Effects of Spent Mushroom Substrate Treated with Plant Growth-Promoting Rhizobacteria on Blueberry Growth and Soil Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PGPR Strains and Detection of SMS Bacteria Toxicity
2.2. Quality Analysis of SMS Treated by PGPR at Room Temperature
2.3. Transplanting of Blueberry Seedlings and Analysis of Growth Status
2.4. Analysis of Nutrient Element Contents and Microbial Diversity in Blueberry Seedlings Rhizosphere Soil
2.5. Statistical Analysis
3. Results
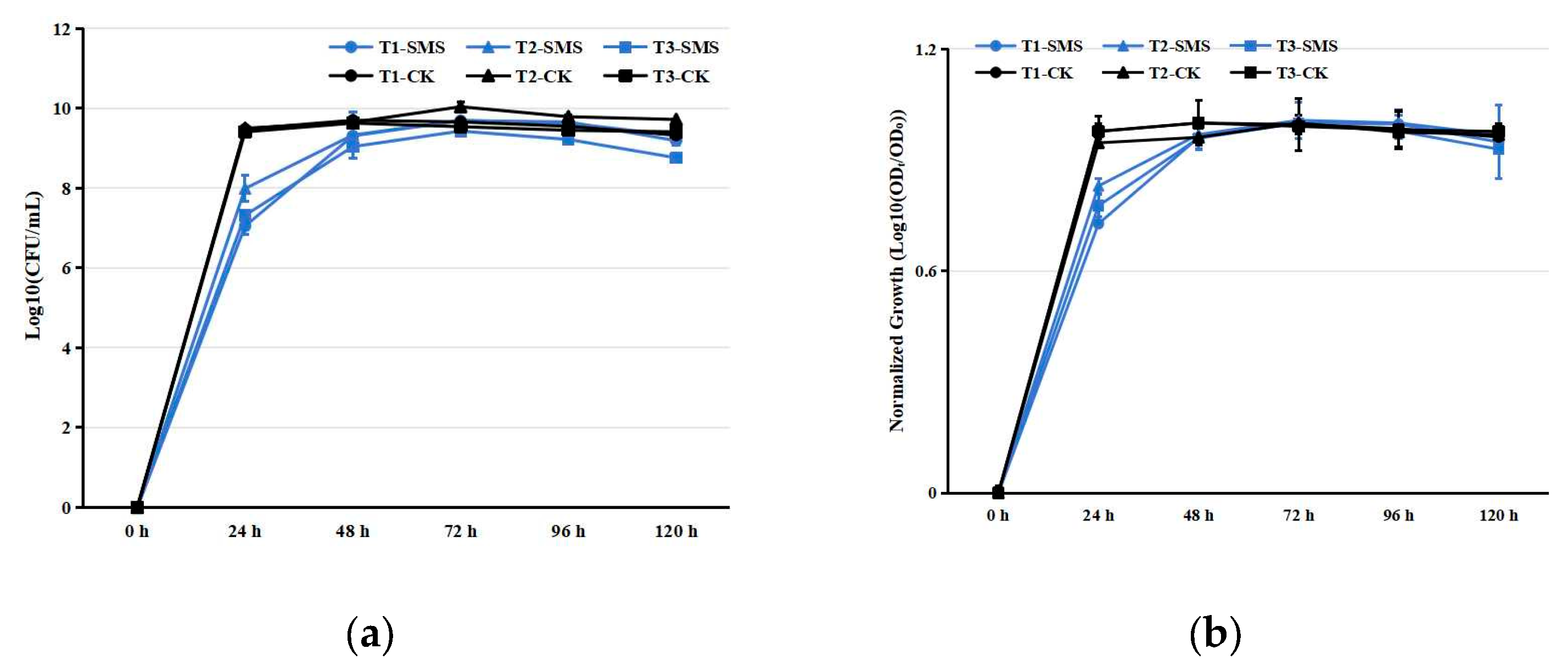
3.1. Assessment of SMS Bacterial Toxicity and Quality Analysis of SMS Treated by PGPRs at Room Temperature
3.2. Analysis of Growth Performance of Blueberry Seedlings
3.3. Analysis of Nutrient Elements in Rhizosphere Soil
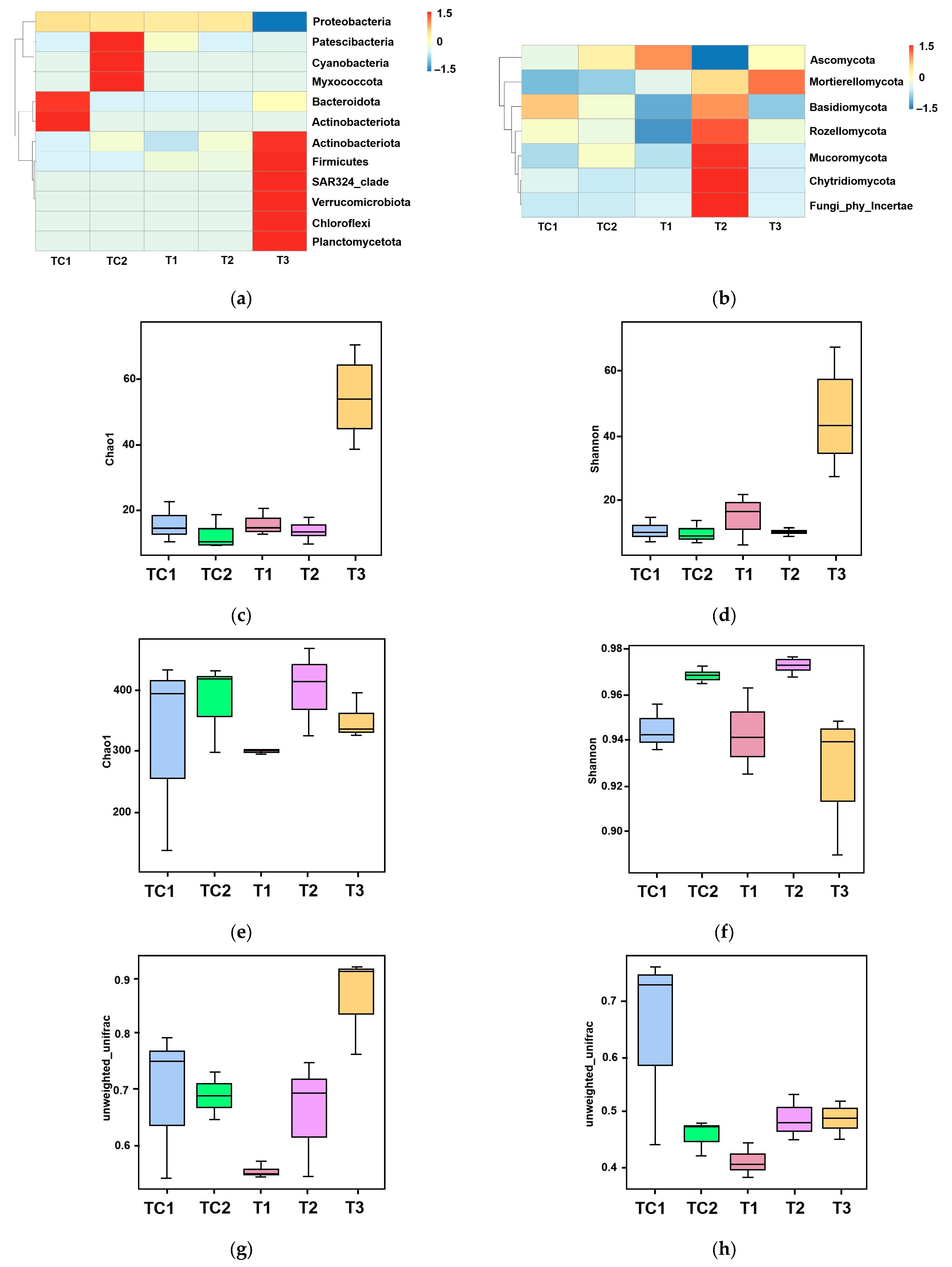
3.4. Analysis of Rhizosphere Soil Microbial Community
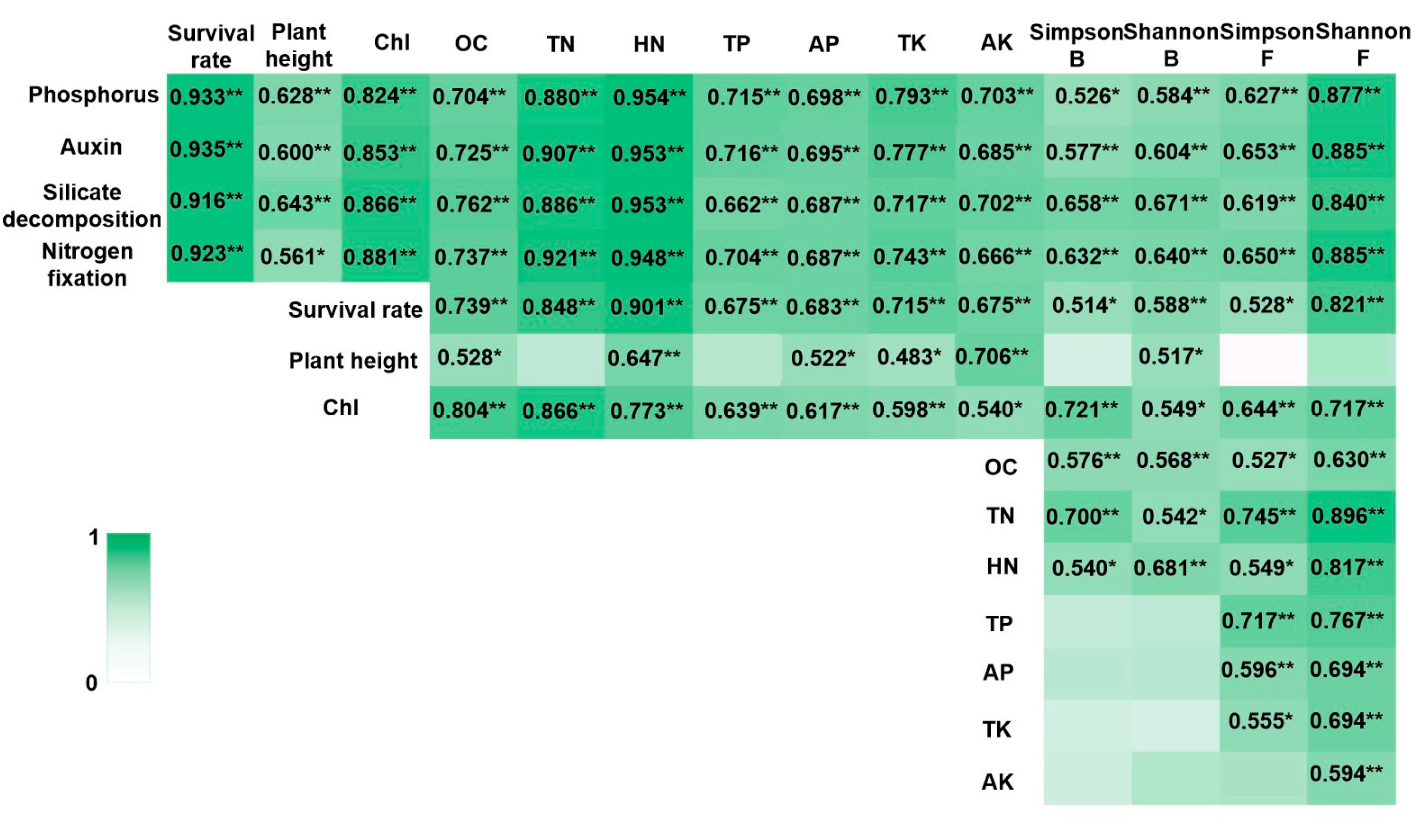
3.5. Correlations Between Growth-Promoting Characteristics of PGPR Strains and Plant Growth, Soil Element Content, and Rhizosphere Microbial Diversity
4. Discussion
4.1. Detection of SMS Bacterial Toxicity and Quality Analysis of SMS Treated by PGPRs at Room Temperature
4.2. PGPR-Treated Substrates Promote the Growth of Transplanted Blueberry Seedlings
4.3. PGPR-Treated Substrates Improve the Properties of Cultivation Medium for Soil Maintenance
4.4. Correlations Between Growth-Promoting Characteristics of PGPR Strains and Plant Growth, Soil Nutrient Content, and Rhizosphere Microbial Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sousa, A.S.; Araújo-Rodrigues, H.; Pintado, M.E. The Health-promoting Potential of Edible Mushroom Proteins. Curr. Pharm. Des. 2023, 29, 804–823. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, B.R.; Wagner, G.V.J.; Rafael, B.P.; Lucas, D.S.A.; Cinthia, E.C.C.; Marcos, A.D.S.F.; Diego, C.Z. A cascade approach to sustainable agriculture: From mushroom mycelium to lettuce harvest. Sci. Total Environ. 2024, 944, 173976. [Google Scholar] [CrossRef]
- Baptista, F.; Almeida, M.; Paié-Ribeiro, J.; Barros, A.N.; Rodrigues, M. Unlocking the Potential of Spent Mushroom Substrate (SMS) for Enhanced Agricultural Sustainability: From Environmental Benefits to Poultry Nutrition. Life 2023, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture (SOFIA) 2022: Towards Blue Transformation. 2022. Available online: https://www.fao.org/3/cc0461en/cc0461en.pdf (accessed on 23 January 2025).
- Grand View Research. Mushroom Market Size, Share & Trends Analysis Report by Product (Button, Shiitake, Oyster), by Form (Fresh, Processed), by Application (Food, Pharmaceuticals), by Region, and Segment Forecasts, 2023–2030 (Report GVR-4-68039-2023). 2023. Available online: https://www.grandviewresearch.com/industry-analysis/mushroom-market (accessed on 23 January 2025).
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent substrate from mushroom cultivation: Exploitation potential toward various applications and value-added products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef]
- Leong, Y.K.; Varjani, S.; Lee, D.J.; Chang, J.S. Valorization of spent mushroom substrate for low-carbon biofuel production: Recent advances and developments. Bioresour. Technol. 2022, 363, 128012. [Google Scholar] [CrossRef]
- Fortin, F.M.; Hijri, M.; Labrecque, M. Short rotation intensive culture of willow, spent mushroom substrate and ramial chipped wood for bioremediation of a contaminated site used for land farming activities of a former petrochemical plant. Plants 2021, 10, 520. [Google Scholar] [CrossRef]
- Alves, L.D.S.; Moreira, B.R.D.A.; Viana, R.D.S.; Pardo-Gimenez, A.; Dias, E.S.; Noble, N.; Zied, D.C. Recycling spent mushroom substrate into fuel pellets for low-emission bioenergy producing systems. J. Clean. Prod. 2021, 313, 127875. [Google Scholar] [CrossRef]
- Alves, L.D.S.; Moreira, B.R.A.; Viana, R.D.S.; Dias, E.S.; Rinker, D.L.; Pardo-Gimenez, A.; Zied, D.C. Spent mushroom substrate is capable of physisorption-chemisorption of CO2. Environ. Res. 2022, 204 Pt A, 111945. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic. Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Bibi, F.; Ilyas, N.; Arshad, M.; Khalid, A.; Saeed, M.; Ansar, S.; Batley, J. Formulation and efficacy testing of bio-organic fertilizer produced through solid-state aerobic decomposition of agro-waste by Burkholderia cenocepacia. Chemosphere 2022, 291 Pt 3, 132762. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Zhao, J.; Huang, R.; Li, R.; Shen, Q. Utilization of different waste proteins to create a novel PGPR-containing bio-organic fertilizer. Sci. Rep. 2015, 5, 7766. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.K.; Wang, Q.Y.; Li, J.S.; Yan, H.W.; Chen, Q.J.; Sun, J.; Liu, C.J.; Han, Y.Y.; Zou, Y.J.; Zhang, G.Q. Biochar immobilized plant growth-promoting rhizobacteria enhanced the physicochemical properties, agronomic characters and microbial communities during lettuce seedling. Front. Microbiol. 2023, 14, 1218205. [Google Scholar] [CrossRef]
- Zhang, X.; Borjigin, Q.; Gao, J.; Yu, X.; Zhang, B.; Hu, S.; Han, S.; Liu, R.; Zhang, S. Community succession and straw degradation characteristics using a microbial decomposer at low temperature. PLoS ONE 2022, 17, e0270162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, H.; Xu, Z. Analysis of blueberry plant rhizosphere bacterial diversity and selection of plant growth promoting rhizobacteria. Curr. Microbiol. 2022, 79, 331. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Dai, H.; Xu, Z. Characterization of plant-growth-promoting rhizobacteria for tea plant (Camellia sinensis) development and soil nutrient enrichment. Plants 2024, 13, 2659. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X. Effects of plant growth-promoting rhizobacteria on blueberry growth and rhizosphere soil microenvironment. PeerJ 2024, 12, e16992. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Xu, L.; Xu, Z.M. Bacterial diversity in tea plant (Camellia sinensis) rhizosphere soil from Qinling Mountains and its relationship with environmental elements. Plant Soil. 2021, 460, 403–415. [Google Scholar] [CrossRef]
- Kaur, S.; Atri, C.; Akhatar, J.; Mittal, M.; Kaur, R.; Banga, S. Genetics of days to fowering, maturity and plant height in natural and derived forms of Brassica rapa L. Theor. Appl. Genet. 2021, 134, 473–487. [Google Scholar] [CrossRef]
- Sehoefs, B.; Darko, E.; Rodermel, S. Photosynthetic Pigments, Photosynthesis and Plastid Ultrastructure in RbcS Antisense DNA Mutants of Tobacco (Nicotiana tabacum). Zeitschrift für Naturforschung C 2001, 56, 11–12. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Zhang, A.; Shao, G.; Ma, Z.; Yu, G.; Qin, C. Structure and assembly mechanisms of the microbial community on an artificial reef surface, Fangchenggang, China. Appl. Microbiol. Biotechnol. 2025, 109, 23. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Bagnoli, D.; Vergalito, F.; Testa, B.; Tremonte, P.; Succi, M.; Pannella, G.; Letizia, F.; Albanese, G.; Lombardi, S.J.; et al. Diversity of fungal communities on Cabernet and Aglianico grapes from vineyards located in Southern Italy. Front. Microbiol. 2024, 15, 1399968. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wu, M.; Che, S.; Yuan, S.; Yang, X.; Li, S.; Tian, P.; Wu, L.; Yang, M.; Wu, Z.J. Effects of Continuous Straw Returning on Soil Functional Microorganisms and Microbial Communities. J. Microbiol. 2023, 61, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lorenzana-Moreno, A.; Lara, H.; Corona, L.; Granados, O.; Márquez-Mota, C.C. Production of 17 strains of edible mushroom grown on corn stover and its effect on the chemical composition and ruminal in vitro digestibility of the residual substrate. PLoS ONE 2023, 18, e0286514. [Google Scholar] [CrossRef]
- Nagrale, D.T.; Chaurasia, A.; Kumar, S.; Gawande, S.P.; Hiremani, N.S.; Shankar, R.; Gokte-Narkhedkar, N.; Renu; Prasad, Y.G. PGPR: The treasure of multifarious beneficial microorganisms for nutrient mobilization, pest biocontrol and plant growth promotion in field crops. World J. Microbiol. Biotechnol. 2023, 39, 100. [Google Scholar] [CrossRef]
- Tobar-Bolaños, G.; Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Blueberry juice: Bioactive compounds, health impact, and concentration technologies-a review. J. Food Sci. 2021, 86, 5062–5077. [Google Scholar] [CrossRef]
- Tkachenko, O.V.; Evseeva, N.V.; Kargapolova, K.Y.; Denisova, A.Y.; Pozdnyakova, N.N.; Kulikov, A.A.; Burygin, G.L. Rhizobacteria Increase the Adaptation Potential of Potato Microclones under Aeroponic Conditions. Microorganisms 2024, 11, 1866. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Cheng, Z.; Zheng, X.; Cai, C.; Wang, H.; Lu, K.; Zhu, C.; Ding, Y. Important Factors Controlling Gibberellin Homeostasis in Plant Height Regulation. J. Agric. Food Chem. 2023, 71, 15895–15907. [Google Scholar] [CrossRef]
- Lamb, J.J.; Eaton-Rye, J.J.; Hohmann-Marriott, M.F. An LED-based fluorometer for chlorophyll quantification in the laboratory and in the field. Photosynth. Res. 2012, 114, 59–68. [Google Scholar] [CrossRef]
- Nurzhanova, A.A.; Pidlisnyuk, V.; Berzhanova, R.; Nurmagambetova, A.S.; Terletskaya, N.; Omirbekova, N.; Berkinbayev, G.; Mamirova, A. PGPR-driven phytoremediation and physiobiochemical response of Miscanthus giganteus to stress induced by the trace elements. Environ. Sci. Pollut. Res. Int. 2023, 30, 96098–96113. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, J.; Yang, Z.; Xu, C.; Liao, P.; Pu, S.; El-Kassaby, Y.A.; Feng, J. Role of soil nutrient elements transport on Camellia oleifera yield under different soil types. BMC Plant Biol. 2023, 23, 378. [Google Scholar] [CrossRef]
- Belimov, A.A.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Safronova, V.I.; Litvinskiy, V.A.; Nosikov, V.V.; Zavalin, A.A.; Tikhonovich, I.A. Microbial Consortium of PGPR, Rhizobia and Arbuscular Mycorrhizal Fungus Makes Pea Mutant SGECd(t) Comparable with Indian Mustard in Cadmium Tolerance and Accumulation. Plants 2024, 9, 975. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Jiang, Y.; Xu, J.; Ren, X.; Zhang, Y.; Wang, H.; Lu, Q.; Yan, J.; Ahmed, T.; et al. The effects of microbial fertilizer based Aspergillus brunneoviolaceus HZ23 on pakchoi growth, soil properties, rhizosphere bacterial community structure, and metabolites in newly reclaimed land. Front. Microbiol. 2023, 14, 1091380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, Y.; Yan, Z.; Yang, Z.; Tian, F.; Liu, X.; Wu, Z. Insight into the microbial mechanisms for the improvement of spent mushroom substrate composting efficiency driven by phosphate-solubilizing Bacillus subtilis. J. Environ. Manag. 2023, 336, 117561. [Google Scholar] [CrossRef] [PubMed]
- Ehinmitan, E.; Losenge, T.; Mamati, E.; Ngumi, V.; Juma, P.; Siamalube, B. BioSolutions for Green Agriculture: Unveiling the Diverse Roles of Plant Growth-Promoting Rhizobacteria. Int. J. Microbiol. 2024, 2024, 6181491. [Google Scholar] [CrossRef]
- De Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere bacterial interactions and impact on plant health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, L.; Nian, H.; Jin, J.; Lian, T. Linking plant functional genes to rhizosphere microbes: A review. Plant Biotechnol. J. 2022, 21, 902–917. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The Application of Arbuscular Mycorrhizal Fungi as Microbial Biostimulant, Sustainable Approaches in Modern Agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Wu, X.; Ye, J.; Cheng, F. Promoting the application of Pinus thunbergii Parl. to enhance the growth and survival rates of post-germination somatic plantlets. BMC Plant Biol. 2023, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lin, G.; Lv, H.; Wang, C.; Yang, Y.; Liao, H. Environmental and genetic regulation of plant height in soybean. BMC Plant Biol. 2021, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, J.; Zhang, R.; Wang, J.; Tian, D.; Niu, S. Environmental factors, bacterial interactions and plant traits jointly regulate epiphytic bacterial community composition of two alpine grassland species. Sci. Total Environ. 2022, 836, 155665. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Sun, D.; Xu, Z. Effects of Spent Mushroom Substrate Treated with Plant Growth-Promoting Rhizobacteria on Blueberry Growth and Soil Quality. Microorganisms 2025, 13, 932. https://doi.org/10.3390/microorganisms13040932
Wang M, Sun D, Xu Z. Effects of Spent Mushroom Substrate Treated with Plant Growth-Promoting Rhizobacteria on Blueberry Growth and Soil Quality. Microorganisms. 2025; 13(4):932. https://doi.org/10.3390/microorganisms13040932
Chicago/Turabian StyleWang, Mengjiao, Desheng Sun, and Zhimin Xu. 2025. "Effects of Spent Mushroom Substrate Treated with Plant Growth-Promoting Rhizobacteria on Blueberry Growth and Soil Quality" Microorganisms 13, no. 4: 932. https://doi.org/10.3390/microorganisms13040932
APA StyleWang, M., Sun, D., & Xu, Z. (2025). Effects of Spent Mushroom Substrate Treated with Plant Growth-Promoting Rhizobacteria on Blueberry Growth and Soil Quality. Microorganisms, 13(4), 932. https://doi.org/10.3390/microorganisms13040932







