The Vaginally Exposed Extracellular Vesicle of Gardnerella vaginalis Induces RANK/RANKL-Involved Systemic Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
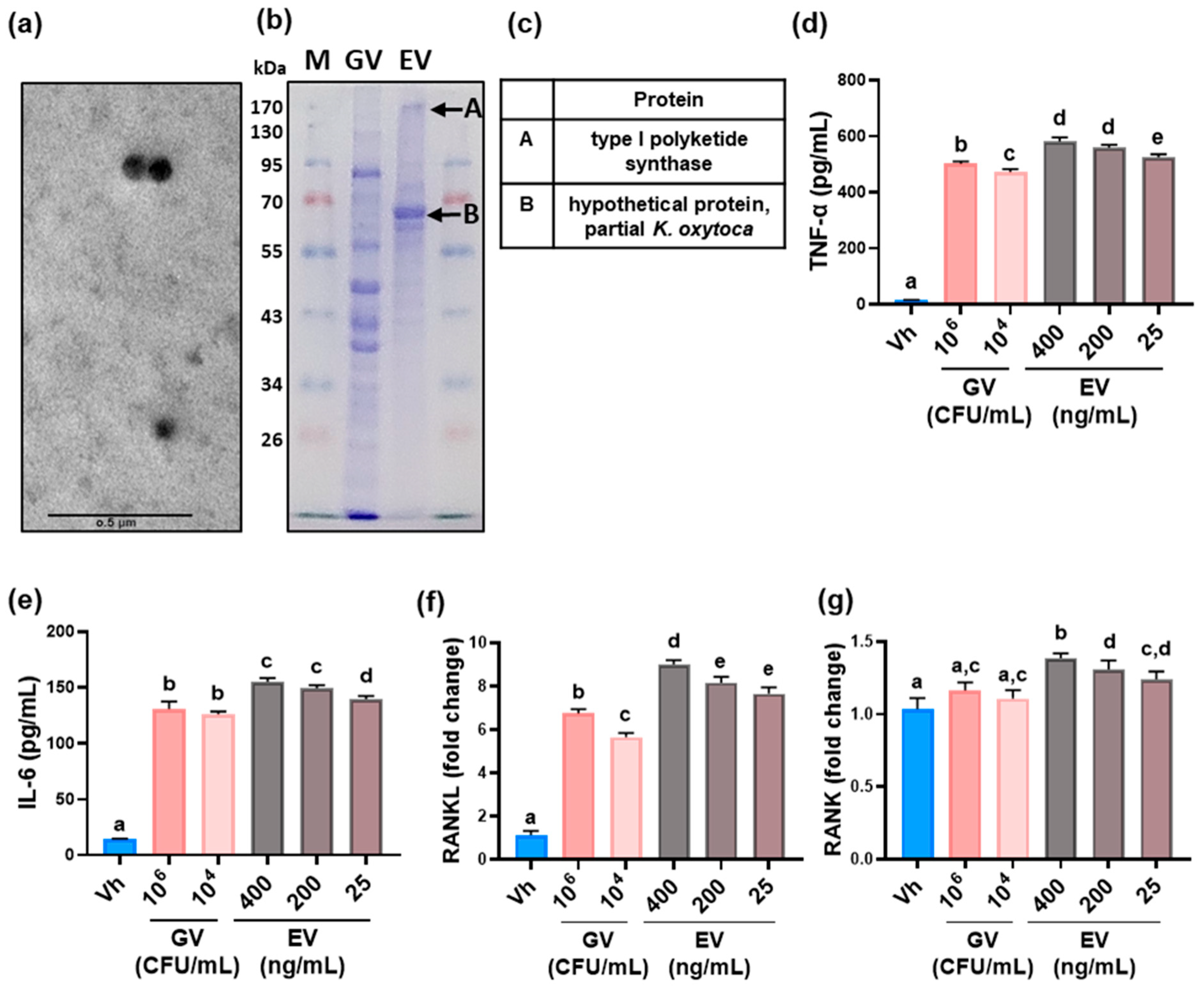
2.1. GV Culture and gEV Preparation
2.2. Animals and Housing
2.3. Macrophages
2.4. Exposure to GV or gEV in the Vagina of Mice
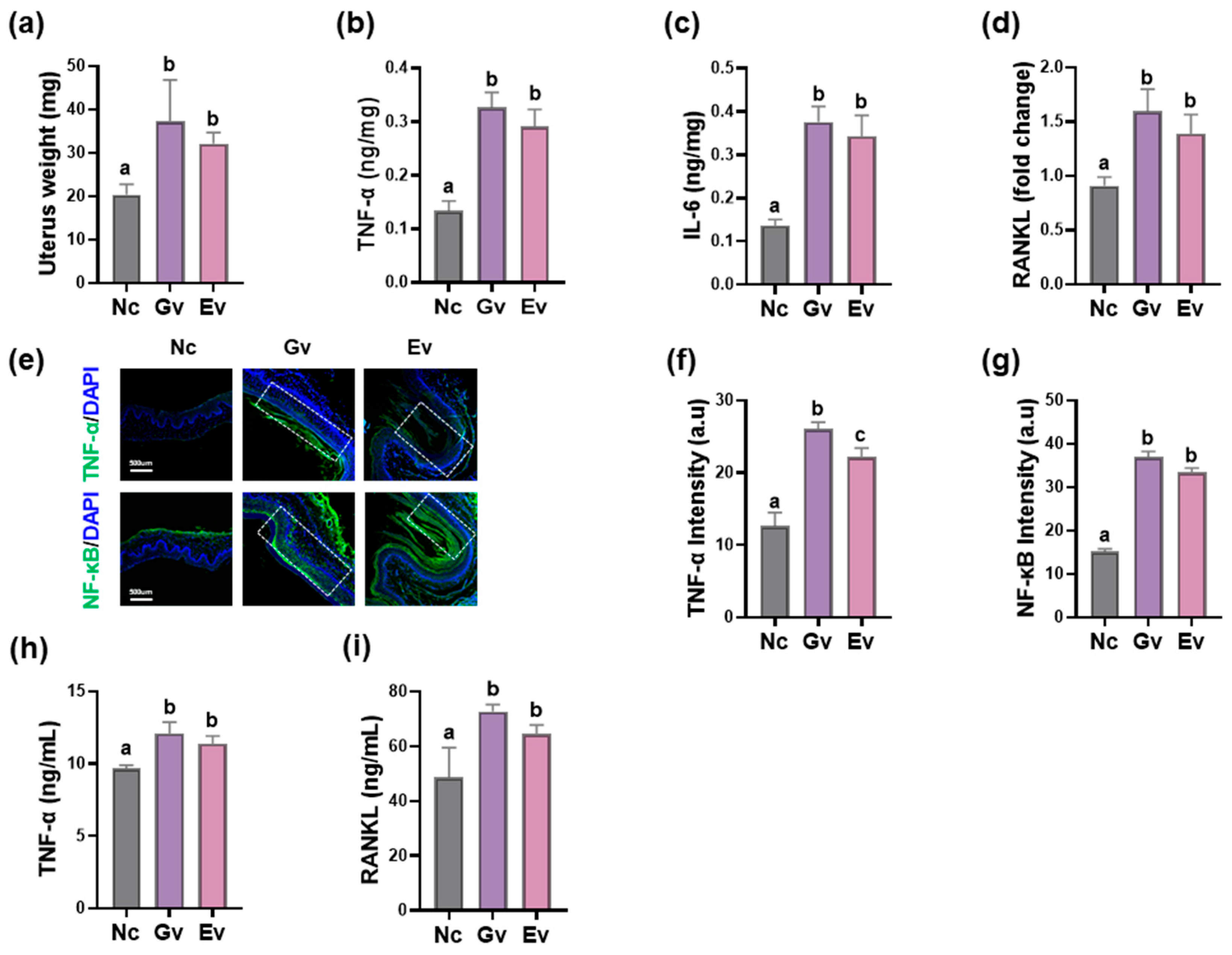
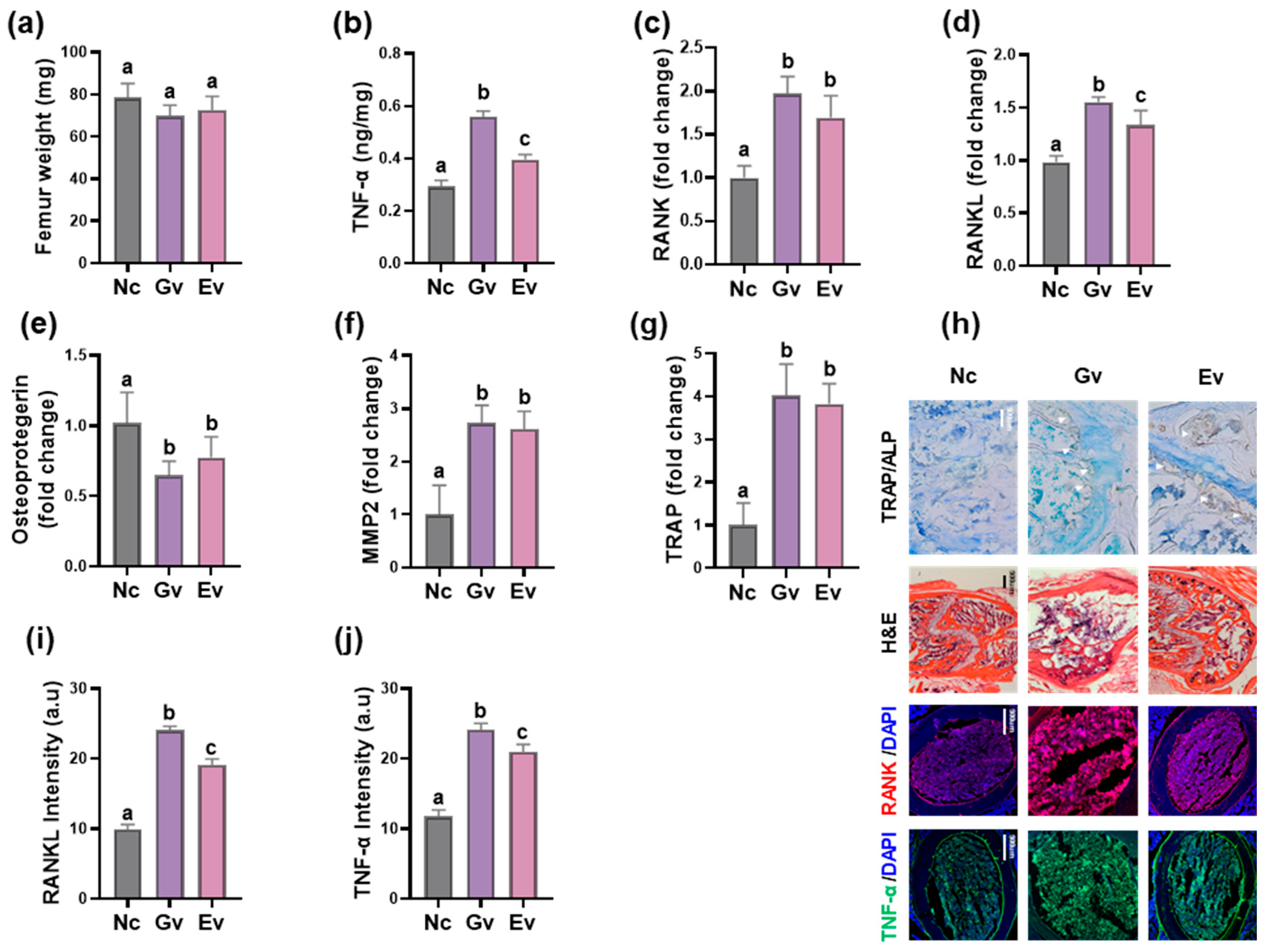
- Experiment 1—To investigate whether gEVs could induce systemic inflammation, mice were divided into three groups: Nc, Gv, and Ev. GV (1 × 106 CFU/0.02 mL) and gEVs (10 µg protein/0.02 mL saline) were vaginally applied to Gv and Ev groups daily for 5 days, respectively. The Nc group received saline alone.
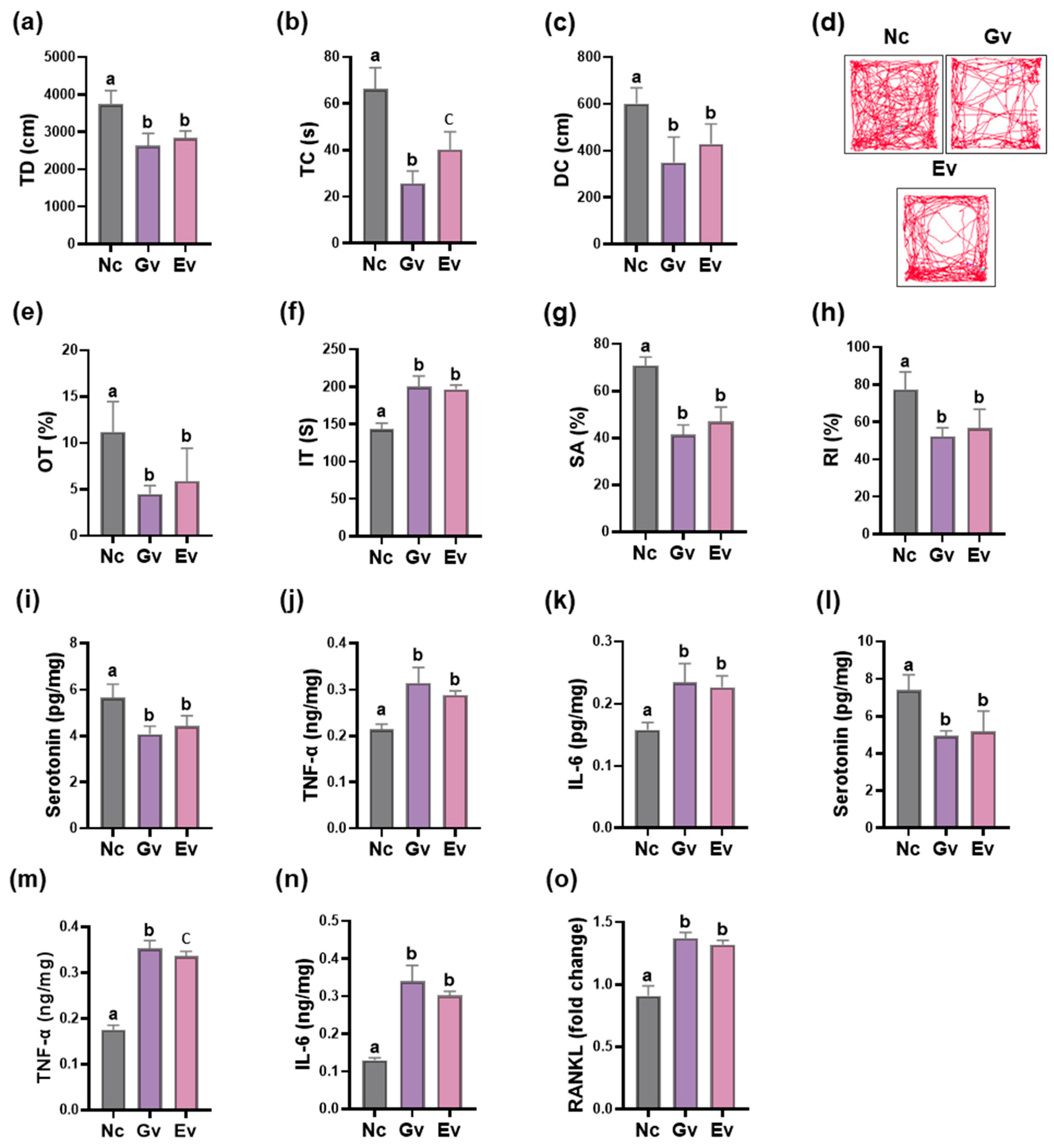
- Experiment 2—To examine the impact of OV on gEV-induced systemic inflammation, mice were divided into four groups: Sham (Sh), Ov, oGv (OV + GV), and oEv (OV + gEV). The Sh group was operated on without the resection of bilateral ovaries. All mice other than the Sh group underwent a bilateral ovary removal operation under 2.5% isoflurane, while Sh mice underwent an operation without ovary removal. GV (1 × 106 CFU/0.02 mL) and gEVs (10 µg protein/0.02 mL saline) were vaginally applied to oGv and oEv groups daily for 5 days, respectively. Sh and Ov mice received sterilized saline.
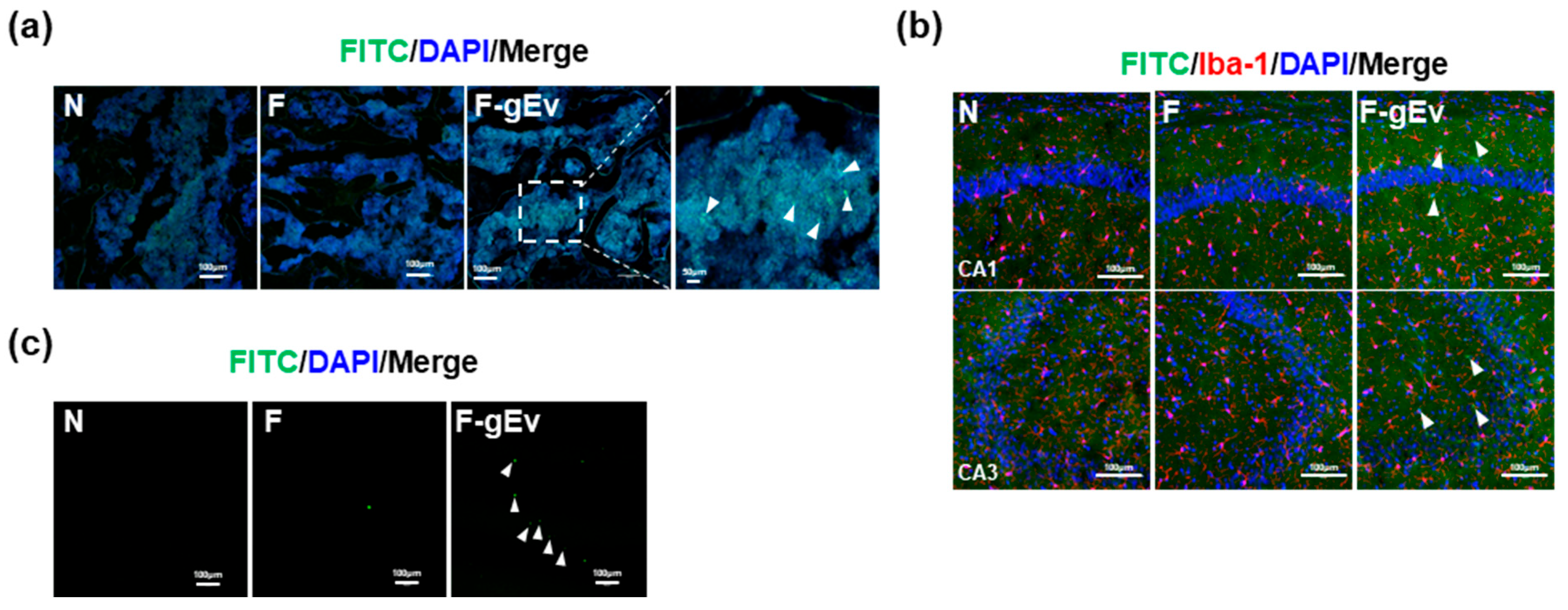
- Experiment 3—To explore the translocated organs of vaginally applied gEVs, mice were divided into three groups: Nc, Ft, and fEv. F-gEV (2 µg/0.02 mL saline/day) was vaginally applied to the fEv mice daily for 3 days. Nc and Ft groups received normal saline and FITC (0.02 mL/day), respectively.
2.5. Behavioral Tasks
2.6. ELISA and qPCR
2.7. Immunofluorescence, Hematoxylin and Eosin (H&E), and Tartrate-Resistant Acid Phosphatase (TRAP)/Alkaline Phosphatase (ALP) Staining
2.8. Statistical Analysis
3. Results
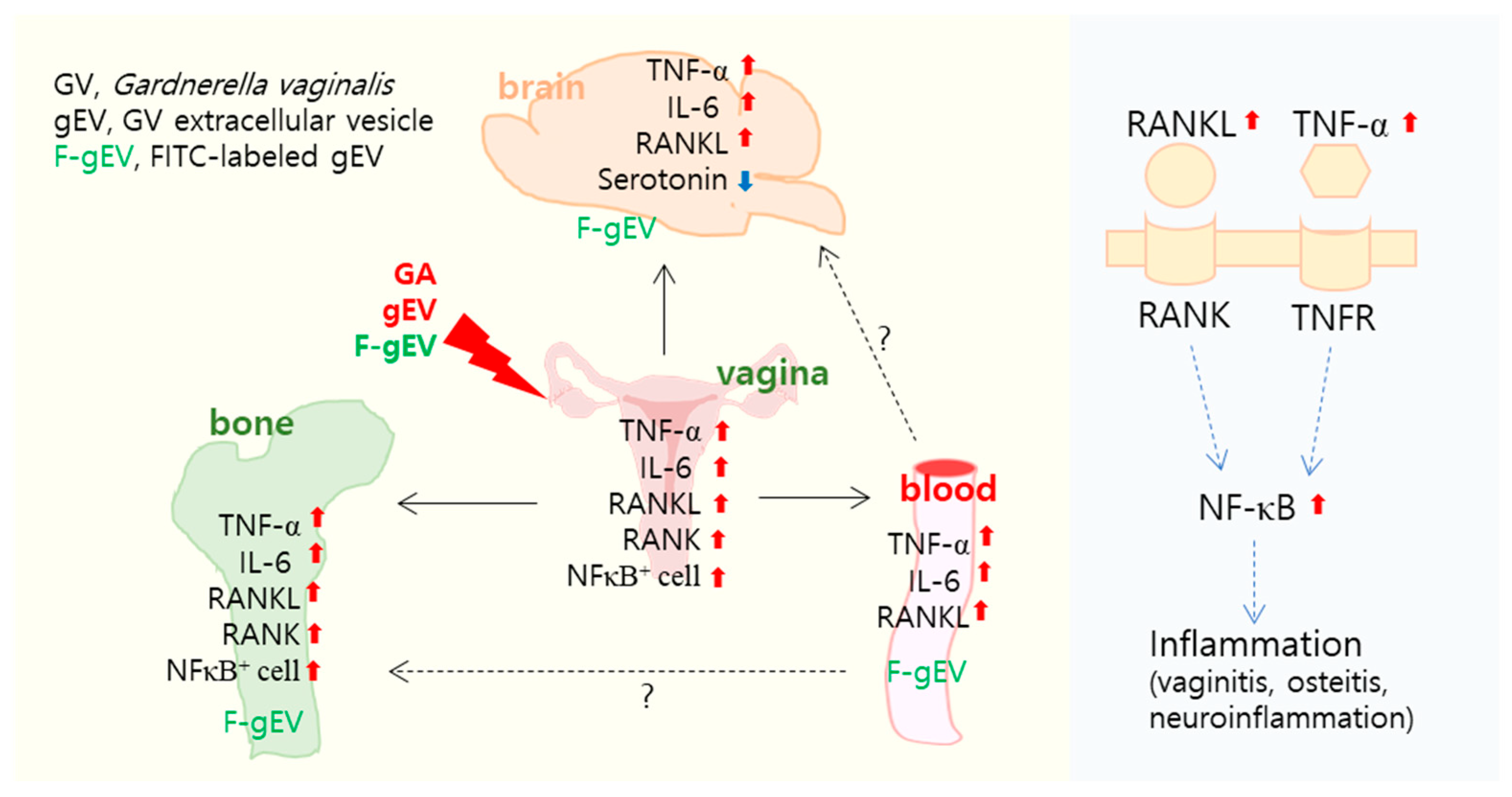
3.1. Vaginally Exposed gEV Caused Vaginitis, Osteitis, and Neuroinflammation in Mice
3.2. Vaginally Exposed gEV Deteriorated Vaginitis, Osteitis, and Neuroinflammation in Ovariectomized Mice
3.3. FITC-Labeled gEV Administered Vaginally Is Transported into the Bloodstream, Hippocampus, and Femur
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease-What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Oliveira, R.; Soares, R.; Barata, P. Influence of gut microbiota dysbiosis on brain function: A systematic review. Porto Biomed. J. 2020, 5, 1. [Google Scholar] [CrossRef]
- Jemimah, S.; Chabib, C.M.M.; Hadjileontiadis, L.; AlShehhi, A. Gut microbiome dysbiosis in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0285346. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.F.; Filippone, R.T.; Stavely, R.; Bornstein, J.C.; Apostolopoulos, V.; Nurgali, K. Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J. Neuroinflamm. 2022, 19, 4. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.K.; Joo, M.K.; Shin, Y.J.; Lee, C.K.; Kim, H.J.; Kim, D.H. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci. Rep. 2021, 11, 20406. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Min, X.; Wang, G.; Cui, Y.; Meng, P.; Hu, X.; Liu, S.; Wang, Y. Association between inflammatory cytokines and symptoms of major depressive disorder in adults. Front. Immunol. 2023, 14, 1110775. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Maeng, S.H.; Hong, H. Inflammation as the Potential Basis in Depression. Int. Neurourol. J. 2019, 23, S63–S71. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Heulsmann, A.; Tondravi, M.M.; Mukherjee, A.; Abu-Amer, Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 2001, 276, 563–568. [Google Scholar] [CrossRef]
- Zha, L.; He, L.; Liang, Y.; Qin, H.; Yu, B.; Chang, L.; Xue, L. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 2018, 102, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Samra, M.S.; Lim, D.H.; Han, M.Y.; Jee, H.M.; Kim, Y.K.; Kim, J.H. Bacterial Microbiota-derived Extracellular Vesicles in Children with Allergic Airway Diseases: Compositional and Functional Features. Allergy Asthma Immunol. Res. 2021, 13, 56–74. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Ma, X.; Shin, Y.J.; Yoo, J.W.; Park, H.S.; Kim, D.H. Extracellular vesicles derived from Porphyromonas gingivalis induce vagus nerve-mediated cognitive impairment. J. Adv. Res. 2023, 54, 293–303. [Google Scholar] [CrossRef]
- Lee, K.E.; Kim, J.K.; Han, S.K.; Lee, D.Y.; Lee, H.J.; Yim, S.V.; Kim, D.H. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome 2020, 8, 107. [Google Scholar] [CrossRef]
- Ma, X.; Park, H.S.; Shin, Y.J.; Kim, J.K.; Hong, J.K.; Han, S.W.; Yoon, I.Y.; Kim, D.H. The extracellular vesicle of depressive patient-derived Escherichia fergusonii induces vagus nerve-mediated neuroinflammation in mice. J. Neuroinflamm. 2024, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Ma, X.; Joo, M.K.; Baek, J.S.; Kim, D.H. Lactococcus lactis and Bifidobacterium bifidum alleviate postmenopausal symptoms by suppressing NF-κB signaling and microbiota dysbiosis. Sci. Rep. 2024, 14, 31675. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, K.E.; Lee, S.A.; Jang, H.M.; Kim, D.H. Interplay Between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice. Front. Immunol. 2020, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Lee, K.E.; Kim, D.H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kim, J.K.; Shin, Y.J.; Park, H.S.; Lee, D.Y.; Yim, S.V.; Kim, D.H. Lipopolysaccharide-producing Veillonella infantium and Escherichia fergusonii cause vagus nerve-mediated cognitive impairment in mice. Brain Behav. Immun. 2024, 118, 136–148. [Google Scholar] [CrossRef]
- Ceddia, T.; Cappa, F.; Cialfi, R.; Gioia, G.; Cassone, A. Prevalence of non-specific vaginitis and correlation with isolation of Gardnerella vaginalis in Italian outpatients. Eur. J. Epidemiol. 1989, 5, 529–531. [Google Scholar] [CrossRef]
- Luo, G.; Li, F.; Li, X.; Wang, Z.G.; Zhang, B. TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol. Med. Rep. 2018, 17, 6605–6611. [Google Scholar] [CrossRef]
- Kohli, S.S.; Kohli, V.S. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–181. [Google Scholar] [CrossRef]
- Brusselmans, J.; De Sutter, A.; Devleesschauwer, B.; Verstraelen, H.; Cools, P. Scoping review of the association between bacterial vaginosis and emotional, sexual and social health. BMC Womens Health 2023, 23, 168. [Google Scholar] [CrossRef]
- Chen, K.; Wang, T.; Tong, X.; Song, Y.; Hong, J.; Sun, Y.; Zhuang, Y.; Shen, H.; Yao, X.I. Osteoporosis is associated with depression among older adults: A nationwide population-based study in the USA from 2005 to 2020. Public Health 2024, 226, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fujita, Y.; Chang, L.; Pu, Y.; Qu, Y.; Wang, S.; Hashimoto, K. Beneficial effects of anti-RANKL antibody in depression-like phenotype, inflammatory bone markers, and bone mineral density in male susceptible mice after chronic social defeat stress. Behav. Brain Res. 2020, 379, 112397. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Chung, Y.; Kim, B.; Lee, J.H.; Lee, K.; Lee, Y.; Lee, Y.H.; Ahn, S.Y.; Kim, Y.G.; Hwang, H.S.; et al. Effect of RANKL on Lower Depressive Symptoms In Hemodialysis Patients. Calcif. Tissue Int. 2024, 115, 124–131. [Google Scholar] [CrossRef]
- Verma, A.; Kohli, G.S.; Harwood, D.T.; Ralph, P.J.; Murray, S.A. Transcriptomic investigation into polyketide toxin synthesis in Ostreopsis (Dinophyceae) species. Environ. Microbiol. 2019, 21, 4196–4211. [Google Scholar] [CrossRef]
- Darby, A.; Lertpiriyapong, K.; Sarkar, U.; Seneviratne, U.; Park, D.S.; Gamazon, E.R.; Batchelder, C.; Cheung, C.; Buckley, E.M.; Taylor, N.S.; et al. Cytotoxic and pathogenic properties of Klebsiella oxytoca isolated from laboratory animals. PLoS ONE 2014, 9, e100542. [Google Scholar] [CrossRef]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Joo, M.K.; Ma, X.; Yoo, J.W.; Shin, Y.J.; Kim, H.J.; Kim, D.H. Patient-derived Enterococcus mundtii and its capsular polysaccharides cause depression through the downregulation of NF-κB-involved serotonin and BDNF expression. Microbes Infect. 2023, 25, 105116. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.-J.; Ma, X.; Baek, J.-S.; Kim, D.-H. The Vaginally Exposed Extracellular Vesicle of Gardnerella vaginalis Induces RANK/RANKL-Involved Systemic Inflammation in Mice. Microorganisms 2025, 13, 955. https://doi.org/10.3390/microorganisms13040955
Shin Y-J, Ma X, Baek J-S, Kim D-H. The Vaginally Exposed Extracellular Vesicle of Gardnerella vaginalis Induces RANK/RANKL-Involved Systemic Inflammation in Mice. Microorganisms. 2025; 13(4):955. https://doi.org/10.3390/microorganisms13040955
Chicago/Turabian StyleShin, Yoon-Jung, Xiaoyang Ma, Ji-Su Baek, and Dong-Hyun Kim. 2025. "The Vaginally Exposed Extracellular Vesicle of Gardnerella vaginalis Induces RANK/RANKL-Involved Systemic Inflammation in Mice" Microorganisms 13, no. 4: 955. https://doi.org/10.3390/microorganisms13040955
APA StyleShin, Y.-J., Ma, X., Baek, J.-S., & Kim, D.-H. (2025). The Vaginally Exposed Extracellular Vesicle of Gardnerella vaginalis Induces RANK/RANKL-Involved Systemic Inflammation in Mice. Microorganisms, 13(4), 955. https://doi.org/10.3390/microorganisms13040955




