than the Proline variant, thus increasing the risk of cervical cancer in Arg72 carriers [157].
Though not usually in the general population, somatic alterations in p16INK4a, a cyclin-dependent kinase inhibitor, occur in cervical precancerous lesions. Loss of the p16INK4a function causes uncontrolled cell cycle progression, thus promoting malignant transformation.
Variations in the estrogen receptor α gene (ESR1), including XbaI (A/G) and PvuII (T/C) polymorphisms, have been linked to changed receptor activity. These alterations could produce a hormonal environment that supports oncogenesis and HPV persistence [158].
2.19. Inflammation Factors in CC Related to HPV
During initial infection, HPV avoids the natural immune reaction, as it replicates without producing notable cytopathic effects or inducing a strong inflammatory response. The virus reduces the release of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), thus postponing the activation of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs). Chronic HPV infection triggers a sustained inflammatory response that significantly influences the cervical tumor microenvironment. The virus alters cytokine production, disturbs natural immune signaling pathways, affects important transcription factors, and increases the expression of protumorigenic mediators.
The activation of PRRs eventually leads to the stimulation of the transcription of genes involved in inflammation (e.g., TNF-α, IL-6, COX-2, ICAM), proliferation (CDK2), cell survival (XIAP, cIAP, BCL2, BCL-xL), and angiogenesis, promoting the recruitment of innate and adaptive immune cells to the site of infection [132]. Ideally, an effective inflammatory response facilitates viral clearance. Indeed, most HPV infections are transient, with approximately 90% resolving within two years [159].
Chronic inflammation and pro-inflammatory cytokines such as IL-1, IL-6, IL-8, IL-18, COX-2, and TNF-α are significantly involved in CC progression. Cytokines like TNF-α and IL-1α, while inhibitory to normal cervical epithelial cells, stimulate proliferation in HPV-immortalized and cervical carcinoma cell lines through an EGFR-dependent pathway. Chronic inflammation may also disrupt cellular senescence, a critical tumor-suppressive mechanism. Senescent cells release cytokines such as IL-6, IL-18, and IL-1, fostering tumorigenesis. HPV-16/18 E6 proteins promote fibroblast senescence through IL-6/STAT3 signaling, thereby altering the TME and supporting the progression from cervical intraepithelial neoplasia (CIN) to invasive cancer.
Immunosuppressive cytokines like IL-10 are overexpressed in CC and premalignant cervical lesions. HPV E2 protein can bind to the IL-10 promoter, enhancing IL-10 expression and contributing to viral persistence and immune evasion. A shift towards Th2-dominant cytokine profiles, characterized by elevated IL-10 and IL-6 and reduced IFN-γ and TNF-α levels, has been associated with poor immune responses and the progression of cervical lesions. Studies have confirmed decreased IFN-γ and increased IL-10 expression correlating with the severity of CIN lesions. Thus, an imbalance between Th1 and Th2 cytokines facilitates persistent HPV infection and drives lesion progression toward cervical cancer [132,141,160].
The aberrant overexpression of Cyclooxygenase-2 (COX-2) has been implicated in the pathogenesis of various malignancies and inflammatory diseases. Emerging evidence indicates that NF-κB is a key regulator of COX-2 gene expression, with the inhibition of NF-κB activation resulting in the downregulation of COX-2 expression. In the context of HPV-associated carcinogenesis, the viral oncoproteins E6 and E7 have been shown to upregulate COX-2 expression, thereby contributing to both tumor initiation and progression.
A study conducted by Kim et al. specifically examined the impact of the HPV16 E5 oncoprotein on COX-2 expression. Their findings demonstrated that E5 enhances COX-2 expression via the activation of the epidermal growth factor receptor (EGFR) signaling cascade. This upregulation was shown to be mediated, at least in part, through the involvement of NF-κB and AP-1 transcription factors. These results suggest that the E5 oncoprotein may contribute to cervical carcinogenesis by promoting COX-2 overexpression. Table 1 provides an overview of the HPV-encoded proteins implicated in inflammatory processes and cervical cancer pathogenesis [82,132].

Table 1.
Influence of HPV oncoproteins on immune system components.
2.20. MicroRNA Regulation by HPV Oncoproteins: Development of Cervical Cancer
Significant insights have been gained over the last thirty years into the role of genetic aberrations, particularly mutations in protein-coding oncogenes and tumor suppressors, which substantially contribute to the onset of CC. The discovery of non-coding RNAs (ncRNAs), particularly microRNAs as critical drivers of cancer, has transformed the traditional perspective of cancer biology.
These small, non-coding RNA molecules play an essential role in the initiation, progression, and prognosis of CC by regulating several cellular processes, including apoptosis, cell cycle progression, metastasis, and treatment resistance.
MicroRNAs (miRNAs), short non-coding RNAs of around 22 nucleotides in length, facilitate the post-transcriptional regulation of gene expression. They primarily function by engaging with the 3′ untranslated regions (UTRs) of target mRNAs, leading to mRNA degradation or translational repression. This technique enables miRNAs to simultaneously influence the expression of several genes, hence regulating networks of genes involved in diverse cellular activities [161,162,163].
Although the roles of oncoproteins E5, E6, and E7 have been well characterized, they contribute to the complexity of the molecular pathways involved in cervical carcinogenesis through their effects on the regulation of miRNAs [164].
HPV16-E5 expression in keratinocytes has been shown to downregulate miR-203 and miR-324-5p while upregulating miR-146a, further complicating the immune evasion process and facilitating viral persistence [164,165]. The production of E6 and E7 has been demonstrated to activate transcription factors like c-Myc and E2F, which can transactivate miRNA expression, hence promoting tumorigenic alterations [92,166].
A prominent instance of miRNA regulation by HPV oncoproteins is the modulation of miR-23b, which is directly affected by the E6 oncoprotein. The E6-mediated downregulation of miR-23b has been linked to the upregulation of the urokinase-type plasminogen activator (uPA), a protein involved in cancer cell migration and metastasis. The miR-23b and uPA correlation highlights the role of HPV-mediated miRNA modulation in driving cancer cells toward high invasive potential. Furthermore, miR-23b downregulation has also shown an association with radioresistance in cancer cells, indicating that miR-23b may play an important role in therapeutic resistance [164,167].
The other miRNA that has been implicated in HPV-related carcinogenesis is miR-34a, a direct transcriptional target of p53 [168]. E6 is accountable for the inactivation of p53; hence, it is anticipated that the expression of miR-34a is often downregulated in CC. MiR-34a targets many cell cycle regulators, such as CDK4, cyclin E2, and E2F1, in addition to pro-apoptotic and metastatic proteins including Bcl-2 and MET [169,170].
miR-203 is another miRNA that is upregulated or downregulated by HPV oncoproteins. This miRNA is known for its role in driving keratinocytes out of a proliferative state into a terminally differentiated, post-mitotic state. MiR-203 targets the transcription factor p63, which maintains a balance between cellular proliferation and differentiation [168,171,172]. The overexpression of E7 by HPV in differentiated epithelial cells results in the inevitable downregulation of miR-203 expression, which is linked to the preservation of a tumorigenic stem-like phenotype. Additionally, miR-203 was reported to play a key role in regulating the stemness and radioresistance of cancer cells. It has been found that the downregulation of the “stemness” potential of cancer cells is suppressed by miR-203 in several preparations [173,174].
E7 protein has been shown to downregulate miR-203, and thus has been proposed to contribute to the maintenance of stem-like phenotypes, which is often correlated with poor prognosis and treatment resistance. Tumor stem cells, capable of self-renewal and tumor repopulation, are more resistant to radiogenic damage, which adds another layer of complexity in treatment outcomes [168,171].
miR-145 is found to be suppressed in HPV-positive cervical tumors, leading to enhanced oncogene expression and tumor progression [99].
Certain oncogenic miRNAs, such as miR-21, are upregulated in HPV-associated cancers and target tumor suppressor genes such as PTEN and PDCD4, promoting cell survival and resistance to apoptosis [175]. miR-155 enhances inflammatory signaling and modulates the immune environment to favor the immune escape of HPV-infected cells [175,176,177].
A significant study documented the expression profiles of 96 cancer-associated miRNAs in a cohort of 102 CC tumor tissues utilizing quantitative RT-PCR. Among the chosen miRNAs, miR-200a and miR-9 had a strong correlation with overall survival [171,178]. These miRNAs were transfected into HeLa for functional evaluation. The research demonstrated that miR-200a regulates cancer cell migration and metastasis, potentially influencing the metastatic capability of CC cells by modulating pathways associated with cell motility [179,180]. In comparison, miR-9 let us discover regulation pathways for metabolism; therefore, we can understand its role in the metabolism balance of rapidly growing cells like cancer cells [164,178].
These miRNAs actively participate in the start, spread, and maintenance of cervical cancer. Knowing the interaction between HPV oncoproteins and host miRNAs helps to better understand the molecular processes of cervical tumorigenesis and points to new biomarkers and therapeutic targets.
2.21. Exosomes, Inflammation, and Microenvironment
Exosomes, nano-sized extracellular vesicles, serve as crucial mediators of intercellular communication within this milieu. They can transport proteins, lipids, mRNA, and microRNA (miRNAs), facilitate horizontal gene transfer, mediate cross-talk between tumor cells and immune cells and other elements of the tumor microenvironment [129,132,181,182,183], generating diverse biological processes, such as tumor progression, immune response, or inflammation [184]. For instance, in HPV-induced CC, exosomes are hypothesized to modulate the balance of pro-inflammatory and anti-inflammatory signals, leading to an environment that promotes immune tolerance and eventual tumor growth [107,185].
Various macromolecules present in exosomes secreted by different CC cell lines, including HeLa cells, have been shown to modulate responses by the immune system. In fact, these exosomes contain anti-apoptotic proteins, like survivin, that are involved in facilitating tumor growth and survival through the prevention of apoptosis. This finding was first published in 2009 and indicated that one of the oncogenic consequences of HPV was the synthesis and release of these extracellular vesicles. In particular, survivin-positive exosomes exerted pro-proliferative properties and had the ability to induce angiogenesis, a critical mechanism implicated in the progression of tumors [132,184,186,187].
Inflammation has an important function in promoting cancer. It is well known that, while the pro-inflammatory cytokines released by immune cells can perpetuate chronic inflammation, chronic inflammation can also perpetuate infection, a process that can be influenced by HPV. For instance, interleukin-36 gamma (IL-36γ), a pro-inflammatory cytokine, has been proposed as a modulator of keratinocyte responses in HPV-induced tissues [132,188,189]. This cytokine triggers signaling pathways, which may play a role in sustaining inflammation, a hallmark of malignant lesions. Moreover, there is evidence that HPV-16 suppresses the expression of certain pro-inflammatory genes, which might indicate that the virus uses mechanisms to avoid immune recognition while promoting inflammation [190].
Exosomes released by HPV-infected cells were shown to transfer inflammatory mediators and mRNAs to neighboring non-infected cells, as described in several recent studies. For example, oncogenic miRNAs like miR-21 and miR-155, which are often upregulated in cancer, are also found in exosomes from HPV-infected cells. The overexpression of these miRNAs has demonstrated anti-apoptotic and pro-proliferative effects, implicating them in immune evasion and tumor survival [132,191,192,193,194]. Another layer of regulatory complexity is added by long non-coding RNAs (lncRNAs), which have been found in exosomes, such as lincRNA-p21 and others, and can modulate gene transcription in accepting cells, potentially promoting inflammation and possibly facilitating tumor growth [191,195,196].
Exosomes stimulate several immune cells, including macrophages, by activating pro-inflammatory signaling pathways. Exosomes derived from cancer cells can stimulate the macrophage NF-κB pathway, resulting in the release of pro-inflammatory cytokines such as TNF-α, IL-6, and CCL2 [130,131,132]. These cytokines then stimulate immune cell recruitment to the tumor site, resulting in a host environment that facilitates tumor progression. Remarkably, exosomes from keratinocytes harboring HPV can additionally transport IL-36γ, a pro-inflammatory cytokine that activates Wnt signaling pathway-dependent pro-inflammatory responses in neighboring keratinocytes. These findings indicate the significant role of exosomes in maintaining an inflammatory cycle within the cervical epithelium that could enhance HPV persistence and tumorigenesis [132,188,189].
The immune landscape in CC is even more complex considering that exosomes also interact with numerous immune cells, including dendritic cells and T cells. Exosomes can deliver viral antigens and cytokines that modify immune cell activation and polarization. The expansion of regulatory T cells or the inhibition of cytotoxic T cell activity can result from this interaction, allowing the tumor to evade immune surveillance [109].
Finally, there is an interest in the therapeutic potency of inhibiting inflammation in the HPV-related CC context. We believe that strategies to either inhibit the generation of exosomes or neutralize their pro-inflammatory effects hold promise for new therapies. HPV is recognized as an infectious agent that induces chronic inflammation, and the involvement of inflammation in cervical carcinogenesis has provided new insights into prevention and treatment through its processes.
2.22. Epigenetic Modifications in CC
Not all HPV infections develop into cancer, suggesting that other factors control carcinogenesis. Among these, epigenetic changes, such as DNA methylation, are most important in controlling host and viral gene expression during HPV infection. Epigenetics is associated with modifications in gene function without changes in the DNA sequence. These changes consist of non-coding RNA-mediated control, histone modification, and DNA methylation.
Epigenetic DNA methylation occurs at the C5 site of cytosine residues inside CpG dinucleotides and is linked to the recruitment of transcriptional repressors or the suppression of transcription factor DNA-binding activity [90,197,198,199]. By changing the expression of important regulating genes, aberrant methylation patterns, including global hypomethylation and the promoter hypermethylation of tumor suppressor genes, help to drive malignant transformation in cancer [200].
Although the exact link between hr-HPV and cellular DNA methylation is still being explored, mounting evidence suggests that hr-HPV may be involved in the mis-regulation of the methylation-associated patterns of miRNA, subsequently resulting in the dysregulation of tumor-suppressive or oncogenic miRNAs [90,201].
The methylation of adenomatous polyposis coli (APC), an important negative regulator of β-catenin, leads to the hypermethylation of the APC promoter in HPV-infected CC cells, promoting β-catenin accumulation in the cell. These findings indicate that APC hypermethylation occurs at early phases in HPV carcinogenesis, but in advanced disease, APC hypermethylation is also intensified and underscores the importance of epigenetic mutations in tumor progression [107,202].
Frequently hypermethylated in CC are tumor suppressor genes, such as KIP1 (p27), TP53 (p53), PTEN, and RASSF1, all of which influence cell cycle control, proliferation, and death. For example, PTEN methylation stimulates PI3K signaling pathway activation, thus promoting cellular proliferation [198,201,203].
Other common epigenetic alterations include the silencing of the CDKN2A gene, which encodes the tumor suppressor protein p16INK4a. The hypermethylation of CDKN2A leads to pRB phosphorylation and results in releasing E2F and aberrant cell cycle progression, contributing to cervical carcinogenesis [201,204,205].
In HPV-induced cervical carcinogenesis, the hypermethylation of the CDH1 promoter is linked to cell adhesion, promoting invasion and metastases and influencing tumor progression. Levels of CDH1 methylation are positively associated with the severity of CC and have therefore been suggested as an epigenetic biomarker for the determination of CC severity and prognosis [201,206].
Oncogene hypomethylation has also been shown to be associated with CC progression, in contrast to the hypermethylation of tumor suppressor genes. In cancer, STK31 expression is found to be upregulated by promoter hypomethylation, which in turn is a predicted driver of cancer cell migration and invasion. The STK31 promoter exhibits substantial methylation in HPV-negative tumors, whereas it undergoes demethylation in high-risk HPV infections, leading to a marked overexpression in CC cell lines triggered by either HPV16 or HPV18 [207].
Invasive cervical carcinoma exhibits an elevated incidence of LCR methylation, particularly in HPV16-positive tumors [208,209]. Furthermore, E2 is modulated by hypermethylation at the E2 binding site (E2BS) inside the LCR, resulting in the overexpression of the E6 and E7 oncogenes, akin to the phenomenon found following the integration of the viral genome [208]. LCR methylation density analysis indicates the subtype-specific epigenetic risk of high-risk HPVs, where HPV16 appears to have the highest level of methylation density compared to other high-risk types like HPV18 and HPV45.
The most promising epigenetic markers include the hypermethylation of hsa-miR-124-2, SOX1, TERT, and LMX1A, which have emerged as potential biomarkers for CC precursors, regardless of high-risk HPV status. Rogeri et al. reported a correlation between hsa-miR-124-2 hypermethylation and the reduced expression of miR-124, and proposed its involvement in cervical carcinogenesis. Beyond miR-124, Yao et al. identified miR-432, miR-1286, miR-641, miR-1290, miR-1287, and miR-95, which were correlated with hr-HPV genetic status, as a panel of hypermethylated miRNA for CC. Wilting et al. also showed that increased promoter methylation resulted in the transcriptional repression of hsa-miR-149, hsa-miR-203, hsa-miR-375, and hsa-miR-638 in CC. Yao et al. further showed that the downregulation of hsa-miR-432, hsa-miR-1286, hsa-miR-641, hsa-miR-1290, hsa-miR-128, and hsa-miR-95 in primary cervical tumors is dependent on their methylation status [175,176,177].
Wilting et al. reported significantly elevated levels of methylation of hsa-miR-203, hsa-miR-149, and hsa-miR-375 in patients with CC, with hsa-miR-203 and hsa-miR-375 showing higher levels in high-grade lesions. Overall, these discoveries highlight the importance of miRNA methylation in cervical carcinogenesis. The methylation of tumor-suppressive miRNAs, such as hsa-miR-124, hsa-miR-203, hsa-miR-375, and hsa-miR-149, is particularly clear and shows aberration in CC progression through the downregulation of these important regulatory pathways [176,177,201].
CIN progression is also associated with methylation changes; hence, methylation markers can be useful for the stratification of lesions and prognostication. Vink et al. showed that when lesions evolved from CIN2 to CIN3, both p16 and Ki-67 expression increased, while E4 expression decreased along with increasing miR-124-2 methylation, indicating their importance in determining disease severity [197,210].
Cervical carcinogenesis caused by HPV could depend on the dysregulation of methylation in both viral and host genomes. Knowing about these epigenetic changes opens doors for early diagnosis, prognosis, and treatment plans in HPV-related tumors.
3. Prevention and Management
Vaccination
Vaccination against HPV has the potential to prevent CC, as well as other disorders that are related with the virus [211]. In 2018, the World Health Organization advocated for global efforts to eradicate CC, establishing a benchmark of four cases per 100,000 women annually [212]. The most effective method of preventing HPV infection is the vaccine, which has been available to medical professionals in the US since 2006 and is now the standard of treatment [213,214].
Recent studies support the extension of the HPV vaccination from a strictly preventive action to a possible therapeutic adjuvant for individuals already infected. Particularly for the genotypes covered by the 9-valent HPV vaccination, the administration of the vaccination in a cohort of HPV-positive women was linked to a noticeably improved clearance rate of the infection. In vaccinated women, clearance rates reached 72.4%; in the unvaccinated cohort, they were 45.7%; and those who had also had surgical removal of high-grade lesions showed the most notable benefits. These data challenge the accepted limit of HPV vaccination to pre-exposure application and show a synergistic benefit when given post treatment, presenting a promising secondary prevention method for patients susceptible to reinfection or lesion recurrence [215].
Especially against high-risk carcinogenic types like HPV-16 and HPV-18, the HPV vaccination has shown notable subtype-specific efficacy. Data from 22 randomized controlled studies comprising more than 95,000 participants revealed a 46% relative decrease in CIN2+ lesions linked to HPV-16/18 and a 73% reduction in CIN3+ lesions, thus underlining the critical importance of the vaccination in the prevention of pre-cancerous cervical abnormalities. The immunological durability of the current vaccination formulations is highlighted by the long-term follow-up data showing continuous protection for over 12 years. These results support the case for broad immunization policy within comprehensive cervical cancer prevention campaigns and confirm the need for early vaccination before HPV exposure [216].
Although the Centers for Disease Control and Prevention has reaffirmed its earlier advice that children between the ages of 11 and 12 should receive the HPV vaccine, only 58.6% of adolescents followed the immunization schedule in the year 2020 [217,218,219].
Although there are now efficient HPV vaccines, reaching enough population-level immunity calls for focused campaigns to increase vaccination acceptance, especially among teenagers. Coverage has been raised with success by school-based immunization programs, community education, and provider advocacy. Attaching herd immunity to several HPV subtypes—especially those not covered by earlier vaccine generations—requires increasing vaccination rates. These treatments help to prevent main infections and lower the frequency of high-risk HPV strains by extending coverage and addressing sociocultural barriers, possibly benefiting persons already exposed by lowering their chances of reinfection or chronic infection [220].
4. Future Directions
4.1. Research Gaps
The adoption of preventative techniques is severely hampered by the low level of information that is available among individuals who are considering careers in the healthcare industry. It is vital to adopt a comprehensive array of measures to meet the World Health Organization’s goal of eradicating CC by the year 2030. These measures include the design of policies, education for high-risk groups, and the provision of training and information for healthcare professionals.
Since its introduction, both supporters and opponents of HPV vaccination have expressed their views [219]. The assertions made by anti-vaccination organizations on social media about the possible adverse effects of vaccines are unsupported by any data [221,222].
Negative coverage of HPV vaccination can cause parents to feel confused and anxious, which can lead to healthcare practitioners assuming that parents do not value the vaccine and create an awkward conversational environment. Understanding successful social media tactics is essential for promoting HPV vaccination and battling bad PR. This entails determining which unfavorable plotlines require attention, as well as when, how, and by whom they should be addressed.
Important gaps are those that deal with systemic strategies (for example, best practices for health insurers and plans), healthcare provider initiatives (for example, getting providers involved in quality improvement efforts in-clinic], or social media and vaccine confidence [for example, using social media to boost vaccine confidence). It is very important to create and test treatments in all these areas in order to fill in the gaps and find the best ways to increase the number of people who get vaccinated against HPV.
The quadrivalent preventive HPV vaccine has shown a 70% decrease in the occurrence of cervical and other HPV-associated cancers in young men and women, with a single dose offering protection for at least ten years [223].
The significance of early delivery is evidenced by the observation that anti-HPV antibody titers were 2–3 times greater in younger persons post immunization [224].
There are significant knowledge gaps among medical professionals on HPV infection, vaccination, and preventative strategies, according to several studies that were conducted in high-income countries [HICs], as well as low-income countries and middle-income countries. To achieve the long-term aims of lowering the number of cancers that are caused by HPV and the WHO’s vision of reaching the goal of eliminating CC by the year 2030, it is necessary to address these discrepancies. The success of immunization efforts will increase because of this, and will also make it possible to accomplish long-term objectives [225,226,227].
First things first, if we want to understand how medical schools and curricula influence the dissemination of accurate information on HPV prevention techniques, we need to evaluate the degree of knowledge that medical students and residents gain. It is imperative that this precaution be taken because of the rising incidence of non-CCs that are caused by HPV, the unwillingness to receive vaccines, and the poor participation rates in CC screening programs.
The analysis reveals major barriers to getting tested for CC, even though CC can be prevented in most cases by appropriate screening and immunization against HPV. Some of the most common concerns include the anticipated discomfort associated with the testing procedure (which was reported by as many as 63% of respondents in certain countries) and the discomfort of disclosing sexual history to healthcare professionals [which was reported by as many as 57% in various communities). These difficulties are further exacerbated in countries with poor incomes, which are locations where ninety percent of deaths from CC occur [14].
4.2. Public Health Implications
Implementing effective control and prevention measures, such as an HPV vaccination and improved screening, should bring about a reduction in the number of malignancies that are caused by HPV. Researchers make use of information obtained from the National Cancer Registry Program (NCRP) in order to assess the degree to which HPV-related measures have improved both outcomes and prognoses.
Healthcare institutions all over India have started to demand screenings for common disorders, such as oral and cervical cancers, as part of their attempts to limit the number of people who are diagnosed with cancer. The objective of this screening program is to bring the cancer rate down to a lower level [228].
Cancer registries maintained in hospitals and communities across India serve as vital sources for assessing the impact of primary (e.g., vaccination) and secondary (e.g., screening) prevention programs. A comprehensive understanding of the epidemiology and transmission dynamics of HPV-related cancers is important to guide preventive measures. Such insights are critical for nationwide efforts aimed at reducing the incidence of these cancers before they occur, ultimately improving public health outcomes. The purpose of this study is to investigate the spread of HPV-related cancers by evaluating data from India’s National Cancer Registry Program, Hospital-Based Cancer Registries (HBCRs), and Population-Based Cancer Registries (PBCRs) [229,230].
In light of the fact that precancerous lesions seldom manifest themselves, it is essential to undergo CC screening on an annual basis, even after receiving an HPV vaccine. A high-performance HPV test and at least two other tests should be administered to every individual before they reach the ages of 35 and 45, respectively, according to international policymakers who have mandated that this be carried out. At the age of thirty, women should begin having checks for CC every five to ten years. This screening should be performed more frequently. It is recommended that women who are HIV positive begin getting tested every three years once they reach the age of 25.
Women may want to collect their own HPV testing samples, because they have been confirmed to be just as reliable as those collected by medical professionals.
The early detection and treatment of CC could lead to a cure. Initially, one can identify the indicators through knowledge, and thereafter, if practicable, pursue medical aid to address any arising difficulties [14].
Usually, formal referral to treatment providers follows the confirmation of a diagnosis. The diagnosis process depends critically on clinical tests and investigations. The prevalence of HPV16/18 and the potential for cross-protection underscore the necessity of incorporating more affordable immunizations in underdeveloped countries.
According to the World Health Organization’s Global Strategy, which sets three targets for 2030, the goal is to reduce the annual incidence of new cases to four or fewer per 100,000 women. This approach directs all nations toward the eradication of the disease in the next decades:
- By the age of 15, 90% of females will have been administered the HPV vaccine;
- By the ages of 35 and 45, 70% of women will have received a high-quality screening;
- Ninety percent of women with cervical disease will receive treatment [14].
If this elimination goal is achieved, modeling indicates that 74 million new CC cases and 62 million fatalities could be prevented by 2120. Analysis of cervical cancer (CC) data compiled by the World Health Organization (WHO) and other international stakeholders highlights a collective global effort aimed at the elimination of cervical cancer and its related health burdens [14].
Identifying and inhibiting carcinogenic HPV genotypes has emerged as a crucial and efficient objective for CC prevention and care, thanks to the substantial decrease in the worldwide incidence of CC caused by HPV vaccines [9,16,231]. Pap smears and other HPV detection methods have been incorporated into standard cervical screening programs in industrialized countries, which has resulted in the complete elimination of invasive CC in these countries. Since the previous staining method was shown to be imprecise, it is reasonable to consider switching to liquid-based cytology [232,233].
The prophylactic HPV vaccine may serve as a cost-effective public health intervention in low- and middle-income countries, which are generally marked by inadequate quality screening programs [234,235]. Historically, the field’s reliance on Pap tests has slowed the implementation of this technology [236].
Because of inequalities in providing education, laboratory resources, and financing, primary HPV testing is not easily accessible to most people. One of the non-emergency treatments that the COVID-19 outbreak impacted is CC screening [237]. There are substantial obstacles to healthcare services, including HPV testing, for women living in low-income neighborhoods.
There have been certain countries in western Europe and sub-Saharan Africa that have started adopting self-sampling at home as an innovative alternative of the screening that takes place in medical facilities [238]. Women in the United States who do not have easy access to doctors or who are scared to obtain the requisite internal exam for screening would benefit tremendously from the broad adoption of this strategy [239].
In order to strike a balance between the inherent advantages of accessibility and the potential drawbacks of incorrect handling and sample collection, regulatory considerations and implementation plans would be required. The provision of instructions that are straightforward, based on images, and at a level of literacy that is functional will improve the conduciveness of the environment, particularly among the most vulnerable groups of women [14,230].
5. Conclusions
HPV stands as the primary viral cause of cervical cancer [CC], which maintains its status as a worldwide public health concern. Extensive research has been conducted to understand HPV-induced carcinogenesis at the molecular level, with particular attention given to the E6 and E7 oncogenes that drive tumorigenesis. Targeted therapeutic approaches against these oncoproteins have demonstrated promising results. However, recent research findings revealing the E5 oncogene’s role in cervical cancer progression indicate the need for broader molecular targeting in therapeutic development.
HPV oncoproteins, along with immune evasion mechanisms and epigenetic modifications, work together to create the complex process of cervical carcinogenesis. The transformation of cervical epithelial cells involves two primary molecular events, including alterations in miRNA expression and DNA methylation. Notably, the hypermethylation of tumor-suppressor miRNA promoters, resulting in the dysregulation of critical cellular pathways, has emerged as a central factor in the advancement of HPV-induced malignancies. Gene expression regulation in CC becomes more complex due to HPV’s ability to modify miRNA expression at critical genomic locations, which requires the development of new therapeutic approaches to address these epigenetic and molecular disruptions.
Research has successfully uncovered HPV-related CC molecular mechanisms, but the clinical application of these findings continues to be difficult. Today’s HPV treatments, including vaccinations and epigenetic inhibitors, encounter multiple barriers in their implementation, including accessibility issues, along with specificity limitations and possible unwanted side effects. Advanced delivery systems combined with epigenetic therapies show great potential for boosting therapeutic outcomes. Future research must focus on refining these approaches to ensure that they are both cost-effective and capable of reducing the global burden of CC.
The prevention of cervical cancer requires an approach that combines multiple protection layers. A preventive strategy must begin with HPV vaccination as the primary intervention, followed by the effective monitoring and treatment of precancerous lesions as the secondary prevention, and then the timely diagnosis and treatment of invasive cancer as the tertiary prevention. All individuals between 9 and 16 years of age must receive the HPV vaccine according to national immunization schedules, to prevent infection-related malignancies.
Medical education should incorporate comprehensive information on HPV, including its role in non-cervical cancers, to raise awareness, advocate for vaccination, and support healthcare professionals with the necessary knowledge to sustain HPV prevention efforts.
In conclusion, the reduction in CC incidence worldwide depends on improved global HPV vaccination programs and ongoing molecular and epigenetic research advancements.
Author Contributions
Conceptualization, A.C. and R.V.; methodology, A.C., L.A.P., and D.E.M.; validation, A.C.; writing—original draft preparation, A.C., L.A.P., N.L.M.-Z., and D.E.M.; writing—review and editing, A.C. and N.L.M.-Z.; visualization, A.C. supervision, A.C.; project administration, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The International Agency for Research on Cancer (IARC). 2022. Available online: https://www.iarc.who.int/cancer-type/cervical-cancer/;900-world-fact-sheet.pdf (accessed on 1 February 2025).
- National Cancer Institute. Cervical Cancer Causes, Risk Factors, and Prevention; National Cancer Institute: Washington, DC, USA, 2021.
- Asiaf, A.; Ahmad, S.T.; Mohammad, S.O.; Zargar, M.A. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur. J. Cancer Prev. 2014, 23, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; Murall, C.L.; Bravo, I.G. Why human papillomavirus acute infections matter. Viruses 2017, 9, 293. [Google Scholar] [CrossRef]
- Tan, S.C.; Ismail, M.P.; Duski, D.R.; Othman, N.H.; Ankathil, R. Prevalence and type distribution of human papillomavirus [HPV] in Malaysian women with and without cervical cancer: An updated estimate. Biosci. Rep. 2018, 38, BSR20171268. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Van Oort, M.G.; Coker, D.G.R.L. Clinical aspects of cervical cancer and HPV infection. J. Infect. Dis. 2012, 206, 965–971. [Google Scholar]
- Tomita, Y.; Koyama, M.; Tsukamoto, H. The molecular basis of cervical cancer progression. Infect. Agents Cancer 2011, 6, 33. [Google Scholar]
- Bao, Y.-P.; Li, N.; Smith, J.S.; Qiao, Y.-L. Human papillomavirus type distribution in women from Asia: A meta-analysis. Int. J. Gynecol. Cancer 2008, 18, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Mirabello, L.; Clarke, M.A.; Nelson, C.W.; Dean, M.; Wentzensen, N.; Yeager, M.; Cullen, M.; Boland, J.F.; Schiffman, M.; Burk, R.D. The Intersection of HPV Epidemiology, Genomics and Mechanistic Studies of HPV-Mediated Carcinogenesis. Viruses 2018, 10, 80. [Google Scholar] [CrossRef]
- Shalaby, N.A.; Tudorescu-Morjan, C.; Manole, C.G.; Iacata, A.-A.; Popovici, M.L.; Grecu, L.I.; Curici, A. Correlation between high-risk HPV infection and p16/Ki-67 abnormalities in Pap samples in a South Eastern Europe cohort. J. Med. Virol. 2024, 96, e29524. [Google Scholar] [CrossRef]
- Human Papillomavirus [HPV] Centre. HPV and Cervical Cancer Statistics; Human Papillomavirus [HPV] Centre: Barcelona, Spain, 2021. [Google Scholar]
- World Health Organization. Cervical Cancer; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Vaccarella, S.; Franceschi, S.; Herrero, R.; Muñoz, N.; Snijders, P.J.F.; Clifford, G.M.; Smith, J.S. Sexual Behavior, Condom Use, and Human Papillomavirus: Pooled Analysis of the IARC Human Papillomavirus Prevalence Surveys. Cancer Epidemiol. Biomark. Prev. 2006, 15, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Muñoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.M.; Lopez, C.C.; Munteanu, D.M.; and Rivas, E.J. Identification of predictive factors for human papillomavirus clearance in women aged 25–40. BMC Med. 2023, 21, 43. [Google Scholar]
- Lee, S.; Park, W.K.; Lee, J.W. The risk factors and clinical outcomes of cervical cancer patients with HPV infections. Cancer Manag. Res. 2019, 11, 529–534. [Google Scholar]
- Costa, M.; Rosa, T.C.; Silva, J.G. Genetic basis of cervical cancer development. Genet. Mol. Res. 2015, 14, 14672–14681. [Google Scholar]
- Park, S.; Lee, J.K.; Kim, C.J. Molecular mechanisms of cervical carcinogenesis. Carcinogenesis 2014, 35, 1430–1443. [Google Scholar]
- Lee, J.W.; Park, W.K.; Lee, S. HPV and its role in cervical cancer. Asian Pac. J. Cancer Prev. 2012, 13, 5489–5496. [Google Scholar]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 38. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Erickson, B.K.; Alvarez, R.D.; Huh, W.K. Human papillomavirus: What every provider should know. Am. J. Obstet. Gynecol. 2013, 208, 169–175. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Franceschi, S.; Diaz, M.; Muñoz, N.; Villa, L.L. HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006, 24 (Suppl. S3), S3/26–S3/34. [Google Scholar] [CrossRef]
- van Hamont, D.; van Ham, M.A.P.C.; Bakkers, J.M.J.E.; Massuger, L.F.A.G.; Melchers, W.J.G. Evaluation of the SPF10-INNO LiPA human papillomavirus [HPV] genotyping test and the Roche Linear Array HPV genotyping test. J. Clin. Microbiol. 2006, 44, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of multiple high-risk human papillomavirus [HPV] infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Vaziri, M.; Sadeghi, F.; Hashemi, F.S.; Haeri, H.; Bokharaei-Salim, F.; Monavari, S.H.; Keyvani, H. Distribution of Human Papillomavirus Genotypes in Iranian Women According to the Severity of the Cervical Lesion. Iran. Red. Crescent Med. J. 2016, 18, e24458. [Google Scholar] [CrossRef]
- You, W.; Li, S.; Du, R.; Zheng, J.; Shen, A. Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp. Ther. Med. 2018, 15, 412–418. [Google Scholar] [CrossRef]
- Sotlar, K.; Stubner, A.; Diemer, D.; Menton, S.; Menton, M.; Dietz, K.; Wallwiener, D.; Kandolf, R.; Bültmann, B. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J. Med. Virol. 2004, 74, 107–116. [Google Scholar] [CrossRef]
- Castle, P.E.; Solomon, D.; Schiffman, M.; Wheeler, C.M. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J. Natl. Cancer Inst. 2005, 97, 1066–1071. [Google Scholar] [CrossRef]
- Bonnez, W.; Reichman, R.C. Papillomaviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 5th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingston: Philadelphia, PA, USA, 2000; pp. 1630–1640. [Google Scholar]
- Weng, S.L.; Huang, K.Y.; Weng, J.T.Y.; Hung, F.Y.; Chang, T.H.; Lee, T.Y. Genome-wide discovery of viral microRNAs based on phylogenetic analysis and structural evolution of various human papillomavirus subtypes. Brief. Bioinform. 2018, 19, 1102–1114. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and Taxonomic Classification of Alphapapillomavirus 7 Complete Genomes: HPV18, HPV39, HPV45, HPV59, HPV68 and HPV70. PLoS ONE 2013, 8, e72565. [Google Scholar] [CrossRef]
- Ong, C.K.; Chan, S.Y.; Campo, M.S.; Fujinaga, K.; Mavromara-Nazos, P.; Labropoulou, V.; Pfister, H.; Tay, S.K.; ter Meulen, J.; Villa, L.L. Evolution of human papillomavirus type 18: An ancient phylogenetic root in Africa and intratype diversity reflect coevolution with human ethnic groups. J. Virol. 1993, 67, 6424–6431. [Google Scholar] [CrossRef]
- Calleja-Macias, I.E.; Kalantari, M.; Allan, B.; Williamson, A.; Chung, L.; Collins, R.J.; Zuna, R.E.; Dunn, S.T.; Ortiz-Lopez, R.; Barrera-Saldaña, H.A.; et al. Papillomavirus Subtypes Are Natural and Old Taxa: Phylogeny of Human Papillomavirus Types 44 and 55 and 68a and -b. J. Virol. 2005, 79, 6565–6569. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/35460940/ (accessed on 5 February 2025).
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef]
- Baker, T.S.; Newcomb, W.W.; Olson, N.H.; Cowsert, L.M.; Olson, C.; Brown, J.C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 1991, 60, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Favre, M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J. Virol. 1975, 15, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A.; Pett, M.R.; Coleman, N. HPV: From infection to cancer. Biochem. Soc. Trans. 2007, 35, 1456–1460. [Google Scholar] [CrossRef]
- Torrisi, A.; Del Mistro, A.; Onnis, G.L.; Merlin, F.; Bertorelle, R.; Minucci, D. Colposcopy, cytology and HPV testing in HIV-positive and HIV-negative women. Eur. J. Gynecol. Oncol. 2000, 21, 168–172. [Google Scholar]
- Apt, D.; Watts, R.M.; Suske, G.; Bernard, U. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transcription correlate with the activation of the HPV-16 promoter. Virology 1996, 224, 281–291. [Google Scholar] [CrossRef]
- Sapp, M.; Volpers, C.; Muller, M.; Streck, R.E. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J. Gen. Virol. 1995, 76, 2407–2412. [Google Scholar] [CrossRef]
- De Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obs. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kundu, R. Human papillomavirus E6 and E7: The cervical cancer hallmarks and targets for therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A.R. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378. [Google Scholar]
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; Upadhye, V.J.; et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675. [Google Scholar] [CrossRef]
- Berg, M.; Stenlund, A. Functional interactions between papillomavirus E1 and E2 proteins. J. Virol. 1997, 71, 3853–3863. [Google Scholar] [CrossRef]
- Bergvall, M.; Melendy, T.; Archambault, J. The E1 proteins. Virology 2013, 445, 35–56. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Manzo-Merino, J.; Muñoz-Bello, J.O.; Olmedo-Nieva, L.; Cedro-Tanda, A.; Alfaro-Ruiz, L.A.; Hidalgo-Miranda, A.; Madrid-Marina, V.; Lizano, M. The Human Papillomavirus (HPV) E1 protein regulates the expression of cellular genes involved in immune response. Sci. Rep. 2019, 9, 13620. [Google Scholar] [CrossRef]
- Chojnacki, M.; Melendy, T. The human papillomavirus DNA helicase E1 binds, stimulates, and confers processivity to cellular DNA polymerase epsilon. Nucl. Acids Res. 2018, 46, 229–241. [Google Scholar] [CrossRef]
- D’Abramo, C.; Archambault, J. Small molecule inhibitors of human papillomavirus protein-protein interactions. Open Virol. J. 2011, 5, 80. [Google Scholar] [CrossRef]
- Goodwin, E.C.; DiMaio, D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 12513–12518. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- White, P.W.; Pelletier, A.; Brault, K.; Titolo, S.; Welchner, E.; Thauvette, L.; Fazekas, M.; Cordingley, M.G.; Archambault, J. Characterization of recombinant HPV6 and 11 E1 helicases: Effect of ATP on the interaction of E1 with E2 and mapping of a minimal helicase domain. J. Biol. Chem. 2001, 276, 22426–22438. [Google Scholar] [CrossRef] [PubMed]
- White, P.W.; Titolo, S.; Brault, K.; Thauvette, L.; Pelletier, A.; Welchner, E.; Bourgon, L.; Doyon, L.; Ogilvie, W.W.; Yoakim, C. Inhibition of human papillomavirus DNA replication by small molecule antagonists of the E1–E2 protein interaction. J. Biol. Chem. 2003, 278, 26765–26772. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular pathways and targets. Curr. Probl. Cancer 2018, 42, 161–174. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Gonzal’ez-García, M.C.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Valverde, M.; Rojas, E.; Rodríguez-Sastre, M.A.; García-Cuellar, C.M.; Lizano, M. Human papillomavirus types 16 and 18 early-expressed proteins differentially modulate the cellular redox state and DNA damage. Int. J. Biol. Sci. 2018, 14, 21. [Google Scholar] [CrossRef]
- Gupta, S.M.; Mania-Pramanik, J. Retracted Article: Molecular mechanisms in progression of HPV-associated cervical carcinogenesis. J. Biomed. Sci. 2019, 26, 28. [Google Scholar] [CrossRef]
- Zahra, K.; Patel, S.; Dey, T.; Pandey, U.; Mishra, S.P. A study of oxidative stress in cervical cancer-an institutional study. Biochem. Biophys. Rep. 2021, 25, 100881. [Google Scholar] [CrossRef] [PubMed]
- Sheila, V. Graham Human Papillomavirus E2 Protein: Linking Replication, Transcription, and RNA Processing. J. Virol. 2016, 90, 8384–8388. [Google Scholar] [CrossRef]
- Khan, S.A. Plasmid rolling-circle replication: Recent developments. Mol. Microbiol. 2000, 37, 477–484. [Google Scholar] [CrossRef]
- Akagi, K.; Symer, D.E.; Mahmoud, M.; Goodwin, S.; Wangsa, D.; Li, Z.; Xiao, W.; Dunn, J.D.; Ried, T.; Coombes, K.R.; et al. Intratumoral heterogeneity and clonal evolution induced by HPV integration. Cancer Discov. 2023, 13, 910–927. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Steenbergen, R.D.M.; Pumpe, A.; Kenyon, A.N.; Melchers, W.J.G. HPV integration and cervical cancer: A failed evolutionary viral trait. Trends Mol. Med. 2024, 30, 890–902. [Google Scholar] [CrossRef]
- Sui, S.; Jiao, Z.; Chen, H.; Niyazi, M.; Wang, L. Association between APOBEC3s and HPV16 E2 gene hypermutation in Uygur females with cervical cancer. Oncol. Lett. 2020, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Lagström, S.; Lovestad, A.H.; Umu, S.U.; Ambur, O.H.; Nygard, M.; Rouge, T.B.; Kraus Christiansen, I. HPV16 and HPV18 type-specific APOBEC3 and integration profiles in different diagnostic categories of cervical samples. Tumour Virus Res. 2021, 12, 200221. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Wang, T.; He, D.; Tian, R.; Cui, Z.; Tian, X.; Gao, Q.; Ma, X.; Yang, J.; et al. In vitro and in vivo growth inhibition of human cervical cancer cells via human papillomavirus E6/E7 mRNAs’ cleavage by CRISPR/Cas13a system. Antivir. Res. 2020, 178, 104794. [Google Scholar] [CrossRef]
- Tian, R.; Liu, J.; Fan, W.; Li, R.; Cui, Z.; Jin, Z.; Huang, Z.; Xie, H.; Li, L.; Huang, Z.; et al. Gene knock-out chain reaction enables high disruption efficiency of HPV18 E6/E7 genes in cervical cancer cells. Mol. Ther. Oncolytics 2022, 24, 171–179. [Google Scholar] [CrossRef]
- Paolini, F.; Amici, C.; Carosi, M.; Bonomo, C.; Di Bonito, P.; Venuti, A.; Accardi, L. Intrabodies targeting human papillomavirus 16 E6 and E7 oncoproteins for therapy of established HPV associated tumors. J. Exp. Clin. Cancer Res. 2021, 40, 37. [Google Scholar] [CrossRef]
- Doorbar, J. The E4 protein; structure, function and patterns of expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef]
- Doorbar, J.; Medcalf, E.; Napthine, S. Analysis of HPV1 E4 complexes and their association with keratinsin vivo. Virology 1996, 218, 114–126. [Google Scholar] [CrossRef]
- Khan, J.; Davy, C.E.; McIntosh, P.B.; Jackson, D.J.; Hinz, S.; Wang, Q.; Doorbar, J. Role of calpain in the formation of human papillomavirus type 16 E1^ E4 amyloid fibers and reorganization of the keratin network. J. Virol. 2011, 85, 9984–9997. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, P.B.; Laskey, P.; Sullivan, K.; Davy, C.; Wang, Q.; Jackson, D.J.; Griffin, H.M.; Doorbar, J. E1^E4-mediated keratin phosphorylation and ubiquitylation: Amechanism for keratin depletion in HPV16-infected epithelium. J. Cell Sci. 2010, 123, 2810–2822. [Google Scholar] [CrossRef]
- Roberts, S.; Hillman, M.L.; Knight, G.L.; Gallimore, P.H. The ND10 component promyelocytic leukemia protein relocates to human papillomavirus type 1 E4 intranuclear inclusion bodies in cultured keratinocytes and in warts. J. Virol. 2003, 77, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Rogel-Gaillard, C.; Pehau-Arnaudet, G.; Breitburd, F.; Orth, G. Cytopathic effect in human papillomavirus type 1–Induced inclusion warts: In vitro analysis of the contribution of two forms of the viral E4 protein. J. Investig. Dermatol. 1993, 101, 843–851. [Google Scholar] [CrossRef]
- Wang, Q.; Kennedy, A.; Das, P.; McIntosh, P.B.; Howell, S.A.; Isaacson, E.R.; Hinz, S.A.; Davy, C.; Doorbar, J. Phosphorylation of the human papillomavirus type 16 E1^ E4 protein at T57 by ERK triggers a structural change that enhances keratin binding and protein stability. J. Virol. 2009, 83, 3668–3683. [Google Scholar] [CrossRef]
- Kim, S.-H.; Oh, J.-M.; No, J.-H.; Bang, Y.-J.; Juhnn, Y.-S.; Song, Y.-S. Involvement of NF-κB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis 2009, 30, 753–757. [Google Scholar] [CrossRef]
- Deng, W.; Lin, B.Y.; Jin, G.; Wheeler, C.G.; Ma, T.; Harper, J.W.; Broker, T.R.; Cho, L.T. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 2004, 78, 13954–13965. [Google Scholar] [CrossRef]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 drive alternative carcinogenic pathways in HPV positive cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef] [PubMed]
- Crook, T.; Vousden, K.H.; Tidy, J.A. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell 1991, 67, 547–556. [Google Scholar] [CrossRef]
- Ganguly, N. Human papillomavirus-16 E5 protein: Oncogenic role and therapeutic value. Cell. Oncol. 2012, 35, 67–76. [Google Scholar] [CrossRef]
- Wang, W.; Sima, N.; Kong, D.; Luo, A.; Gao, Q.; Liao, S.; Li, W.; Han, L.; Wang, J.; Wang, S. Selective targeting of HPV-16 E6/E7 in cervical cancer cells with a potent oncolytic adenovirus and its enhanced effect with radiotherapy in vitro and vivo. Cancer Lett. 2010, 291, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.H.; Haghshenas, M.R.; Marchetti, B.; O’Brien, P.M.; Campo, M.S. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer 2005, 113, 276–283. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 protein of human papillomavirus 16 downregulates HLA class Iand interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 2006, 119, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Vizcaino, G.; Sánchez-Torres, C.; Lizano, M. The Natural History of Cervical Cancer and the Case for MicroRNAs: Is Human Papillomavirus Infection the Whole Story? Int. J. Mol. Sci. 2024, 25, 12991. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus [HPV] infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Vallejo-Ruiz, V.; Gutiérrez-Xicotencatl, L.; Medina-Contreras, O.; Lizano, M. Molecular aspects of cervical cancer: A pathogenesis update. Front. Oncol. 2024, 14, 1356581. [Google Scholar] [CrossRef]
- Celegato, M.; Messa, L.; Bertagnin, C.; Mercorelli, B.; Loregian, A. Targeted disruption of E6/p53 binding exerts broad activity and synergism with paclitaxel and topotecan against HPV-transformed cancer cells. Cancers 2022, 14, 193. [Google Scholar] [CrossRef]
- De Nola, R.; Loizzi, V.; Cicinelli, E.; Cormio, G. Dynamic crosstalk within the tumor microenvironment of uterine cervical carcinoma: Baseline network, iatrogenic alterations, and translational implications. Crit. Rev. Oncol. Hematol. 2021, 162, 103343. [Google Scholar] [CrossRef]
- Vats, A.; Skrabar, N.; Del Sal, G.; Banks, L. Loss of the E6AP ubiquitin ligase induces p53-dependent phosphorylation of HPV-18 E6 in cells derived from cervical cancer. J. Virol. 2022, 96, e0150321. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, L.; Zuo, L.; Gao, S.; Jiang, X.; Han, Y.; Lin, J.; Peng, M.; Wu, N.; Tang, Y.; et al. HPV E6/E7: Insights into their regulatory role and mechanism in signaling pathways in HPV-associated tumor. Cancer Gene Ther. 2024, 31, 9–17. [Google Scholar] [CrossRef]
- Branca, M.; Ciotti, M.; Santini, D.; Di Bonito, L.; Benedetto, A.; Giorgi, C.; Paba, P.; Favalli, C.; Costa, S.; Agarossi, A. Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am. J. Clin. Pathol. 2004, 122, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nature reviews. Clin. Oncol. 2013, 10, 143–153. [Google Scholar]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Pol, S.V.; Podjarny, A. Structure of the E6/ E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Kato, I.; Kim, H.-R.C. A novel function of HPV16-E6/E7 in epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 2013, 435, 339–344. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Kim, S.; Choi, Y.-L.; Cho, Y.J.; Oh, E.; Choi, J.-J.; Jung, K.; Song, J.-Y.; Ahn, S.E.; Kim, B.-G. HER2 as a novel therapeutic target for cervical cancer. Oncotarget 2015, 6, 36219. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lin, B.; Liu, X.; Zhang, W.; Zhang, E.; Hu, L.; Ma, Y.; Li, X.; Tang, X. ERK signaling pathway is involved in HPV-16 E6 but not E7 oncoprotein-induced HIF-1α protein accumulation in NSCLC cells. Oncol. Res. 2016, 23, 109. [Google Scholar] [CrossRef]
- Jones, D.L.; Münger, K. Interactions of the human papillomavirus E7 protein with cell cycle regulators. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 327–337. [Google Scholar]
- Kennedy, E.M.; Kornepati, A.V.; Goldstein, M.; Bogerd, H.P.; Poling, B.C.; Whisnant, A.W.; Kastan, M.B.; Cullen, B.R. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014, 88, 11965–11972. [Google Scholar] [CrossRef]
- Chen, X.; He, H.; Xiao, Y.; Hasim, A.; Yuan, J.; Ye, M.; Li, X.; Hao, Y.; Guo, X. CXCL10 Produced by HPV-Positive Cervical Cancer Cells Stimulates Exosomal PDL1 Expression by Fibroblasts via CXCR3 and JAK-STAT Pathways. Front. Oncol. 2021, 11, 629350. [Google Scholar] [CrossRef]
- Huang, X.; Huo, L.; Xiao, B.; Ouyang, Y.; Chen, F.; Li, J.; Zheng, X.; Wei, D.; Wu, Y.; Zhang, R.; et al. Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer. Cell Death Differ. 2024, 31, 78–89. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Hemmat, N.; Baradaran, B.; Mokhtarzadeh, A.; Poortahmasebi, V.; Oskuee, M.A.; Baghi, H.B. The effect of Wnt/β-catenin signaling on PD-1/PDL-1 axis in HPV-related cervical cancer. Oncol. Res. 2023, 30, 99–116. [Google Scholar] [CrossRef]
- Hu, C.; Liu, T.; Han, C.; Xuan, Y.; Jiang, D.; Sun, Y.; Zhang, X.; Zhang, W.; Xu, Y.; Liu, Y.; et al. HPV E6/E7 promotes aerobic glycolysis in cervical cancer by regulating IGF2BP2 to stabilize m6A-MYC expression. Int. J. Biol. Sci. 2022, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Taguchi, A.; Sone, K.; Mori, M.; Osuga, Y. Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int. J. Clin. Oncol. 2023, 28, 965–974. [Google Scholar] [CrossRef]
- Singini, M.G.; Singh, E.; Bradshaw, D.; Ramaliba, T.; Chen, W.C.; Motlhale, M.; Kamiza, A.B.; de Villiers, C.B.; Muchengeti, M.; Mathew, C.G.; et al. Usefulness of high-risk HPV early oncoprotein [E6 and E7] serological markers in the detection of cervical cancer: A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e27900. [Google Scholar] [CrossRef]
- Balaji, D.; Kalarani, I.B.; Mohammed, V.; Veerabathiran, R. Potential role of human papillomavirus proteins associated with the development of cancer. Virus Dis. 2022, 33, 322–333. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keifer, T.R.; Sapp, M. Human papillomavirus major capsid protein L1 remains associated with the incoming viral genome throughout the entry process. J Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Caodaglio, A.S.; Sichero, L. Regulation of HPV transcription. Clinics 2018, 73, e486s. [Google Scholar] [CrossRef]
- Song, Z.; Ding, L.; Ren, Z.; Sun, X.; Yang, Q.; Wang, L.; Feng, M.; Liu, C.; Wang, J. Effects of Src on cervical cancer cells proliferation and apoptosis through ERK signal transduction pathway. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2017, 38, 1246–1251. [Google Scholar] [PubMed]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Brand, T.M.; Iida, M.; Corrigan, K.L.; Braverman, C.M.; Coan, J.P.; Flanigan, B.G.; Stein, A.P.; Salgia, R.; Rolff, J.; Kimple, R.J. The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor. Sci. Signal. 2017, 10, eaag1064. [Google Scholar] [CrossRef]
- Manzo-Merino, J.; Contreras-Paredes, A.; V’azquez-Ulloa, E.; Rocha-Zavaleta, L.; Fuentes-Gonzalez, A.M.; Lizano, M. The role of signaling pathways in cervical cancer and molecular therapeutic targets. Arch. Med. Res. 2014, 45, 525–539. [Google Scholar] [CrossRef]
- Talora, C.; Cialfi, S.; Segatto, O.; Morrone, S.; Choi, J.K.; Frati, L.; Dotto, G.P.; Gulino, A.; Screpanti, I. Constitutively active Notch1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathways. Exp. Cell Res. 2005, 305, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Moon, R.T.; Kohn, A.D.; De Ferrari, G.V.; Kaykas, A. WNT and β-catenin signalling: Diseases and therapies. Nat. Rev. Gen. 2004, 5, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavalu, K.; Subbaiah, V.K.; Srivastava, S.; Chakrabarti, O.; Syal, R.; Krishna, S. Complementation of human papillomavirus type 16 E6 and E7 by Jagged1-specific Notch1-phosphatidylinositol 3-kinase signaling involves pleiotropic oncogenic functions independent of CBF1; Su [H]; Lag-1 activation. J. Virol. 2005, 79, 7889–7898. [Google Scholar] [CrossRef]
- Menges, C.W.; Baglia, L.A.; Lapoint, R.; McCance, D.J. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006, 66, 5555–5559. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.A.; Ahmad, A.; Farooq, N.; Priya, M.V.; Siddiqui, A.H.; Asif, M.; Manzoor, R.; Ishqi, H.M.; Alomar, S.Y.; Rahaman, P.F. Increased expression of HPV-E7 oncoprotein correlates with a reduced level of pRb proteins via high viral load in cervical cancer. Sci. Rep. 2023, 13, 15075. [Google Scholar] [CrossRef]
- Morgan, E.L.; Macdonald, A. Manipulation of JAK/STAT signalling by high-risk HPVs: Potential therapeutic targets for HPV-associated malignancies. Viruses 2020, 12, 977. [Google Scholar] [CrossRef]
- Cheng, Y.-M.; Chou, C.-Y.; Hsu, Y.-C.; Chen, M.-J. Influence of HPV16 E6/7 on the expression of FGF2 and FGFR type B in cervical carcinogenesis. Reprod. Sci. 2012, 19, 580–586. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef]
- McBride, A.A. Oncogenic human papillomaviruses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 2016027397. [Google Scholar] [CrossRef]
- Kusakabe, M.; Taguchi, A.; Tanikawa, M.; Wagatsuma, R.; Yamazaki, M.; Tsuchimochi, S.; Toyohara, Y.; Kawata, A.; Baba, S.; Uen, T.; et al. Cells with stem-like properties are associated with the development of HPV18-positive cervical cancer. Cancer Sci. 2023, 114, 885–895. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.L.; Cho, W.C.S.; Yee, B.K.; Kwan, Y.W.; Tai, W.C.S. Exosomes in inflammation and inflammatory disease. Proteomics 2019, 19, 1800149. [Google Scholar] [CrossRef]
- Chow, A.; Zhou, W.; Liu, L.; Fong, M.Y.; Champer, J.; Van Haute, D.; Chin, A.R.; Ren, X.; Gugiu, B.G.; Meng, Z.; et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci. Rep. 2014, 4, 5750. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Zhang, B.; Shi, H.; Yuan, X.; Sun, Y.; Pan, Z.; Qian, H.; Xu, W. Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumor Biol. 2016, 37, 12169–12180. [Google Scholar] [CrossRef]
- Nahand, J.S.; Moghoofei, M.; Salmaninejad, A.; Bahmanpour, Z.; Karimzadeh, M.; Nasiri, M.; Mirzaei, H.R.; Pourhanifeh, M.H.; Bokharaei-Salim, F.; Mirzaei, H.; et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. Int. J. Cancer 2020, 146, 305–320. [Google Scholar] [CrossRef]
- Miller, L.S.; Modlin, R.L. Human keratinocyte Toll-like receptors promote distinct immune responses. J. Investig. Dermatol. 2007, 127, 262–263. [Google Scholar] [CrossRef]
- Kovachev, A.M. A Review on Inosine Pranobex Immunotherapy for Cervical HPV-Positive Patients. Infect. Drug Resist. 2021, 14, 2039–2049. [Google Scholar] [CrossRef]
- Le Borgne, M.; Etchart, N.; Goubier, A.; Lira, S.A.; Sirard, J.C.; van Rooijen, N.; Caux, C.; Ait-Yahia, S.; Vicari, A.; Kaiserlian, D.; et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 2006, 24, 191–201. [Google Scholar] [CrossRef]
- Smola, S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses 2017, 9, 254. [Google Scholar] [CrossRef]
- Singh, M.; Thakral, D.; Rishi, N.; Kar, H.K.; Mitra, D.K. Functional characterization of CD4 and CD8 T cell responses among human papillomavirus infected patients with anogenital warts. Virus Dis. 2017, 28, 133–140. [Google Scholar] [CrossRef]
- Dugué, P.A.; Rebolj, M.; Garred, P.; Lynge, E. Immunosuppression and risk of cervical cancer. Expert Rev Anticancer Ther. 2013, 13, 29–42. [Google Scholar] [CrossRef]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 delivered by engineered exosomes elicits a protective CD8[+] T cell-mediated immune response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.C.; Zhang, Z.S.; Chen, F. MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3719–3726. [Google Scholar]
- Torres-Poveda, K.; Bahena-Román, M.; Madrid-González, C.; Burguete-García, A.I.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753. [Google Scholar] [CrossRef]
- Paradkar, P.H.; Joshi, J.V.; Mertia, P.N.; Agashe, S.V.; Vaidya, R.A. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 3851–3864. [Google Scholar] [CrossRef]
- Kawachi, A.; Yoshida, H.; Kitano, S.; Ino, Y.; Kato, T.; Hiraoka, N. Tumor-associated CD204+ M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018, 109, 863–870. [Google Scholar] [CrossRef]
- Chen, X.J.; Han, L.F.; Wu, X.G.; Wei, W.-F.; Wu, L.-F.; Yi, H.-Y.; Yan, R.-M.; Bai, X.-Y.; Zhong, M.; Yu, Y.-H.; et al. Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J. Cancer 2017, 8, 3868–3875. [Google Scholar] [CrossRef]
- NK Cells Directly Kill Their Targets by Releasing Cytotoxic Granules (Perforin and Granzymes) and Pro-Inflammatory Cytokines Such as IFN-γ [Which Activate the Broader Immune Response] upon Activation. Available online: www.frontiersin.org (accessed on 15 March 2025).
- Mah, A.Y.; Cooper, M.A. Metabolic Regulation of Natural Killer Cell IFN- Production. Crit. Rev. Immunol. 2016, 36, 131–147. [Google Scholar] [CrossRef]
- Ferns, D.M.; Heeren, A.M.; Samuels, S.; Bleeker, M.C.G.; de Gruijl, T.D.; Kenter, G.G.; Jordanova, E.S. Classical and Non-Classical HLA Class I Aberrations in Primary Cervical Squamous- and Adenocarcinomas and Paired Lymph Node Metastases. J. Immunother. Cancer 2016, 4, 78. [Google Scholar] [CrossRef]
- Cho, H.; Chung, J.-Y.; Kim, S.; Braunschweig, T.; Kang, T.H.; Kim, J.; Chung, E.J.; Hewitt, S.M.; Kim, J.-H. MICA/B and ULBP1 NKG2D Ligands Are Independent Predictors of Good Prognosis in Cervical Cancer. BMC Cancer 2014, 14, 957. [Google Scholar] [CrossRef]
- Utami, T. NK-Cell Count and Its Function in Producing Interferon Gamma Associated with the Cervical Cancer Natural History. Glob. J. Reprod. Med. 2018, 8, 1000462. [Google Scholar]
- Zhu, M.; Wang, J.; Zhang, Y.; Li, Y. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. [Google Scholar] [CrossRef]
- Conesa-Zamora, P. Immune responses against virus and tumor in cervical carcinogenesis: Treatment strategies for avoiding the HPV-induced immune escape. Gynecol. Oncol. 2013, 131, 480–488. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Broker, T.R.; Chow, L.T.; Alvarez, R.D.; Vu, H.L.; Andrasi, J.; Brewer, L.R.; Jin, G. Immune responses to human papillomavirus in genital tract of women with cervical cancer. Gynecol. Oncol. 2005, 96, 452–461. [Google Scholar] [CrossRef]
- Paaso, A.; Jaakola, A.; Syrjänen, S.; Louvanto, K. From HPV infection to lesion progression: The role of HLA alleles and host immunity. Acta Cytol. 2019, 63, 148–158. [Google Scholar] [CrossRef]
- Kojima, S.; Kawana, K.; Tomio, K.; Taguchi, A.; Miura, S.; Adachi, K.; Nagamatsu, T.; Nagasaka, K.; Matsumoto, Y.; Arimoto, T.; et al. The prevalence of cervical regulatory T cells in HPV-related cervical intraepithelial neoplasia [CIN] correlates inversely with spontaneous regression of CIN. Am. J. Reprod. Immunol. 2013, 69, 134–141. [Google Scholar] [CrossRef]
- Szöke, K.; Szalmás, A.; Szládek, G.; Veress, G.; Gergely, L.; Tóth, F.D.; Kónya, J. IL-10 promoter nt -1082A/G polymorphism and human papillomavirus infection in cytologic abnormalities of the uterine cervix. J. Interferon. Cytokine. Res. 2004, 24, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mittal, R.D.; Srivastava, M.; Srivastava, K.; Singh, S.; Srivastava, S.; Mittal, B. Impact of toll-like receptors [TLR] 4 gene polymorphisms in susceptibility of cervical cancer in North Indian women. Gynecol. Oncol. 2009, 114, 501–505. [Google Scholar] [CrossRef]
- Storey, A.; Thomas, M.; Kalita, A.; Harwood, C.; Gardiol, D.; Mantovani, F.; Breuer, J.; Leigh, I.M.; Matlashewski, G.; Banks, L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 1998, 393, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Tayeb, F.J.; Barnawi, J.; Jalal, M.M.; Saeedi, N.H.; Hamadi, A.; Altayar, M.A.; Alshammari, S.E.; Mtiraoui, N.; Ali, M.E.; et al. Biochemical Characterization and Molecular Determination of Estrogen Receptor-α (ESR1 PvuII-rs2234693 T>C) and MiRNA-146a (rs2910164 C>G) Polymorphic Gene Variations and Their Association with the Risk of Polycystic Ovary Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 3114. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Pathology and epidemiology of HPV infection in females. Gynecol. Oncol. 2010, 117, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Morales, V.; Peralta-Zaragoza, O.; Alcocer-González, J.; Moreno, J.; Madrid-Marina, V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol. Med. Rep. 2011, 4, 369–375. [Google Scholar] [PubMed]
- Chauhan, P.; Pramodh, S.; Hussain, A.; Elsori, D.; Lakhanpal, S.; Kumar, R.; Alsaweed, M.; Iqbal, D.; Pandey, P.; Al Othaim, A.; et al. Understanding the role of miRNAs in cervical cancer pathogenesis and therapeutic responses. Front. Cell Dev. Biol. 2024, 12, 1397945. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in breastmilk and the lactating breast: Potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef] [PubMed]
- Mok, E.T.Y.; Chitty, J.L.; Cox, T.R. miRNAs in pancreatic cancer progression and metastasis. Clin. Exp. Metastasis 2024, 41, 163–186. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, S.; Liu, P.; Wang, J.; Du, H. Potential role of microRNAs in the treatment and diagnosis of cervical cancer. Cancer Genet. 2020, 248–249, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-M.; Wang, X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim. Biophys. Acta 2011, 1809, 668–677. [Google Scholar] [CrossRef]
- Zhang, Y.; Dakic, A.; Chen, R.; Dai, Y.; Schlegel, R.; Liu, X. Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation. Oncotarget 2017, 8, 96323–96339. [Google Scholar] [CrossRef]
- Au Yeung, C.L.; Tsang, T.Y.; Yau, P.L.; Kwok, T.T. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 2011, 30, 2401–2410. [Google Scholar] [CrossRef]
- Ivanov, M.K.; Titov, S.E.; Dzyubenko, V.V.; Glushkov, S.A.; Krasilnikov, S.E.; Mansurova, A.S.; Malek, A.V.; Berlev, I.V.; Prisyazhnaya, T.S.; Kuleshova, S.V.; et al. Detection of Cervical Lesions and Cancer in Air-Dried Cytologic Smears by Combined Analysis of mRNA and miRNA Expression Levels. J. Mol. Diagn. 2021, 23, 541–554. [Google Scholar] [CrossRef]
- Greco, D.; Kivi, N.; Qian, K.; Leivonen, S.K.; Auvinen, P.; Auvinen, E. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS ONE 2011, 6, e21646. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Geng, L.; Zhao, L.; Zuo, P.; Wang, J. Human papillomavirus E6-regulated microRNA-20b promotes invasion in cervical cancer by targeting tissue inhibitor of metalloproteinase 2. Mol. Med. Rep. 2017, 16, 5464–5470. [Google Scholar] [CrossRef]
- Pedroza-Torres, A.; López-Urrutia, E.; García-Castillo, V.; Jacobo-Herrera, N.; Herrera, L.A.; Peralta-Zaragoza, O.; López-Camarillo, C.; De Leon, D.C.; Fernández-Retana, J.; Cerna-Cortés, J.F.; et al. MicroRNAs in cervical cancer: Evidences for a miRNA profile deregulated by HPV and its impact on radio-resistance. Molecules 2014, 19, 6263–6281. [Google Scholar] [CrossRef]
- Melar-New, M.; Laimins, L.A. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J. Virol. 2010, 84, 5212–5221. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Chiou, S.-H.; Su, Y. Maintenance of the stemness in CD44[+] HCT-15 and HCT-116 human colon cancer cells requires miR-203 suppression. Stem Cell Res. 2014, 12, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Fullár, A.; Karászi, K.; Hollósi, P.; Lendvai, G.; Reszegi, L.O.A.; Papp, Z.; Sobel, G.; Dudás, J.; Kovalszky, I. Two ways of epigenetic silencing of TFPI2 in cervical cancer. PLoS ONE 2020, 15, e0234873. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Rao, Q.; Liu, L.; Zheng, C.; Xie, Q.; Liang, J.; Lin, Z. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in cervical cancer. Virol. J. 2013, 10, 175. [Google Scholar] [CrossRef]
- Wilting, S.M.; van Boerdonk, R.A.; Henken, F.E.; Meijer, C.J.; Diosdado, B.; Meijer, G.A.; le Sage, C.; Agami, R.; Snijders, P.J.; Steenbergen, R.D. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol. Cancer 2010, 9, 167. [Google Scholar] [CrossRef]
- Wilting, S.M.; Verlaat, W.; Jaspers, A.; Makazaji, N.A.; Agami, R.; Meijer, C.J.; Snijders, P.J.; Steenbergen, R.D. Methylation-mediated transcriptional repression of microRNAs during cervical carcinogenesis. Epigenetics 2013, 8, 220–228. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial tom esenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Pereira, P.M.; Marques, J.P.; Soares, A.R.; Carreto, L.; Santos, M.A. MicroRNA expression variability in human cervical tissues. PLoS ONE 2010, 5, e11780. [Google Scholar] [CrossRef] [PubMed]
- Causin, R.L.; Freitas, A.J.A.D.; Trovo Hidalgo Filho, C.M.; Reis, R.D.; Reis, R.M.; Marques, M.M.C. A Systematic Review of MicroRNAs Involved in Cervical Cancer Progression. Cells 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Alenquer, M.; Amorim, M. Exosome biogenesis, regulation, and function in viral infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793. [Google Scholar] [CrossRef]
- Mirzaei, H.; Sahebkar, A.; Jaafari, M.R.; Goodarzi, M.; Mirzaei, H.R. Diagnostic and Therapeutic Potential of Exosomes in Cancer: The Beginning of a New Tale? J. Cell. Physiol. 2017, 232, 3251–3260. [Google Scholar] [CrossRef]
- Guenat, D.; Hermetet, F.; Prétet, J.-L.; Mougin, C. Exosomes and Other Extracellular Vesicles in HPV Transmission and Carcinogenesis. Viruses 2017, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of CDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Khan, S.; Aspe, J.R.; Asumen, M.G.; Almaguel, F.; Odumosu, O.; Acevedo-Martinez, S.; De Leon, M.; Langridge, W.H.; Wall, N.R. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br. J. Cancer 2009, 100, 1073–1086. [Google Scholar] [CrossRef]
- Khan, S.; Jutzy, J.M.; Aspe, J.R.; McGregor, D.W.; Neidigh, J.W.; Wall, N.R. Survivin is released from cancer cells via exosomes. Apoptosis Int. J. Program. Cell Death 2011, 16, 1–12. [Google Scholar] [CrossRef]
- Bridgewood, C.; Stacey, M.; Alase, A.; Lagos, D.; Graham, A.; Wittmann, M. IL-36γ has proinflammatory effects on human endothelial cells. Exp. Dermatol. 2017, 26, 402–408. [Google Scholar] [CrossRef]
- Wang, W.; Yu, X.; Wu, C.; Jin, H. IL-36γ inhibits differentiation and induces inflammation of keratinocyte via WNT signaling pathway in psoriasis. Int. J. Med. Sci. 2017, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Merola, M.; Ferrario, E.; Trabattoni, D.; Villa, M.L.; Stefanon, B.; Venzon, D.J.; Shearer, G.M.; De Palo, G.; Clerici, E. Cytokine production patterns in cervical intraepithelial neoplasia: Association with human papillomavirus infection. J. Natl. Cancer Inst. 1997, 89, 245–250. [Google Scholar] [CrossRef]
- Gezer, U.; Ozgur, E.; Cetinkaya, M.; Isin, M.; Dalay, N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol. Int. 2014, 38, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sultmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef]
- Chiantore, M.V.; Mangino, G.; Iuliano, M.; Zangrillo, M.S.; De Lillis, I.; Vaccari, G.; Accardi, R.; Tommasino, M.; Columba Cabezas, S.; Federico, M.; et al. Human papillomavirus E6 and E7 oncoproteins affect the expression of cancer-related microRNAs: Additional evidence in HPV-induced tumorigenesis. J. Cancer Res. Clin. Oncol. 2016, 142, 1751–1763. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 2017, 508, 63–69. [Google Scholar] [CrossRef]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M.; et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Console, L.; Scalise, M.; Indiveri, C. Exosomes in inflammation and role as biomarkers. Clin. Chim. Acta 2018, 488, 165–171. [Google Scholar] [CrossRef]
- Pulliero, A.; Cassatella, G.; Astuni, P.; Izzotti, A. The Role of microRNA Expression and DNA Methylation in HPV-Related Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12714. [Google Scholar] [CrossRef]
- Jiménez-Wences, H.; Peralta-zaragoza, O.; Fernández-Tilapa, G. Human papilloma virus, DNA methylation and microRNA expression in cervical cancer. Oncol. Rep. 2014, 31, 2467–2476. [Google Scholar] [CrossRef]
- Chaiwongkot, A.; Vinokurova, S.; Pientong, C.; Ekalaksananan, T.; Kongyingyoes, B.; Kleebkaow, P.; Chumworathayi, B.; Patarapadungkit, N.; Reuschenbach, M.; von Knebel, D.M. Differential methylation of E2 binding sites in episomal and integrated HPV 16 genomes in preinvasive and invasive cervical lesions. Int. J. Cancer 2013, 132, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Burdier, F.R.; Waheed, D.-E.-N.; Nedjai, B.; Steenbergen, R.D.M.; Poljak, M.; Baay, M.; Vorsters, A.; Van Keer, S. DNA methylation as a triage tool for cervical cancer screening—A meeting report. Prev. Med. Rep. 2024, 41, 102678. [Google Scholar] [CrossRef]
- Sen, P.; Ganguly, P.; Ganguly, N. Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncol. Lett. 2018, 15, 11–22. [Google Scholar] [CrossRef]
- Ayala-Calvillo, E.; Mojica-Vázquez, L.H.; García-Carrancá, A.; González-Maya, L. Wnt/β-catenin pathway activation and silencing of the APC gene in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2018, 17, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Ohtani, N.; Yamakoshi, K.; Takahashi, A.; Hara, E. The p16INK4a-RB pathway: Molecular link between cellular senescence and tumor suppression. J. Med. Investig. 2004, 51, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Vink, F.J.; Dick, S.; Heideman, D.A.M.; De Strooper, L.M.A.; Steenbergen, R.D.M.; Lissenberg-Witte, B.I.; Floore, A.; Bonde, J.H.; Oštrbenk Valenčak, A.; Poljak, M.; et al. Classification of High-Grade Cervical Intraepithelial Neoplasia by P16[Ink4a], Ki-67, HPV E4 and FAM19A4/MiR124-2 Methylation Status Demonstrates Considerable Heterogeneity with Potential Consequences for Management. Int. J. Cancer 2021, 149, 707–716. [Google Scholar] [CrossRef]
- Narayan, G.; Arias-Pulido, H.; Koul, S.; Vargas, H.; Zhang, F.F.; Villella, J.; Schneider, A.; Terry, M.B.; Mansukhani, M.; Murty, V.V. Frequent promoter methylation of CDH1, DAPK, RARB, and HIC1 genes in carcinoma of cervix uteri: Its relationship to clinical outcome. Mol. Cancer 2003, 2, 24. [Google Scholar] [CrossRef]
- Yin, F.F.; Wang, N.; Bi, X.N.; Yu, X.; Xu, X.H.; Wang, Y.L.; Zhao, C.Q.; Luo, B.; Wang, Y.K. Serine/threonine kinases 31[STK31] may be a novel cellular target gene for the HPV16 oncogene E7 with potential as a DNA hypomethylation biomarker in cervical cancer. Virol. J. 2016, 13, 60. [Google Scholar] [CrossRef]
- Da Silva, M.L.R.; De Albuquerque, B.H.D.R.; De Medeiros Fernandes Allyrio, T.A.; De Almeida, V.D.; De Oliveira Cobucci, R.N.; Bezerra, F.L.; Andrade, V.S.; Lanza, D.C.F.; De Azevedo, J.C.V.; De Araújo, J.M.G.; et al. The methylation of the HPV genome is significant as it influences viral replication and the advancement of HPV-related cervical lesions. Biomed. Rep. 2021, 15, 60. [Google Scholar] [CrossRef]
- Filho, S.M.; Bertoni, N.; Brant, A.C.; Vidal, J.P.; Felix, S.P.; Cavalcanti, S.M.; Carestiato, F.N.; Martins, L.F.; Almeida, L.M.; Moreira, M.A. Methylation at 3’LCR of HPV16 can be affected by patient age and disruption of E1 or E2 genes. Virus Res. 2017, 232, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Vink, F.J.; Meijer, C.J.L.M.; Clifford, G.M.; Poljak, M.; Oštrbenk, A.; Petry, K.U.; Rothe, B.; Bonde, J.; Pedersen, H.; de Sanjosé, S.; et al. FAM19A4/MiR124-2 Methylation in Invasive Cervical Cancer: A Retrospective Cross-Sectional Worldwide Study. Int. J. Cancer 2020, 147, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft: Global Strategy Towards Eliminating Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/m/item/draft-global-strategy-towards-eliminating-cervical-cancer-as-a-public-health-problem (accessed on 20 March 2025).
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.R.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef]
- Chatterjee, A. The next generation of HPV vaccines: Nonavalent vaccine V503 on the horizon. Expert. Rev. Vaccines 2014, 13, 1279–1290. [Google Scholar] [CrossRef]
- Dominik, P.; Sonja, M.K.; Robert, J.; Przybylski, M. Effect of vaccination against HPV in the HPV-positive patients not covered by primary prevention on the disappearance of infection. Sci. Rep. 2025, 15, 12642. [Google Scholar] [CrossRef]
- Jørgensen, L.; Gøtzsche, P.C.; Jefferson, T. Benefits and harms of the human papillomavirus (HPV) vaccines: Systematic review with meta-analyses of trial data from clinical study reports. Syst. Rev. 2020, 9, 43. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Lawson, H.W.; Chesson, H.; Unger, E.R.; Centers for Disease Control and Prevention [CDC]; Advisory Committee on Immunization Practices [ACIP]. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices [ACIP]. MMWR Recomm. Rep. 2007, 56, 1–30. [Google Scholar]
- Centers for Disease Control and Prevention [CDC]. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices [ACIP], 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1705–1708. [Google Scholar]
- American College of Obstetricians and Gynecologists’ Committee on Adolescent Health Care; American College of Obstetricians and Gynecologists’ Immunization, Infectious Disease, and Public Health Preparedness Expert Work Group. Human Papillomavirus Vaccination: ACOG Committee Opinion, Number 809. Obstet. Gynecol. 2020, 136, e15–e21. [Google Scholar] [CrossRef]
- Abdullahi, L.H.; Kagina, B.M.; Ndze, V.N.; Hussey, G.D.; Wiysonge, C.S. Improving vaccination uptake among adolescents. Cochrane Database Syst. Rev. 2020, 1, CD011895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadaf, A.; Richards, J.L.; Glanz, J.; Salmon, D.A.; Omer, S.B. A systematic review of interventions for reducingparental vaccine refusal and vaccine hesitancy. Vaccine 2013, 31, 4293–4304. [Google Scholar] [CrossRef]
- Dunn, A.G.; Leask, J.; Zhou, X.; Mandl, K.D.; Coiera, E. Associations between exposure to and expression of negative opinions about human papillomavirus vaccines on social media: An observational study. J. Med. Internet Res. 2015, 17, e144. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Singh, S.V. HPV Vaccine Scenario in India—A Short Report. Madridge J. Vaccines 2018, 2, 62–63. [Google Scholar] [CrossRef]
- Basu, P.; Malvi, S.G.; Joshi, S.; Bhatla, N.; Muwonge, R.; Lucas, E. Vaccine efficacy against persistent human papillomavirus [HPV] 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multi-centre, prospective, cohort study. Lancet Oncol. 2021, 22, 1518–1529. [Google Scholar] [CrossRef]
- Afonso, N.M.; Kavanagh, M.J.; Swanberg, S.M.; Schulte, J.M.; Wunderlich, T.; Lucia, V.C. Will they lead by example? Assessment of vaccination rates and attitudes to human papilloma virus in millennial medical students. BMC Public Health 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.W.A.; Lam, P.L.; Chan, T.K.; Chau, K.W.; Hsu, M.L.; Lim, Y.M.; Lo, C.H.; Siu, L.; Tang, H.F.; Tong, A.M.J.M.; et al. A Cross-Sectional Study on Knowledge, Attitude and Practice related to Human Papillomavirus Vaccination for Cervical Cancer Prevention between Medical and Non-Medical Students in Hong Kong. Asian Pac. J. Cancer Prev. 2017, 18, 1689–1695. [Google Scholar]
- Singh, J.; Baliga, S.S. Knowledge regarding cervical cancer and HPV vaccine among medical students: A cross-sectional study. Clin. Epidem Glob. Health 2021, 9, 289–292. [Google Scholar] [CrossRef]
- National Health Mission. National Programme for Prevention & Control of Cancer, Diabetes, Cardiovascular Diseases & Stroke [NPCDCS]; National Health Mission: Bhopal, India, 2024.
- Indian Council of Medical Research, National Centre for Disease Informatics and Research. National Cancer Registry Programme Report; Indian Council of Medical Research, National Centre for Disease Informatics and Research: Bangalore, India, 2020.
- ICMR-National Centre for Disease Informatics and Research. Clinicopathological Profile of Cancers in India: A Report of the Hospital Based Cancer Registries, 2021; ICMR-National Centre for Disease Informatics and Research: Bangalore, India, 2021; Available online: https://ncdirindia.org/All_Reports/HBCR_2021/Default.aspx (accessed on 5 February 2025).
- Clifford, G.M.; Rana, R.K.; Franceschi, S.; Smith, J.S.; Gough, G.; Pimenta, J.M. Distribution of human papillomavirus genotypes in low-grade cervical lesions: Comparison by geographical regions and with cervical cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1157–1164. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Naz, S.; Ahmed, O.; Yaqeen, S.R.; Irfan, M.; Asif, M.G.; Kamal, A.; Faridi, N. Comparison of Liquid-Based Cytology and Conventional Papanicolaou Smear for Cervical Cancer Screening: An Experience from Pakistan. Cureus 2020, 12, e12293. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shashikala, N.; Khan, M.A.; Srinivas, V.; Bhat, K.; Karnam, R.; Madhivanan, P. Understanding Knowledge Gaps in HPV Infection and Vaccination among Medical Students and Its Implications on Current and Future Trends of HPV Prevention Strategy in India. Int. J. Pharm. Clin. Res. 2025, 17, 141–150. [Google Scholar]
- Kaarthigeyan, K. Cervical cancer in India and HPV vaccination. Indian. J. Med. Paediatr. Oncol. 2012, 33, 7–12. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, K.L.; Jacobson, R.M.; Radecki Breitkopf, C.; Wilson, P.M.; Jacobson, D.J.; Fan, C.; St Sauver, J.L.; Finney Rutten, L.J. Trends Over Time in Pap and Pap-HPV Cotesting for Cervical Cancer Screening. J. Womens Health 2019, 28, 244–249. [Google Scholar] [CrossRef]
- Wentzensen, N.; Clarke, M.A.; Perkins, R.B. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improve resilience and reduce disparities. Prev. Med. 2021, 151, 106596. [Google Scholar] [CrossRef]
- Tranberg, M.; Bech, B.H.; Blaakær, J.; Jensen, J.S.; Svanholm, H.; Andersen, B. Preventing cervical cancer using HPV self-sampling: Direct mailing of test-kits increases screening participation more than timely opt-in procedures—A randomized controlled trial. BMC Cancer 2018, 18, 273. [Google Scholar] [CrossRef]
- Arbyn, M.; de Sanjose, S.; Weiderpass, E. HPV-based cervical cancer screening, including self-sampling, versus screening with cytology in Argentina. Lancet Glob. Health 2019, 7, e688–e689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).