Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications
Abstract
1. Introduction
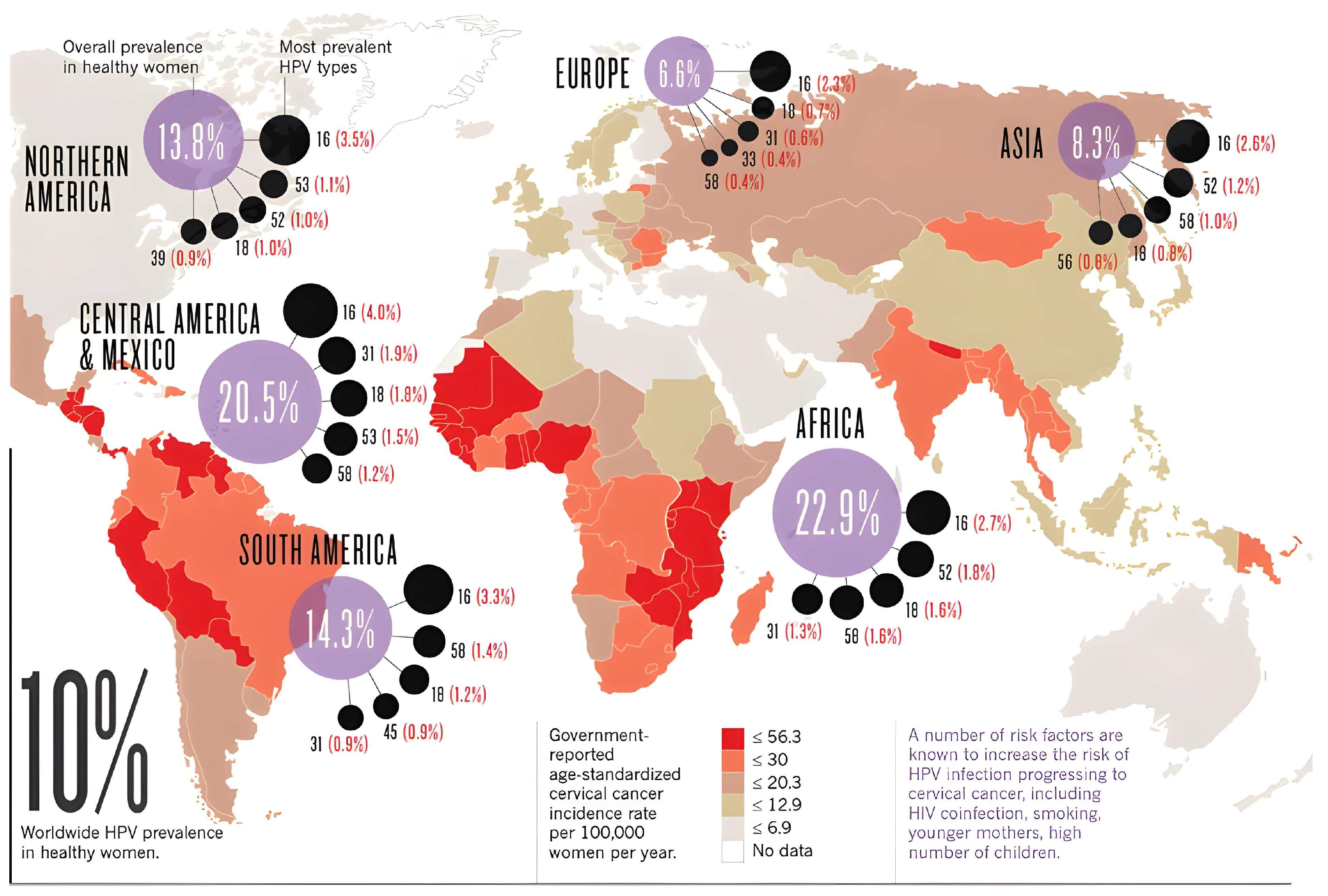
1.1. Prevalence and the Impact of HPV on Cervical Cancer
1.2. HPV Classification
- Mechanism: E6/E7-mediated p53/Rb degradation, genomic instability.
- Group 2A (Probably carcinogenic): HPV 68 (previously listed here; now reclassified to Group 1), with no other genital HPV types currently assigned to Group 2A by IARC [27,29]. Some studies propose HPV 26, 53, or 66 for Group 2A, but IARC retains them in Group 2B due to insufficient evidence [26,28].
1.3. Significance of HPV as a Public Health Concern, Particularly Its Association with CC
- Cytology co-testing (e.g., Pap smear for HPV-positive cases);
- p16/Ki-67 dual staining (improves specificity for CIN2+);
- Methylation markers (e.g., FAM19A4/miR124-2) for progressive lesions.
1.4. Pathogenesis of HPV
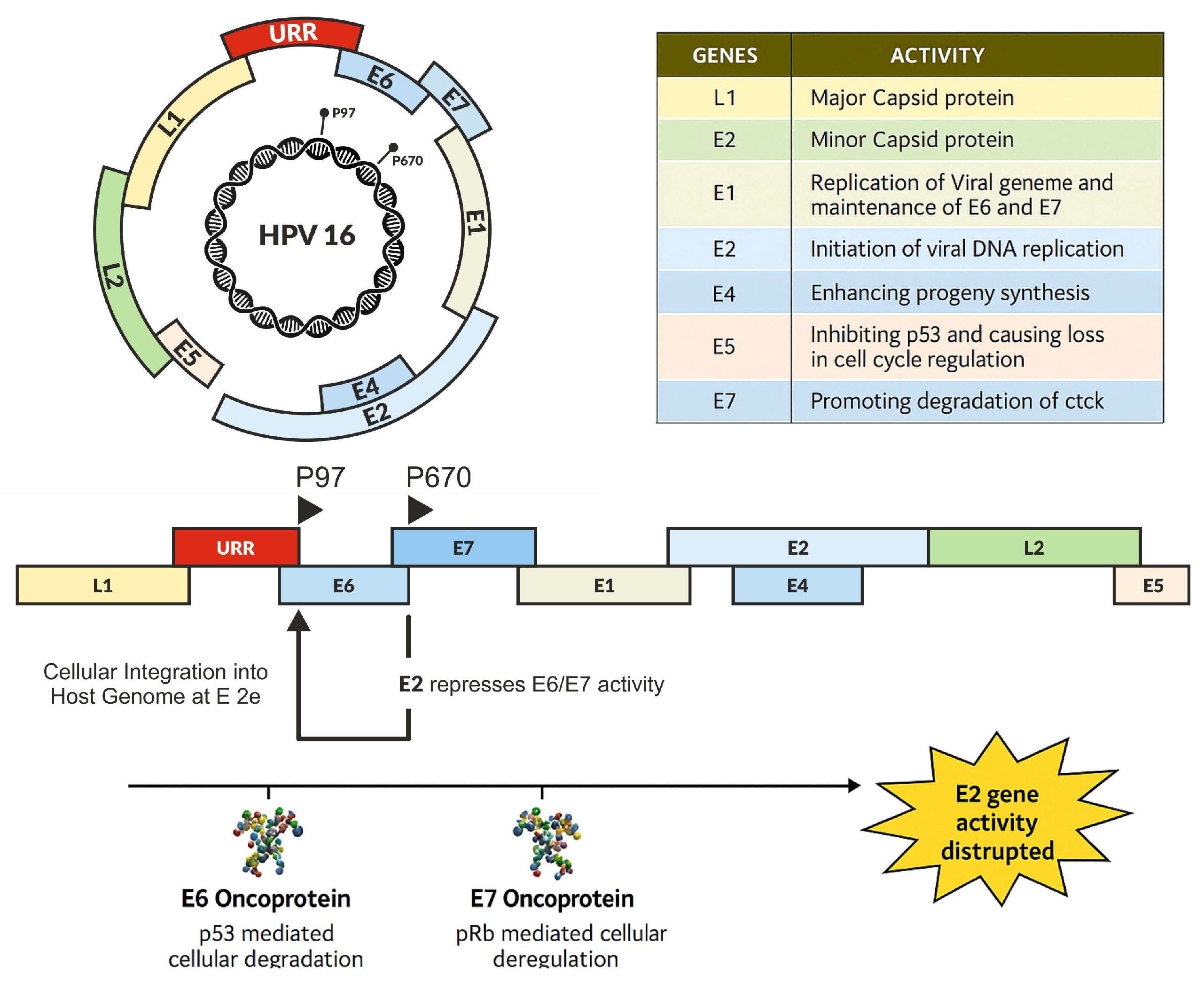
2. Early Proteins and Viral Integration Process
2.1. E1 and E2 Proteins: Initiation of HPV Replication and Viral Integration
2.2. E4 Protein: Role in Cytopathic Effects and Viral Release
2.3. E5 Protein: Modulation of Immune Response and Cell Growth
2.4. E6 Oncoprotein: Regulator of Tumor Suppression and DNA Repair
2.5. E7 Oncoprotein: A Driver of Cell Cycle and Genome Instability
2.6. Late Proteins—L1 and L2
2.7. HPV Signaling Pathway
2.8. MAPK-ERK Pathway
2.9. PI3K/AKT, Wnt, and Notch Pathways
2.10. STAT3 and EMT Pathways in HPV-Induced Cancer
2.11. Role of AP-1 and Notch Signaling Pathways in Cervical Carcinogenesis
2.12. Strategies of Immune Evasion by HPV-Infected Cells
2.13. Evasion of Antigen Presentation and Adaptive Immunity
2.14. Innate Immunity and Pattern Recognition Receptors (PRRs)
2.15. T Cell Responses and Adaptive Immunity
- Th1 cells, which are CD4-positive T helper cells responsible for beginning cellular immune responses, generally facilitate viral clearance after HPV infection. HPV utilizes many mechanisms to avoid host immune responses, mostly facilitated by the viral oncoproteins E5, E6, and E7. Th2 cells are also involved in this process. Nonetheless, HPV infection induces a change in the differentiation of Th1 and Th2 cells towards a Th2 phenotype. This shift likely contributes to both the persistence of HPV infection and the development of cervical lesions. This modification results in a diminished immune response, perhaps accounting for both instances [137];
- CD8+ Cytotoxic T Cell (CTL): CTLs are important for directly killing HPV-infected cells. The E5 and E7 oncoproteins diminish the production of MHC class I molecules, obstructing antigen presentation and resulting in weak or missing HPV-specific CTL responses, commonly seen in persistent HPV infections [139]. In those who have genital warts or CIN, HPV-specific CD8+ T cells can be correlated with the regression of lesions and clearance of the virus. In cases of persistent infection, however, HPV-specific CTL responses are frequently weak or absent, and therefore contribute to an inability to clear the infection [139];
- Tregs function in immune suppression, and their higher numbers in the tumor microenvironment correlate with poor immune responses, as well as persistent HPV. E7 has been associated with the promotion of Treg growth, wherein Tregs secrete IL-10 and TGF-β, which downregulate effector T cell responses and enhance viral immune tolerance. E5 and E6 concurrently facilitate the recruitment of tumor-associated macrophages (TAMs), which release pro-tumor cytokines (including IL-10, VEGF, and TGF-β) and inhibit anti-tumor immune responses [140,141,142,143,144].
2.16. Activation and Mechanisms of Cytotoxicity of NK Cells
2.17. Humoral Immunity and Antibody Responses
2.18. Immunosuppressive Microenvironment and Host Genetic Factors
2.19. Inflammation Factors in CC Related to HPV
2.20. MicroRNA Regulation by HPV Oncoproteins: Development of Cervical Cancer
2.21. Exosomes, Inflammation, and Microenvironment
2.22. Epigenetic Modifications in CC
3. Prevention and Management
Vaccination
4. Future Directions
4.1. Research Gaps
4.2. Public Health Implications
- By the age of 15, 90% of females will have been administered the HPV vaccine;
- By the ages of 35 and 45, 70% of women will have received a high-quality screening;
- Ninety percent of women with cervical disease will receive treatment [14].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC). 2022. Available online: https://www.iarc.who.int/cancer-type/cervical-cancer/;900-world-fact-sheet.pdf (accessed on 1 February 2025).
- National Cancer Institute. Cervical Cancer Causes, Risk Factors, and Prevention; National Cancer Institute: Washington, DC, USA, 2021.
- Asiaf, A.; Ahmad, S.T.; Mohammad, S.O.; Zargar, M.A. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur. J. Cancer Prev. 2014, 23, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; Murall, C.L.; Bravo, I.G. Why human papillomavirus acute infections matter. Viruses 2017, 9, 293. [Google Scholar] [CrossRef]
- Tan, S.C.; Ismail, M.P.; Duski, D.R.; Othman, N.H.; Ankathil, R. Prevalence and type distribution of human papillomavirus [HPV] in Malaysian women with and without cervical cancer: An updated estimate. Biosci. Rep. 2018, 38, BSR20171268. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Van Oort, M.G.; Coker, D.G.R.L. Clinical aspects of cervical cancer and HPV infection. J. Infect. Dis. 2012, 206, 965–971. [Google Scholar]
- Tomita, Y.; Koyama, M.; Tsukamoto, H. The molecular basis of cervical cancer progression. Infect. Agents Cancer 2011, 6, 33. [Google Scholar]
- Bao, Y.-P.; Li, N.; Smith, J.S.; Qiao, Y.-L. Human papillomavirus type distribution in women from Asia: A meta-analysis. Int. J. Gynecol. Cancer 2008, 18, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Mirabello, L.; Clarke, M.A.; Nelson, C.W.; Dean, M.; Wentzensen, N.; Yeager, M.; Cullen, M.; Boland, J.F.; Schiffman, M.; Burk, R.D. The Intersection of HPV Epidemiology, Genomics and Mechanistic Studies of HPV-Mediated Carcinogenesis. Viruses 2018, 10, 80. [Google Scholar] [CrossRef]
- Shalaby, N.A.; Tudorescu-Morjan, C.; Manole, C.G.; Iacata, A.-A.; Popovici, M.L.; Grecu, L.I.; Curici, A. Correlation between high-risk HPV infection and p16/Ki-67 abnormalities in Pap samples in a South Eastern Europe cohort. J. Med. Virol. 2024, 96, e29524. [Google Scholar] [CrossRef]
- Human Papillomavirus [HPV] Centre. HPV and Cervical Cancer Statistics; Human Papillomavirus [HPV] Centre: Barcelona, Spain, 2021. [Google Scholar]
- World Health Organization. Cervical Cancer; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Vaccarella, S.; Franceschi, S.; Herrero, R.; Muñoz, N.; Snijders, P.J.F.; Clifford, G.M.; Smith, J.S. Sexual Behavior, Condom Use, and Human Papillomavirus: Pooled Analysis of the IARC Human Papillomavirus Prevalence Surveys. Cancer Epidemiol. Biomark. Prev. 2006, 15, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Muñoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.M.; Lopez, C.C.; Munteanu, D.M.; and Rivas, E.J. Identification of predictive factors for human papillomavirus clearance in women aged 25–40. BMC Med. 2023, 21, 43. [Google Scholar]
- Lee, S.; Park, W.K.; Lee, J.W. The risk factors and clinical outcomes of cervical cancer patients with HPV infections. Cancer Manag. Res. 2019, 11, 529–534. [Google Scholar]
- Costa, M.; Rosa, T.C.; Silva, J.G. Genetic basis of cervical cancer development. Genet. Mol. Res. 2015, 14, 14672–14681. [Google Scholar]
- Park, S.; Lee, J.K.; Kim, C.J. Molecular mechanisms of cervical carcinogenesis. Carcinogenesis 2014, 35, 1430–1443. [Google Scholar]
- Lee, J.W.; Park, W.K.; Lee, S. HPV and its role in cervical cancer. Asian Pac. J. Cancer Prev. 2012, 13, 5489–5496. [Google Scholar]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 38. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Erickson, B.K.; Alvarez, R.D.; Huh, W.K. Human papillomavirus: What every provider should know. Am. J. Obstet. Gynecol. 2013, 208, 169–175. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Franceschi, S.; Diaz, M.; Muñoz, N.; Villa, L.L. HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006, 24 (Suppl. S3), S3/26–S3/34. [Google Scholar] [CrossRef]
- van Hamont, D.; van Ham, M.A.P.C.; Bakkers, J.M.J.E.; Massuger, L.F.A.G.; Melchers, W.J.G. Evaluation of the SPF10-INNO LiPA human papillomavirus [HPV] genotyping test and the Roche Linear Array HPV genotyping test. J. Clin. Microbiol. 2006, 44, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of multiple high-risk human papillomavirus [HPV] infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Vaziri, M.; Sadeghi, F.; Hashemi, F.S.; Haeri, H.; Bokharaei-Salim, F.; Monavari, S.H.; Keyvani, H. Distribution of Human Papillomavirus Genotypes in Iranian Women According to the Severity of the Cervical Lesion. Iran. Red. Crescent Med. J. 2016, 18, e24458. [Google Scholar] [CrossRef]
- You, W.; Li, S.; Du, R.; Zheng, J.; Shen, A. Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp. Ther. Med. 2018, 15, 412–418. [Google Scholar] [CrossRef]
- Sotlar, K.; Stubner, A.; Diemer, D.; Menton, S.; Menton, M.; Dietz, K.; Wallwiener, D.; Kandolf, R.; Bültmann, B. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J. Med. Virol. 2004, 74, 107–116. [Google Scholar] [CrossRef]
- Castle, P.E.; Solomon, D.; Schiffman, M.; Wheeler, C.M. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J. Natl. Cancer Inst. 2005, 97, 1066–1071. [Google Scholar] [CrossRef]
- Bonnez, W.; Reichman, R.C. Papillomaviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 5th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingston: Philadelphia, PA, USA, 2000; pp. 1630–1640. [Google Scholar]
- Weng, S.L.; Huang, K.Y.; Weng, J.T.Y.; Hung, F.Y.; Chang, T.H.; Lee, T.Y. Genome-wide discovery of viral microRNAs based on phylogenetic analysis and structural evolution of various human papillomavirus subtypes. Brief. Bioinform. 2018, 19, 1102–1114. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and Taxonomic Classification of Alphapapillomavirus 7 Complete Genomes: HPV18, HPV39, HPV45, HPV59, HPV68 and HPV70. PLoS ONE 2013, 8, e72565. [Google Scholar] [CrossRef]
- Ong, C.K.; Chan, S.Y.; Campo, M.S.; Fujinaga, K.; Mavromara-Nazos, P.; Labropoulou, V.; Pfister, H.; Tay, S.K.; ter Meulen, J.; Villa, L.L. Evolution of human papillomavirus type 18: An ancient phylogenetic root in Africa and intratype diversity reflect coevolution with human ethnic groups. J. Virol. 1993, 67, 6424–6431. [Google Scholar] [CrossRef]
- Calleja-Macias, I.E.; Kalantari, M.; Allan, B.; Williamson, A.; Chung, L.; Collins, R.J.; Zuna, R.E.; Dunn, S.T.; Ortiz-Lopez, R.; Barrera-Saldaña, H.A.; et al. Papillomavirus Subtypes Are Natural and Old Taxa: Phylogeny of Human Papillomavirus Types 44 and 55 and 68a and -b. J. Virol. 2005, 79, 6565–6569. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/35460940/ (accessed on 5 February 2025).
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef]
- Baker, T.S.; Newcomb, W.W.; Olson, N.H.; Cowsert, L.M.; Olson, C.; Brown, J.C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 1991, 60, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Favre, M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J. Virol. 1975, 15, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A.; Pett, M.R.; Coleman, N. HPV: From infection to cancer. Biochem. Soc. Trans. 2007, 35, 1456–1460. [Google Scholar] [CrossRef]
- Torrisi, A.; Del Mistro, A.; Onnis, G.L.; Merlin, F.; Bertorelle, R.; Minucci, D. Colposcopy, cytology and HPV testing in HIV-positive and HIV-negative women. Eur. J. Gynecol. Oncol. 2000, 21, 168–172. [Google Scholar]
- Apt, D.; Watts, R.M.; Suske, G.; Bernard, U. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transcription correlate with the activation of the HPV-16 promoter. Virology 1996, 224, 281–291. [Google Scholar] [CrossRef]
- Sapp, M.; Volpers, C.; Muller, M.; Streck, R.E. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J. Gen. Virol. 1995, 76, 2407–2412. [Google Scholar] [CrossRef]
- De Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obs. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kundu, R. Human papillomavirus E6 and E7: The cervical cancer hallmarks and targets for therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A.R. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378. [Google Scholar]
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; Upadhye, V.J.; et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675. [Google Scholar] [CrossRef]
- Berg, M.; Stenlund, A. Functional interactions between papillomavirus E1 and E2 proteins. J. Virol. 1997, 71, 3853–3863. [Google Scholar] [CrossRef]
- Bergvall, M.; Melendy, T.; Archambault, J. The E1 proteins. Virology 2013, 445, 35–56. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Manzo-Merino, J.; Muñoz-Bello, J.O.; Olmedo-Nieva, L.; Cedro-Tanda, A.; Alfaro-Ruiz, L.A.; Hidalgo-Miranda, A.; Madrid-Marina, V.; Lizano, M. The Human Papillomavirus (HPV) E1 protein regulates the expression of cellular genes involved in immune response. Sci. Rep. 2019, 9, 13620. [Google Scholar] [CrossRef]
- Chojnacki, M.; Melendy, T. The human papillomavirus DNA helicase E1 binds, stimulates, and confers processivity to cellular DNA polymerase epsilon. Nucl. Acids Res. 2018, 46, 229–241. [Google Scholar] [CrossRef]
- D’Abramo, C.; Archambault, J. Small molecule inhibitors of human papillomavirus protein-protein interactions. Open Virol. J. 2011, 5, 80. [Google Scholar] [CrossRef]
- Goodwin, E.C.; DiMaio, D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 12513–12518. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- White, P.W.; Pelletier, A.; Brault, K.; Titolo, S.; Welchner, E.; Thauvette, L.; Fazekas, M.; Cordingley, M.G.; Archambault, J. Characterization of recombinant HPV6 and 11 E1 helicases: Effect of ATP on the interaction of E1 with E2 and mapping of a minimal helicase domain. J. Biol. Chem. 2001, 276, 22426–22438. [Google Scholar] [CrossRef] [PubMed]
- White, P.W.; Titolo, S.; Brault, K.; Thauvette, L.; Pelletier, A.; Welchner, E.; Bourgon, L.; Doyon, L.; Ogilvie, W.W.; Yoakim, C. Inhibition of human papillomavirus DNA replication by small molecule antagonists of the E1–E2 protein interaction. J. Biol. Chem. 2003, 278, 26765–26772. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular pathways and targets. Curr. Probl. Cancer 2018, 42, 161–174. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Gonzal’ez-García, M.C.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Valverde, M.; Rojas, E.; Rodríguez-Sastre, M.A.; García-Cuellar, C.M.; Lizano, M. Human papillomavirus types 16 and 18 early-expressed proteins differentially modulate the cellular redox state and DNA damage. Int. J. Biol. Sci. 2018, 14, 21. [Google Scholar] [CrossRef]
- Gupta, S.M.; Mania-Pramanik, J. Retracted Article: Molecular mechanisms in progression of HPV-associated cervical carcinogenesis. J. Biomed. Sci. 2019, 26, 28. [Google Scholar] [CrossRef]
- Zahra, K.; Patel, S.; Dey, T.; Pandey, U.; Mishra, S.P. A study of oxidative stress in cervical cancer-an institutional study. Biochem. Biophys. Rep. 2021, 25, 100881. [Google Scholar] [CrossRef] [PubMed]
- Sheila, V. Graham Human Papillomavirus E2 Protein: Linking Replication, Transcription, and RNA Processing. J. Virol. 2016, 90, 8384–8388. [Google Scholar] [CrossRef]
- Khan, S.A. Plasmid rolling-circle replication: Recent developments. Mol. Microbiol. 2000, 37, 477–484. [Google Scholar] [CrossRef]
- Akagi, K.; Symer, D.E.; Mahmoud, M.; Goodwin, S.; Wangsa, D.; Li, Z.; Xiao, W.; Dunn, J.D.; Ried, T.; Coombes, K.R.; et al. Intratumoral heterogeneity and clonal evolution induced by HPV integration. Cancer Discov. 2023, 13, 910–927. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Steenbergen, R.D.M.; Pumpe, A.; Kenyon, A.N.; Melchers, W.J.G. HPV integration and cervical cancer: A failed evolutionary viral trait. Trends Mol. Med. 2024, 30, 890–902. [Google Scholar] [CrossRef]
- Sui, S.; Jiao, Z.; Chen, H.; Niyazi, M.; Wang, L. Association between APOBEC3s and HPV16 E2 gene hypermutation in Uygur females with cervical cancer. Oncol. Lett. 2020, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Lagström, S.; Lovestad, A.H.; Umu, S.U.; Ambur, O.H.; Nygard, M.; Rouge, T.B.; Kraus Christiansen, I. HPV16 and HPV18 type-specific APOBEC3 and integration profiles in different diagnostic categories of cervical samples. Tumour Virus Res. 2021, 12, 200221. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Wang, T.; He, D.; Tian, R.; Cui, Z.; Tian, X.; Gao, Q.; Ma, X.; Yang, J.; et al. In vitro and in vivo growth inhibition of human cervical cancer cells via human papillomavirus E6/E7 mRNAs’ cleavage by CRISPR/Cas13a system. Antivir. Res. 2020, 178, 104794. [Google Scholar] [CrossRef]
- Tian, R.; Liu, J.; Fan, W.; Li, R.; Cui, Z.; Jin, Z.; Huang, Z.; Xie, H.; Li, L.; Huang, Z.; et al. Gene knock-out chain reaction enables high disruption efficiency of HPV18 E6/E7 genes in cervical cancer cells. Mol. Ther. Oncolytics 2022, 24, 171–179. [Google Scholar] [CrossRef]
- Paolini, F.; Amici, C.; Carosi, M.; Bonomo, C.; Di Bonito, P.; Venuti, A.; Accardi, L. Intrabodies targeting human papillomavirus 16 E6 and E7 oncoproteins for therapy of established HPV associated tumors. J. Exp. Clin. Cancer Res. 2021, 40, 37. [Google Scholar] [CrossRef]
- Doorbar, J. The E4 protein; structure, function and patterns of expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef]
- Doorbar, J.; Medcalf, E.; Napthine, S. Analysis of HPV1 E4 complexes and their association with keratinsin vivo. Virology 1996, 218, 114–126. [Google Scholar] [CrossRef]
- Khan, J.; Davy, C.E.; McIntosh, P.B.; Jackson, D.J.; Hinz, S.; Wang, Q.; Doorbar, J. Role of calpain in the formation of human papillomavirus type 16 E1^ E4 amyloid fibers and reorganization of the keratin network. J. Virol. 2011, 85, 9984–9997. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, P.B.; Laskey, P.; Sullivan, K.; Davy, C.; Wang, Q.; Jackson, D.J.; Griffin, H.M.; Doorbar, J. E1^E4-mediated keratin phosphorylation and ubiquitylation: Amechanism for keratin depletion in HPV16-infected epithelium. J. Cell Sci. 2010, 123, 2810–2822. [Google Scholar] [CrossRef]
- Roberts, S.; Hillman, M.L.; Knight, G.L.; Gallimore, P.H. The ND10 component promyelocytic leukemia protein relocates to human papillomavirus type 1 E4 intranuclear inclusion bodies in cultured keratinocytes and in warts. J. Virol. 2003, 77, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Rogel-Gaillard, C.; Pehau-Arnaudet, G.; Breitburd, F.; Orth, G. Cytopathic effect in human papillomavirus type 1–Induced inclusion warts: In vitro analysis of the contribution of two forms of the viral E4 protein. J. Investig. Dermatol. 1993, 101, 843–851. [Google Scholar] [CrossRef]
- Wang, Q.; Kennedy, A.; Das, P.; McIntosh, P.B.; Howell, S.A.; Isaacson, E.R.; Hinz, S.A.; Davy, C.; Doorbar, J. Phosphorylation of the human papillomavirus type 16 E1^ E4 protein at T57 by ERK triggers a structural change that enhances keratin binding and protein stability. J. Virol. 2009, 83, 3668–3683. [Google Scholar] [CrossRef]
- Kim, S.-H.; Oh, J.-M.; No, J.-H.; Bang, Y.-J.; Juhnn, Y.-S.; Song, Y.-S. Involvement of NF-κB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis 2009, 30, 753–757. [Google Scholar] [CrossRef]
- Deng, W.; Lin, B.Y.; Jin, G.; Wheeler, C.G.; Ma, T.; Harper, J.W.; Broker, T.R.; Cho, L.T. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 2004, 78, 13954–13965. [Google Scholar] [CrossRef]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 drive alternative carcinogenic pathways in HPV positive cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef] [PubMed]
- Crook, T.; Vousden, K.H.; Tidy, J.A. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell 1991, 67, 547–556. [Google Scholar] [CrossRef]
- Ganguly, N. Human papillomavirus-16 E5 protein: Oncogenic role and therapeutic value. Cell. Oncol. 2012, 35, 67–76. [Google Scholar] [CrossRef]
- Wang, W.; Sima, N.; Kong, D.; Luo, A.; Gao, Q.; Liao, S.; Li, W.; Han, L.; Wang, J.; Wang, S. Selective targeting of HPV-16 E6/E7 in cervical cancer cells with a potent oncolytic adenovirus and its enhanced effect with radiotherapy in vitro and vivo. Cancer Lett. 2010, 291, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.H.; Haghshenas, M.R.; Marchetti, B.; O’Brien, P.M.; Campo, M.S. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer 2005, 113, 276–283. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 protein of human papillomavirus 16 downregulates HLA class Iand interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 2006, 119, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Vizcaino, G.; Sánchez-Torres, C.; Lizano, M. The Natural History of Cervical Cancer and the Case for MicroRNAs: Is Human Papillomavirus Infection the Whole Story? Int. J. Mol. Sci. 2024, 25, 12991. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus [HPV] infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Vallejo-Ruiz, V.; Gutiérrez-Xicotencatl, L.; Medina-Contreras, O.; Lizano, M. Molecular aspects of cervical cancer: A pathogenesis update. Front. Oncol. 2024, 14, 1356581. [Google Scholar] [CrossRef]
- Celegato, M.; Messa, L.; Bertagnin, C.; Mercorelli, B.; Loregian, A. Targeted disruption of E6/p53 binding exerts broad activity and synergism with paclitaxel and topotecan against HPV-transformed cancer cells. Cancers 2022, 14, 193. [Google Scholar] [CrossRef]
- De Nola, R.; Loizzi, V.; Cicinelli, E.; Cormio, G. Dynamic crosstalk within the tumor microenvironment of uterine cervical carcinoma: Baseline network, iatrogenic alterations, and translational implications. Crit. Rev. Oncol. Hematol. 2021, 162, 103343. [Google Scholar] [CrossRef]
- Vats, A.; Skrabar, N.; Del Sal, G.; Banks, L. Loss of the E6AP ubiquitin ligase induces p53-dependent phosphorylation of HPV-18 E6 in cells derived from cervical cancer. J. Virol. 2022, 96, e0150321. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, L.; Zuo, L.; Gao, S.; Jiang, X.; Han, Y.; Lin, J.; Peng, M.; Wu, N.; Tang, Y.; et al. HPV E6/E7: Insights into their regulatory role and mechanism in signaling pathways in HPV-associated tumor. Cancer Gene Ther. 2024, 31, 9–17. [Google Scholar] [CrossRef]
- Branca, M.; Ciotti, M.; Santini, D.; Di Bonito, L.; Benedetto, A.; Giorgi, C.; Paba, P.; Favalli, C.; Costa, S.; Agarossi, A. Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am. J. Clin. Pathol. 2004, 122, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nature reviews. Clin. Oncol. 2013, 10, 143–153. [Google Scholar]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Pol, S.V.; Podjarny, A. Structure of the E6/ E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Kato, I.; Kim, H.-R.C. A novel function of HPV16-E6/E7 in epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 2013, 435, 339–344. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Kim, S.; Choi, Y.-L.; Cho, Y.J.; Oh, E.; Choi, J.-J.; Jung, K.; Song, J.-Y.; Ahn, S.E.; Kim, B.-G. HER2 as a novel therapeutic target for cervical cancer. Oncotarget 2015, 6, 36219. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lin, B.; Liu, X.; Zhang, W.; Zhang, E.; Hu, L.; Ma, Y.; Li, X.; Tang, X. ERK signaling pathway is involved in HPV-16 E6 but not E7 oncoprotein-induced HIF-1α protein accumulation in NSCLC cells. Oncol. Res. 2016, 23, 109. [Google Scholar] [CrossRef]
- Jones, D.L.; Münger, K. Interactions of the human papillomavirus E7 protein with cell cycle regulators. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 327–337. [Google Scholar]
- Kennedy, E.M.; Kornepati, A.V.; Goldstein, M.; Bogerd, H.P.; Poling, B.C.; Whisnant, A.W.; Kastan, M.B.; Cullen, B.R. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014, 88, 11965–11972. [Google Scholar] [CrossRef]
- Chen, X.; He, H.; Xiao, Y.; Hasim, A.; Yuan, J.; Ye, M.; Li, X.; Hao, Y.; Guo, X. CXCL10 Produced by HPV-Positive Cervical Cancer Cells Stimulates Exosomal PDL1 Expression by Fibroblasts via CXCR3 and JAK-STAT Pathways. Front. Oncol. 2021, 11, 629350. [Google Scholar] [CrossRef]
- Huang, X.; Huo, L.; Xiao, B.; Ouyang, Y.; Chen, F.; Li, J.; Zheng, X.; Wei, D.; Wu, Y.; Zhang, R.; et al. Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer. Cell Death Differ. 2024, 31, 78–89. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Hemmat, N.; Baradaran, B.; Mokhtarzadeh, A.; Poortahmasebi, V.; Oskuee, M.A.; Baghi, H.B. The effect of Wnt/β-catenin signaling on PD-1/PDL-1 axis in HPV-related cervical cancer. Oncol. Res. 2023, 30, 99–116. [Google Scholar] [CrossRef]
- Hu, C.; Liu, T.; Han, C.; Xuan, Y.; Jiang, D.; Sun, Y.; Zhang, X.; Zhang, W.; Xu, Y.; Liu, Y.; et al. HPV E6/E7 promotes aerobic glycolysis in cervical cancer by regulating IGF2BP2 to stabilize m6A-MYC expression. Int. J. Biol. Sci. 2022, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Taguchi, A.; Sone, K.; Mori, M.; Osuga, Y. Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int. J. Clin. Oncol. 2023, 28, 965–974. [Google Scholar] [CrossRef]
- Singini, M.G.; Singh, E.; Bradshaw, D.; Ramaliba, T.; Chen, W.C.; Motlhale, M.; Kamiza, A.B.; de Villiers, C.B.; Muchengeti, M.; Mathew, C.G.; et al. Usefulness of high-risk HPV early oncoprotein [E6 and E7] serological markers in the detection of cervical cancer: A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e27900. [Google Scholar] [CrossRef]
- Balaji, D.; Kalarani, I.B.; Mohammed, V.; Veerabathiran, R. Potential role of human papillomavirus proteins associated with the development of cancer. Virus Dis. 2022, 33, 322–333. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keifer, T.R.; Sapp, M. Human papillomavirus major capsid protein L1 remains associated with the incoming viral genome throughout the entry process. J Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Caodaglio, A.S.; Sichero, L. Regulation of HPV transcription. Clinics 2018, 73, e486s. [Google Scholar] [CrossRef]
- Song, Z.; Ding, L.; Ren, Z.; Sun, X.; Yang, Q.; Wang, L.; Feng, M.; Liu, C.; Wang, J. Effects of Src on cervical cancer cells proliferation and apoptosis through ERK signal transduction pathway. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2017, 38, 1246–1251. [Google Scholar] [PubMed]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Brand, T.M.; Iida, M.; Corrigan, K.L.; Braverman, C.M.; Coan, J.P.; Flanigan, B.G.; Stein, A.P.; Salgia, R.; Rolff, J.; Kimple, R.J. The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor. Sci. Signal. 2017, 10, eaag1064. [Google Scholar] [CrossRef]
- Manzo-Merino, J.; Contreras-Paredes, A.; V’azquez-Ulloa, E.; Rocha-Zavaleta, L.; Fuentes-Gonzalez, A.M.; Lizano, M. The role of signaling pathways in cervical cancer and molecular therapeutic targets. Arch. Med. Res. 2014, 45, 525–539. [Google Scholar] [CrossRef]
- Talora, C.; Cialfi, S.; Segatto, O.; Morrone, S.; Choi, J.K.; Frati, L.; Dotto, G.P.; Gulino, A.; Screpanti, I. Constitutively active Notch1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathways. Exp. Cell Res. 2005, 305, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Moon, R.T.; Kohn, A.D.; De Ferrari, G.V.; Kaykas, A. WNT and β-catenin signalling: Diseases and therapies. Nat. Rev. Gen. 2004, 5, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavalu, K.; Subbaiah, V.K.; Srivastava, S.; Chakrabarti, O.; Syal, R.; Krishna, S. Complementation of human papillomavirus type 16 E6 and E7 by Jagged1-specific Notch1-phosphatidylinositol 3-kinase signaling involves pleiotropic oncogenic functions independent of CBF1; Su [H]; Lag-1 activation. J. Virol. 2005, 79, 7889–7898. [Google Scholar] [CrossRef]
- Menges, C.W.; Baglia, L.A.; Lapoint, R.; McCance, D.J. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006, 66, 5555–5559. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.A.; Ahmad, A.; Farooq, N.; Priya, M.V.; Siddiqui, A.H.; Asif, M.; Manzoor, R.; Ishqi, H.M.; Alomar, S.Y.; Rahaman, P.F. Increased expression of HPV-E7 oncoprotein correlates with a reduced level of pRb proteins via high viral load in cervical cancer. Sci. Rep. 2023, 13, 15075. [Google Scholar] [CrossRef]
- Morgan, E.L.; Macdonald, A. Manipulation of JAK/STAT signalling by high-risk HPVs: Potential therapeutic targets for HPV-associated malignancies. Viruses 2020, 12, 977. [Google Scholar] [CrossRef]
- Cheng, Y.-M.; Chou, C.-Y.; Hsu, Y.-C.; Chen, M.-J. Influence of HPV16 E6/7 on the expression of FGF2 and FGFR type B in cervical carcinogenesis. Reprod. Sci. 2012, 19, 580–586. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef]
- McBride, A.A. Oncogenic human papillomaviruses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 2016027397. [Google Scholar] [CrossRef]
- Kusakabe, M.; Taguchi, A.; Tanikawa, M.; Wagatsuma, R.; Yamazaki, M.; Tsuchimochi, S.; Toyohara, Y.; Kawata, A.; Baba, S.; Uen, T.; et al. Cells with stem-like properties are associated with the development of HPV18-positive cervical cancer. Cancer Sci. 2023, 114, 885–895. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.L.; Cho, W.C.S.; Yee, B.K.; Kwan, Y.W.; Tai, W.C.S. Exosomes in inflammation and inflammatory disease. Proteomics 2019, 19, 1800149. [Google Scholar] [CrossRef]
- Chow, A.; Zhou, W.; Liu, L.; Fong, M.Y.; Champer, J.; Van Haute, D.; Chin, A.R.; Ren, X.; Gugiu, B.G.; Meng, Z.; et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci. Rep. 2014, 4, 5750. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Zhang, B.; Shi, H.; Yuan, X.; Sun, Y.; Pan, Z.; Qian, H.; Xu, W. Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumor Biol. 2016, 37, 12169–12180. [Google Scholar] [CrossRef]
- Nahand, J.S.; Moghoofei, M.; Salmaninejad, A.; Bahmanpour, Z.; Karimzadeh, M.; Nasiri, M.; Mirzaei, H.R.; Pourhanifeh, M.H.; Bokharaei-Salim, F.; Mirzaei, H.; et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. Int. J. Cancer 2020, 146, 305–320. [Google Scholar] [CrossRef]
- Miller, L.S.; Modlin, R.L. Human keratinocyte Toll-like receptors promote distinct immune responses. J. Investig. Dermatol. 2007, 127, 262–263. [Google Scholar] [CrossRef]
- Kovachev, A.M. A Review on Inosine Pranobex Immunotherapy for Cervical HPV-Positive Patients. Infect. Drug Resist. 2021, 14, 2039–2049. [Google Scholar] [CrossRef]
- Le Borgne, M.; Etchart, N.; Goubier, A.; Lira, S.A.; Sirard, J.C.; van Rooijen, N.; Caux, C.; Ait-Yahia, S.; Vicari, A.; Kaiserlian, D.; et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 2006, 24, 191–201. [Google Scholar] [CrossRef]
- Smola, S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses 2017, 9, 254. [Google Scholar] [CrossRef]
- Singh, M.; Thakral, D.; Rishi, N.; Kar, H.K.; Mitra, D.K. Functional characterization of CD4 and CD8 T cell responses among human papillomavirus infected patients with anogenital warts. Virus Dis. 2017, 28, 133–140. [Google Scholar] [CrossRef]
- Dugué, P.A.; Rebolj, M.; Garred, P.; Lynge, E. Immunosuppression and risk of cervical cancer. Expert Rev Anticancer Ther. 2013, 13, 29–42. [Google Scholar] [CrossRef]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 delivered by engineered exosomes elicits a protective CD8[+] T cell-mediated immune response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.C.; Zhang, Z.S.; Chen, F. MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3719–3726. [Google Scholar]
- Torres-Poveda, K.; Bahena-Román, M.; Madrid-González, C.; Burguete-García, A.I.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753. [Google Scholar] [CrossRef]
- Paradkar, P.H.; Joshi, J.V.; Mertia, P.N.; Agashe, S.V.; Vaidya, R.A. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 3851–3864. [Google Scholar] [CrossRef]
- Kawachi, A.; Yoshida, H.; Kitano, S.; Ino, Y.; Kato, T.; Hiraoka, N. Tumor-associated CD204+ M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018, 109, 863–870. [Google Scholar] [CrossRef]
- Chen, X.J.; Han, L.F.; Wu, X.G.; Wei, W.-F.; Wu, L.-F.; Yi, H.-Y.; Yan, R.-M.; Bai, X.-Y.; Zhong, M.; Yu, Y.-H.; et al. Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J. Cancer 2017, 8, 3868–3875. [Google Scholar] [CrossRef]
- NK Cells Directly Kill Their Targets by Releasing Cytotoxic Granules (Perforin and Granzymes) and Pro-Inflammatory Cytokines Such as IFN-γ [Which Activate the Broader Immune Response] upon Activation. Available online: www.frontiersin.org (accessed on 15 March 2025).
- Mah, A.Y.; Cooper, M.A. Metabolic Regulation of Natural Killer Cell IFN- Production. Crit. Rev. Immunol. 2016, 36, 131–147. [Google Scholar] [CrossRef]
- Ferns, D.M.; Heeren, A.M.; Samuels, S.; Bleeker, M.C.G.; de Gruijl, T.D.; Kenter, G.G.; Jordanova, E.S. Classical and Non-Classical HLA Class I Aberrations in Primary Cervical Squamous- and Adenocarcinomas and Paired Lymph Node Metastases. J. Immunother. Cancer 2016, 4, 78. [Google Scholar] [CrossRef]
- Cho, H.; Chung, J.-Y.; Kim, S.; Braunschweig, T.; Kang, T.H.; Kim, J.; Chung, E.J.; Hewitt, S.M.; Kim, J.-H. MICA/B and ULBP1 NKG2D Ligands Are Independent Predictors of Good Prognosis in Cervical Cancer. BMC Cancer 2014, 14, 957. [Google Scholar] [CrossRef]
- Utami, T. NK-Cell Count and Its Function in Producing Interferon Gamma Associated with the Cervical Cancer Natural History. Glob. J. Reprod. Med. 2018, 8, 1000462. [Google Scholar]
- Zhu, M.; Wang, J.; Zhang, Y.; Li, Y. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. [Google Scholar] [CrossRef]
- Conesa-Zamora, P. Immune responses against virus and tumor in cervical carcinogenesis: Treatment strategies for avoiding the HPV-induced immune escape. Gynecol. Oncol. 2013, 131, 480–488. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Broker, T.R.; Chow, L.T.; Alvarez, R.D.; Vu, H.L.; Andrasi, J.; Brewer, L.R.; Jin, G. Immune responses to human papillomavirus in genital tract of women with cervical cancer. Gynecol. Oncol. 2005, 96, 452–461. [Google Scholar] [CrossRef]
- Paaso, A.; Jaakola, A.; Syrjänen, S.; Louvanto, K. From HPV infection to lesion progression: The role of HLA alleles and host immunity. Acta Cytol. 2019, 63, 148–158. [Google Scholar] [CrossRef]
- Kojima, S.; Kawana, K.; Tomio, K.; Taguchi, A.; Miura, S.; Adachi, K.; Nagamatsu, T.; Nagasaka, K.; Matsumoto, Y.; Arimoto, T.; et al. The prevalence of cervical regulatory T cells in HPV-related cervical intraepithelial neoplasia [CIN] correlates inversely with spontaneous regression of CIN. Am. J. Reprod. Immunol. 2013, 69, 134–141. [Google Scholar] [CrossRef]
- Szöke, K.; Szalmás, A.; Szládek, G.; Veress, G.; Gergely, L.; Tóth, F.D.; Kónya, J. IL-10 promoter nt -1082A/G polymorphism and human papillomavirus infection in cytologic abnormalities of the uterine cervix. J. Interferon. Cytokine. Res. 2004, 24, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mittal, R.D.; Srivastava, M.; Srivastava, K.; Singh, S.; Srivastava, S.; Mittal, B. Impact of toll-like receptors [TLR] 4 gene polymorphisms in susceptibility of cervical cancer in North Indian women. Gynecol. Oncol. 2009, 114, 501–505. [Google Scholar] [CrossRef]
- Storey, A.; Thomas, M.; Kalita, A.; Harwood, C.; Gardiol, D.; Mantovani, F.; Breuer, J.; Leigh, I.M.; Matlashewski, G.; Banks, L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 1998, 393, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Tayeb, F.J.; Barnawi, J.; Jalal, M.M.; Saeedi, N.H.; Hamadi, A.; Altayar, M.A.; Alshammari, S.E.; Mtiraoui, N.; Ali, M.E.; et al. Biochemical Characterization and Molecular Determination of Estrogen Receptor-α (ESR1 PvuII-rs2234693 T>C) and MiRNA-146a (rs2910164 C>G) Polymorphic Gene Variations and Their Association with the Risk of Polycystic Ovary Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 3114. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Pathology and epidemiology of HPV infection in females. Gynecol. Oncol. 2010, 117, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Morales, V.; Peralta-Zaragoza, O.; Alcocer-González, J.; Moreno, J.; Madrid-Marina, V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol. Med. Rep. 2011, 4, 369–375. [Google Scholar] [PubMed]
- Chauhan, P.; Pramodh, S.; Hussain, A.; Elsori, D.; Lakhanpal, S.; Kumar, R.; Alsaweed, M.; Iqbal, D.; Pandey, P.; Al Othaim, A.; et al. Understanding the role of miRNAs in cervical cancer pathogenesis and therapeutic responses. Front. Cell Dev. Biol. 2024, 12, 1397945. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in breastmilk and the lactating breast: Potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef] [PubMed]
- Mok, E.T.Y.; Chitty, J.L.; Cox, T.R. miRNAs in pancreatic cancer progression and metastasis. Clin. Exp. Metastasis 2024, 41, 163–186. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, S.; Liu, P.; Wang, J.; Du, H. Potential role of microRNAs in the treatment and diagnosis of cervical cancer. Cancer Genet. 2020, 248–249, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-M.; Wang, X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim. Biophys. Acta 2011, 1809, 668–677. [Google Scholar] [CrossRef]
- Zhang, Y.; Dakic, A.; Chen, R.; Dai, Y.; Schlegel, R.; Liu, X. Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation. Oncotarget 2017, 8, 96323–96339. [Google Scholar] [CrossRef]
- Au Yeung, C.L.; Tsang, T.Y.; Yau, P.L.; Kwok, T.T. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 2011, 30, 2401–2410. [Google Scholar] [CrossRef]
- Ivanov, M.K.; Titov, S.E.; Dzyubenko, V.V.; Glushkov, S.A.; Krasilnikov, S.E.; Mansurova, A.S.; Malek, A.V.; Berlev, I.V.; Prisyazhnaya, T.S.; Kuleshova, S.V.; et al. Detection of Cervical Lesions and Cancer in Air-Dried Cytologic Smears by Combined Analysis of mRNA and miRNA Expression Levels. J. Mol. Diagn. 2021, 23, 541–554. [Google Scholar] [CrossRef]
- Greco, D.; Kivi, N.; Qian, K.; Leivonen, S.K.; Auvinen, P.; Auvinen, E. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS ONE 2011, 6, e21646. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Geng, L.; Zhao, L.; Zuo, P.; Wang, J. Human papillomavirus E6-regulated microRNA-20b promotes invasion in cervical cancer by targeting tissue inhibitor of metalloproteinase 2. Mol. Med. Rep. 2017, 16, 5464–5470. [Google Scholar] [CrossRef]
- Pedroza-Torres, A.; López-Urrutia, E.; García-Castillo, V.; Jacobo-Herrera, N.; Herrera, L.A.; Peralta-Zaragoza, O.; López-Camarillo, C.; De Leon, D.C.; Fernández-Retana, J.; Cerna-Cortés, J.F.; et al. MicroRNAs in cervical cancer: Evidences for a miRNA profile deregulated by HPV and its impact on radio-resistance. Molecules 2014, 19, 6263–6281. [Google Scholar] [CrossRef]
- Melar-New, M.; Laimins, L.A. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J. Virol. 2010, 84, 5212–5221. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Chiou, S.-H.; Su, Y. Maintenance of the stemness in CD44[+] HCT-15 and HCT-116 human colon cancer cells requires miR-203 suppression. Stem Cell Res. 2014, 12, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Fullár, A.; Karászi, K.; Hollósi, P.; Lendvai, G.; Reszegi, L.O.A.; Papp, Z.; Sobel, G.; Dudás, J.; Kovalszky, I. Two ways of epigenetic silencing of TFPI2 in cervical cancer. PLoS ONE 2020, 15, e0234873. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Rao, Q.; Liu, L.; Zheng, C.; Xie, Q.; Liang, J.; Lin, Z. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in cervical cancer. Virol. J. 2013, 10, 175. [Google Scholar] [CrossRef]
- Wilting, S.M.; van Boerdonk, R.A.; Henken, F.E.; Meijer, C.J.; Diosdado, B.; Meijer, G.A.; le Sage, C.; Agami, R.; Snijders, P.J.; Steenbergen, R.D. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol. Cancer 2010, 9, 167. [Google Scholar] [CrossRef]
- Wilting, S.M.; Verlaat, W.; Jaspers, A.; Makazaji, N.A.; Agami, R.; Meijer, C.J.; Snijders, P.J.; Steenbergen, R.D. Methylation-mediated transcriptional repression of microRNAs during cervical carcinogenesis. Epigenetics 2013, 8, 220–228. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial tom esenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Pereira, P.M.; Marques, J.P.; Soares, A.R.; Carreto, L.; Santos, M.A. MicroRNA expression variability in human cervical tissues. PLoS ONE 2010, 5, e11780. [Google Scholar] [CrossRef] [PubMed]
- Causin, R.L.; Freitas, A.J.A.D.; Trovo Hidalgo Filho, C.M.; Reis, R.D.; Reis, R.M.; Marques, M.M.C. A Systematic Review of MicroRNAs Involved in Cervical Cancer Progression. Cells 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Alenquer, M.; Amorim, M. Exosome biogenesis, regulation, and function in viral infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793. [Google Scholar] [CrossRef]
- Mirzaei, H.; Sahebkar, A.; Jaafari, M.R.; Goodarzi, M.; Mirzaei, H.R. Diagnostic and Therapeutic Potential of Exosomes in Cancer: The Beginning of a New Tale? J. Cell. Physiol. 2017, 232, 3251–3260. [Google Scholar] [CrossRef]
- Guenat, D.; Hermetet, F.; Prétet, J.-L.; Mougin, C. Exosomes and Other Extracellular Vesicles in HPV Transmission and Carcinogenesis. Viruses 2017, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of CDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Khan, S.; Aspe, J.R.; Asumen, M.G.; Almaguel, F.; Odumosu, O.; Acevedo-Martinez, S.; De Leon, M.; Langridge, W.H.; Wall, N.R. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br. J. Cancer 2009, 100, 1073–1086. [Google Scholar] [CrossRef]
- Khan, S.; Jutzy, J.M.; Aspe, J.R.; McGregor, D.W.; Neidigh, J.W.; Wall, N.R. Survivin is released from cancer cells via exosomes. Apoptosis Int. J. Program. Cell Death 2011, 16, 1–12. [Google Scholar] [CrossRef]
- Bridgewood, C.; Stacey, M.; Alase, A.; Lagos, D.; Graham, A.; Wittmann, M. IL-36γ has proinflammatory effects on human endothelial cells. Exp. Dermatol. 2017, 26, 402–408. [Google Scholar] [CrossRef]
- Wang, W.; Yu, X.; Wu, C.; Jin, H. IL-36γ inhibits differentiation and induces inflammation of keratinocyte via WNT signaling pathway in psoriasis. Int. J. Med. Sci. 2017, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Merola, M.; Ferrario, E.; Trabattoni, D.; Villa, M.L.; Stefanon, B.; Venzon, D.J.; Shearer, G.M.; De Palo, G.; Clerici, E. Cytokine production patterns in cervical intraepithelial neoplasia: Association with human papillomavirus infection. J. Natl. Cancer Inst. 1997, 89, 245–250. [Google Scholar] [CrossRef]
- Gezer, U.; Ozgur, E.; Cetinkaya, M.; Isin, M.; Dalay, N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol. Int. 2014, 38, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sultmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef]
- Chiantore, M.V.; Mangino, G.; Iuliano, M.; Zangrillo, M.S.; De Lillis, I.; Vaccari, G.; Accardi, R.; Tommasino, M.; Columba Cabezas, S.; Federico, M.; et al. Human papillomavirus E6 and E7 oncoproteins affect the expression of cancer-related microRNAs: Additional evidence in HPV-induced tumorigenesis. J. Cancer Res. Clin. Oncol. 2016, 142, 1751–1763. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 2017, 508, 63–69. [Google Scholar] [CrossRef]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M.; et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Console, L.; Scalise, M.; Indiveri, C. Exosomes in inflammation and role as biomarkers. Clin. Chim. Acta 2018, 488, 165–171. [Google Scholar] [CrossRef]
- Pulliero, A.; Cassatella, G.; Astuni, P.; Izzotti, A. The Role of microRNA Expression and DNA Methylation in HPV-Related Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12714. [Google Scholar] [CrossRef]
- Jiménez-Wences, H.; Peralta-zaragoza, O.; Fernández-Tilapa, G. Human papilloma virus, DNA methylation and microRNA expression in cervical cancer. Oncol. Rep. 2014, 31, 2467–2476. [Google Scholar] [CrossRef]
- Chaiwongkot, A.; Vinokurova, S.; Pientong, C.; Ekalaksananan, T.; Kongyingyoes, B.; Kleebkaow, P.; Chumworathayi, B.; Patarapadungkit, N.; Reuschenbach, M.; von Knebel, D.M. Differential methylation of E2 binding sites in episomal and integrated HPV 16 genomes in preinvasive and invasive cervical lesions. Int. J. Cancer 2013, 132, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Burdier, F.R.; Waheed, D.-E.-N.; Nedjai, B.; Steenbergen, R.D.M.; Poljak, M.; Baay, M.; Vorsters, A.; Van Keer, S. DNA methylation as a triage tool for cervical cancer screening—A meeting report. Prev. Med. Rep. 2024, 41, 102678. [Google Scholar] [CrossRef]
- Sen, P.; Ganguly, P.; Ganguly, N. Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncol. Lett. 2018, 15, 11–22. [Google Scholar] [CrossRef]
- Ayala-Calvillo, E.; Mojica-Vázquez, L.H.; García-Carrancá, A.; González-Maya, L. Wnt/β-catenin pathway activation and silencing of the APC gene in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2018, 17, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Ohtani, N.; Yamakoshi, K.; Takahashi, A.; Hara, E. The p16INK4a-RB pathway: Molecular link between cellular senescence and tumor suppression. J. Med. Investig. 2004, 51, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Vink, F.J.; Dick, S.; Heideman, D.A.M.; De Strooper, L.M.A.; Steenbergen, R.D.M.; Lissenberg-Witte, B.I.; Floore, A.; Bonde, J.H.; Oštrbenk Valenčak, A.; Poljak, M.; et al. Classification of High-Grade Cervical Intraepithelial Neoplasia by P16[Ink4a], Ki-67, HPV E4 and FAM19A4/MiR124-2 Methylation Status Demonstrates Considerable Heterogeneity with Potential Consequences for Management. Int. J. Cancer 2021, 149, 707–716. [Google Scholar] [CrossRef]
- Narayan, G.; Arias-Pulido, H.; Koul, S.; Vargas, H.; Zhang, F.F.; Villella, J.; Schneider, A.; Terry, M.B.; Mansukhani, M.; Murty, V.V. Frequent promoter methylation of CDH1, DAPK, RARB, and HIC1 genes in carcinoma of cervix uteri: Its relationship to clinical outcome. Mol. Cancer 2003, 2, 24. [Google Scholar] [CrossRef]
- Yin, F.F.; Wang, N.; Bi, X.N.; Yu, X.; Xu, X.H.; Wang, Y.L.; Zhao, C.Q.; Luo, B.; Wang, Y.K. Serine/threonine kinases 31[STK31] may be a novel cellular target gene for the HPV16 oncogene E7 with potential as a DNA hypomethylation biomarker in cervical cancer. Virol. J. 2016, 13, 60. [Google Scholar] [CrossRef]
- Da Silva, M.L.R.; De Albuquerque, B.H.D.R.; De Medeiros Fernandes Allyrio, T.A.; De Almeida, V.D.; De Oliveira Cobucci, R.N.; Bezerra, F.L.; Andrade, V.S.; Lanza, D.C.F.; De Azevedo, J.C.V.; De Araújo, J.M.G.; et al. The methylation of the HPV genome is significant as it influences viral replication and the advancement of HPV-related cervical lesions. Biomed. Rep. 2021, 15, 60. [Google Scholar] [CrossRef]
- Filho, S.M.; Bertoni, N.; Brant, A.C.; Vidal, J.P.; Felix, S.P.; Cavalcanti, S.M.; Carestiato, F.N.; Martins, L.F.; Almeida, L.M.; Moreira, M.A. Methylation at 3’LCR of HPV16 can be affected by patient age and disruption of E1 or E2 genes. Virus Res. 2017, 232, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Vink, F.J.; Meijer, C.J.L.M.; Clifford, G.M.; Poljak, M.; Oštrbenk, A.; Petry, K.U.; Rothe, B.; Bonde, J.; Pedersen, H.; de Sanjosé, S.; et al. FAM19A4/MiR124-2 Methylation in Invasive Cervical Cancer: A Retrospective Cross-Sectional Worldwide Study. Int. J. Cancer 2020, 147, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft: Global Strategy Towards Eliminating Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/m/item/draft-global-strategy-towards-eliminating-cervical-cancer-as-a-public-health-problem (accessed on 20 March 2025).
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.R.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef]
- Chatterjee, A. The next generation of HPV vaccines: Nonavalent vaccine V503 on the horizon. Expert. Rev. Vaccines 2014, 13, 1279–1290. [Google Scholar] [CrossRef]
- Dominik, P.; Sonja, M.K.; Robert, J.; Przybylski, M. Effect of vaccination against HPV in the HPV-positive patients not covered by primary prevention on the disappearance of infection. Sci. Rep. 2025, 15, 12642. [Google Scholar] [CrossRef]
- Jørgensen, L.; Gøtzsche, P.C.; Jefferson, T. Benefits and harms of the human papillomavirus (HPV) vaccines: Systematic review with meta-analyses of trial data from clinical study reports. Syst. Rev. 2020, 9, 43. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Lawson, H.W.; Chesson, H.; Unger, E.R.; Centers for Disease Control and Prevention [CDC]; Advisory Committee on Immunization Practices [ACIP]. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices [ACIP]. MMWR Recomm. Rep. 2007, 56, 1–30. [Google Scholar]
- Centers for Disease Control and Prevention [CDC]. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices [ACIP], 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1705–1708. [Google Scholar]
- American College of Obstetricians and Gynecologists’ Committee on Adolescent Health Care; American College of Obstetricians and Gynecologists’ Immunization, Infectious Disease, and Public Health Preparedness Expert Work Group. Human Papillomavirus Vaccination: ACOG Committee Opinion, Number 809. Obstet. Gynecol. 2020, 136, e15–e21. [Google Scholar] [CrossRef]
- Abdullahi, L.H.; Kagina, B.M.; Ndze, V.N.; Hussey, G.D.; Wiysonge, C.S. Improving vaccination uptake among adolescents. Cochrane Database Syst. Rev. 2020, 1, CD011895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadaf, A.; Richards, J.L.; Glanz, J.; Salmon, D.A.; Omer, S.B. A systematic review of interventions for reducingparental vaccine refusal and vaccine hesitancy. Vaccine 2013, 31, 4293–4304. [Google Scholar] [CrossRef]
- Dunn, A.G.; Leask, J.; Zhou, X.; Mandl, K.D.; Coiera, E. Associations between exposure to and expression of negative opinions about human papillomavirus vaccines on social media: An observational study. J. Med. Internet Res. 2015, 17, e144. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Singh, S.V. HPV Vaccine Scenario in India—A Short Report. Madridge J. Vaccines 2018, 2, 62–63. [Google Scholar] [CrossRef]
- Basu, P.; Malvi, S.G.; Joshi, S.; Bhatla, N.; Muwonge, R.; Lucas, E. Vaccine efficacy against persistent human papillomavirus [HPV] 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multi-centre, prospective, cohort study. Lancet Oncol. 2021, 22, 1518–1529. [Google Scholar] [CrossRef]
- Afonso, N.M.; Kavanagh, M.J.; Swanberg, S.M.; Schulte, J.M.; Wunderlich, T.; Lucia, V.C. Will they lead by example? Assessment of vaccination rates and attitudes to human papilloma virus in millennial medical students. BMC Public Health 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.W.A.; Lam, P.L.; Chan, T.K.; Chau, K.W.; Hsu, M.L.; Lim, Y.M.; Lo, C.H.; Siu, L.; Tang, H.F.; Tong, A.M.J.M.; et al. A Cross-Sectional Study on Knowledge, Attitude and Practice related to Human Papillomavirus Vaccination for Cervical Cancer Prevention between Medical and Non-Medical Students in Hong Kong. Asian Pac. J. Cancer Prev. 2017, 18, 1689–1695. [Google Scholar]
- Singh, J.; Baliga, S.S. Knowledge regarding cervical cancer and HPV vaccine among medical students: A cross-sectional study. Clin. Epidem Glob. Health 2021, 9, 289–292. [Google Scholar] [CrossRef]
- National Health Mission. National Programme for Prevention & Control of Cancer, Diabetes, Cardiovascular Diseases & Stroke [NPCDCS]; National Health Mission: Bhopal, India, 2024.
- Indian Council of Medical Research, National Centre for Disease Informatics and Research. National Cancer Registry Programme Report; Indian Council of Medical Research, National Centre for Disease Informatics and Research: Bangalore, India, 2020.
- ICMR-National Centre for Disease Informatics and Research. Clinicopathological Profile of Cancers in India: A Report of the Hospital Based Cancer Registries, 2021; ICMR-National Centre for Disease Informatics and Research: Bangalore, India, 2021; Available online: https://ncdirindia.org/All_Reports/HBCR_2021/Default.aspx (accessed on 5 February 2025).
- Clifford, G.M.; Rana, R.K.; Franceschi, S.; Smith, J.S.; Gough, G.; Pimenta, J.M. Distribution of human papillomavirus genotypes in low-grade cervical lesions: Comparison by geographical regions and with cervical cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1157–1164. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Naz, S.; Ahmed, O.; Yaqeen, S.R.; Irfan, M.; Asif, M.G.; Kamal, A.; Faridi, N. Comparison of Liquid-Based Cytology and Conventional Papanicolaou Smear for Cervical Cancer Screening: An Experience from Pakistan. Cureus 2020, 12, e12293. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shashikala, N.; Khan, M.A.; Srinivas, V.; Bhat, K.; Karnam, R.; Madhivanan, P. Understanding Knowledge Gaps in HPV Infection and Vaccination among Medical Students and Its Implications on Current and Future Trends of HPV Prevention Strategy in India. Int. J. Pharm. Clin. Res. 2025, 17, 141–150. [Google Scholar]
- Kaarthigeyan, K. Cervical cancer in India and HPV vaccination. Indian. J. Med. Paediatr. Oncol. 2012, 33, 7–12. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, K.L.; Jacobson, R.M.; Radecki Breitkopf, C.; Wilson, P.M.; Jacobson, D.J.; Fan, C.; St Sauver, J.L.; Finney Rutten, L.J. Trends Over Time in Pap and Pap-HPV Cotesting for Cervical Cancer Screening. J. Womens Health 2019, 28, 244–249. [Google Scholar] [CrossRef]
- Wentzensen, N.; Clarke, M.A.; Perkins, R.B. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improve resilience and reduce disparities. Prev. Med. 2021, 151, 106596. [Google Scholar] [CrossRef]
- Tranberg, M.; Bech, B.H.; Blaakær, J.; Jensen, J.S.; Svanholm, H.; Andersen, B. Preventing cervical cancer using HPV self-sampling: Direct mailing of test-kits increases screening participation more than timely opt-in procedures—A randomized controlled trial. BMC Cancer 2018, 18, 273. [Google Scholar] [CrossRef]
- Arbyn, M.; de Sanjose, S.; Weiderpass, E. HPV-based cervical cancer screening, including self-sampling, versus screening with cytology in Argentina. Lancet Glob. Health 2019, 7, e688–e689. [Google Scholar] [CrossRef]
| Immune Component | HPV Strategy | Involved Viral Proteins |
|---|---|---|
| Th1/Th2 balance | Shift to Th2 phenotype (immunosuppression) | E6, E7 |
| CD8⁺ CTLs | Impaired antigen presentation via MHC-I downregulation | E5, E7 |
| Tregs | Expansion and increased IL-10/TGF-β production | E7 |
| TAMs | Recruitment and pro-tumor cytokine secretion | E5, E6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavelescu, L.A.; Mititelu-Zafiu, N.L.; Mindru, D.E.; Vladareanu, R.; Curici, A. Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications. Microorganisms 2025, 13, 1000. https://doi.org/10.3390/microorganisms13051000
Pavelescu LA, Mititelu-Zafiu NL, Mindru DE, Vladareanu R, Curici A. Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications. Microorganisms. 2025; 13(5):1000. https://doi.org/10.3390/microorganisms13051000
Chicago/Turabian StylePavelescu, Luciana Alexandra, Nicoleta Larisa Mititelu-Zafiu, Dana Elena Mindru, Radu Vladareanu, and Antoanela Curici. 2025. "Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications" Microorganisms 13, no. 5: 1000. https://doi.org/10.3390/microorganisms13051000
APA StylePavelescu, L. A., Mititelu-Zafiu, N. L., Mindru, D. E., Vladareanu, R., & Curici, A. (2025). Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications. Microorganisms, 13(5), 1000. https://doi.org/10.3390/microorganisms13051000





