Case Report: From Misdiagnosis to Accurate Identification: Managing a Case Series of Trichophyton rubrum Infections
Abstract
1. Introduction
2. Case Presentation Section: Patients and Therapy
2.1. First Patient
2.2. Second Patient
2.3. Third Patient
2.4. Fourth Patient
2.5. Fifth Patient
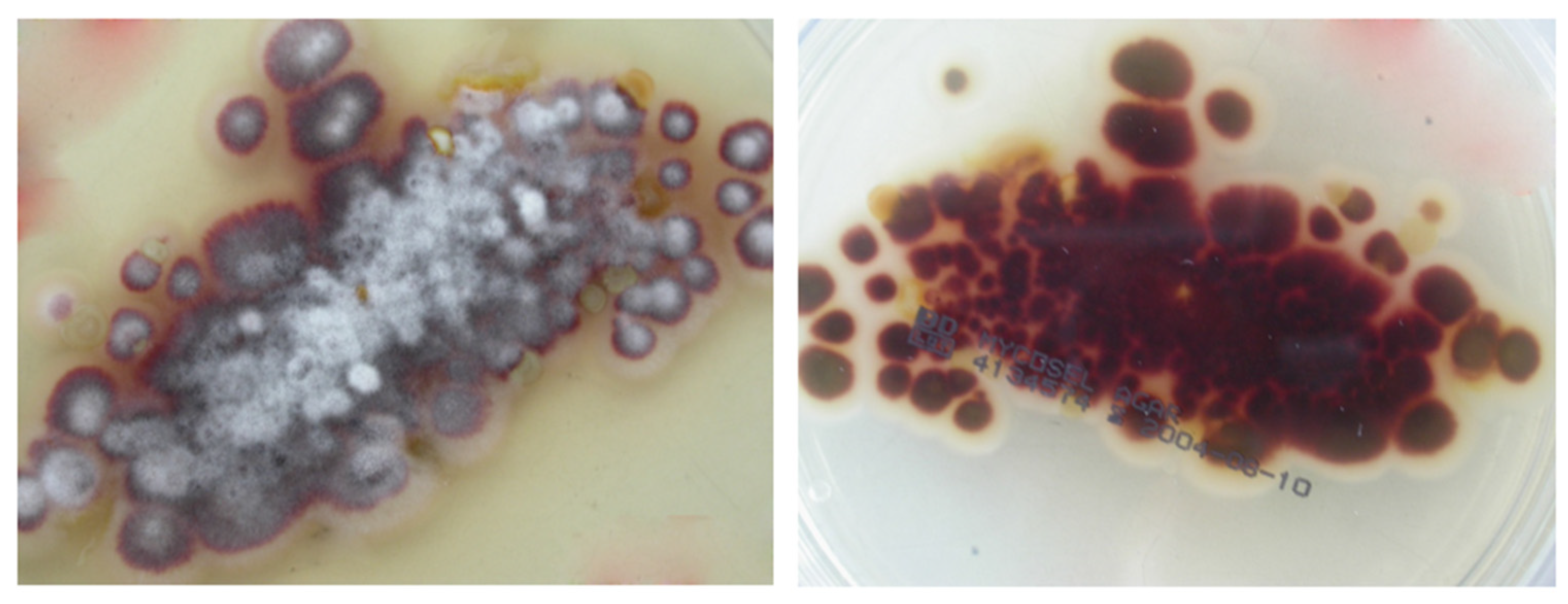
3. Mycological Study and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 12, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Li, H. The human skin microbiome in health and skin diseases. In Metagenomics of the Human Body; Nelson Karen, E., Ed.; Springer: Cham, Switzerland, 2011; pp. 145–163. [Google Scholar]
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Mahajan, R. Management of Tinea corporis, Tinea cruris, and Tinea pedis: A comprehensive review. Indian. Dermatol. Online J. 2016, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Pereira, F.; Gomes, S.M.; Lima da Silva, S.; de Castro Teixeira, P.A.; Lima, I.O. The prevalence of dermatophytoses in Brazil: A systematic review. J. Med. Microbiol. 2021, 70. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Bitencourt, T.A.; Martins, M.P.; Rossi, A. State-of-the-Art Dermatophyte Infections: Epidemiology Aspects, Pathophysiology, and Resistance Mechanisms. J. Fungi 2021, 7, 629. [Google Scholar] [CrossRef]
- Sy, O.; Diongue, K.; Ba, O.; Ahmed, C.B.; Elbechir, M.A.; Abdallahi, M.S.M.; Brahim, M.; Baidy, B.L.; Ndiaye, D. Tinea capitis in school children from Mauritania: A comparative study between urban and rural areas. J. Mycol. Med. 2021, 31, 101048. [Google Scholar] [CrossRef]
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef]
- Sacheli, R.; Cuypers, L.; Seidel, L.; Darfouf, R.; Adjetey, C.; Lagrou, K.; Hayette, M.P. Epidemiology of Dermatophytes in Belgium: A 5 Years’ Survey. Mycopathologia 2021, 186, 399–409, Erratum in Mycopathologia 2021, 186, 897. https://doi.org/10.1007/s11046-021-00590-w. [Google Scholar] [CrossRef]
- Gawdzik, A.; Nowogrodzka, K.; Hryncewicz-Gwóźdź, A.; Szepietowski, J.; Maj, J.; Jankowska-Konsur, A. Epidemiology of dermatophytoses in paediatric population in Southwestern Poland, 2011–2016. Postepy Dermatol. Alergol. 2021, 38, 91–95. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Mann, A.; Polla Ravi, S.; Talukder, M.; Lincoln, S.A.; Foreman, H.C.; Kaplan, B.; Galili, E.; Piguet, V.; et al. Antifungal resistance in dermatophytes—Review of the epidemiology, diagnostic challenges and treatment strategies for managing Trichophyton indotineae infections. Expert Rev. Anti Infect. Ther. 2024, 18, 739–751. [Google Scholar] [CrossRef]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 2024, 5, e594–e605. [Google Scholar] [CrossRef] [PubMed]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Celestrino, G.A.; Verrinder Veasey, J.; Benard, G.; Sousa, M.G.T. Host immune responses in dermatophytes infection. Mycoses 2021, 64, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe dermatophytosis and Acquired or Innate Immunodeficiency: A review. J. Fungi 2016, 2, 4. [Google Scholar] [CrossRef]
- Waldman, A.; Segal, R.; Berdicevsky, I.; Gilhar, A. CD4+ and CD8+ T cells mediated direct cytotoxic effect against Trichophyton rubrum and Trichophyton mentagrophytes. Int. J. Dermatol. 2010, 49, 149–157. [Google Scholar] [CrossRef]
- Larruskain, J.; Piñeiro, L.; Idigoras, P.; Perez-Trallero, E. Dermatophytosis with concurrent lesions in distant locations. Prognostic and therapeutic significance. Enferm. Infecc. Microbiol. Clin. 2005, 23, 191–193. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A. Major challenges and perspectives in the diagnostics and treatment of dermatophyte infections. J. Appl. Microbiol. 2020, 129, 212–232. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Cervetti, O.; Panzone, M.; Tullio, V. A case report of Tinea capitis in infant in first year of life. BMC Pediatr. 2019, 19, 65. [Google Scholar] [CrossRef]
- Bovio, E.; Garzoli, L.; Poli, A.; Prigione, V.; Firsova, D.; McCormack, G.P.; Varese, G.C. The culturable mycobiota associated with three Atlantic sponges, including two new species: Thelebolus balaustiformis and T. spongiae. Fungal Syst. Evol. 2018, 1, 141–167. [Google Scholar] [CrossRef]
- Huang, C.; Peng, Y.; Zhang, Y.; Li, R.; Wan, Z.; Wang, X. Deep dermatophytosis caused by Trichophyton rubrum. Lancet Infect. Dis. 2019, 19, 1380. [Google Scholar] [CrossRef]
- Patro, N.; Panda, M.; Jena, A.K. The menace of superficial dermatophytosis on the quality of life of patients attending Referral Hospital in Eastern India: A cross-sectional observational study. Indian Dermatol. Online J. 2019, 10, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S. Cutaneous fungal infections caused by dermatophytes and non-dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical presentations, and diagnostic testing. J. Fungi 2023, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Guo, K.; Qi, X.; Wang, H.; Chen, S. Deep dermatophytosis caused by Trichophyton rubrum. Mycopathologia 2021, 186, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Blutfield, M.S.; Lohre, J.M.; Pawich, D.A.; Vlahovic, T.C. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J. Fungi 2015, 1, 130–137. [Google Scholar] [CrossRef]
- Zaias, N.; Rebell, G. Chronic dermatophytosis syndrome due to Trichophyton rubrum. Int. J. Dermatol. 1996, 35, 614–617. [Google Scholar] [CrossRef]
- Kong, Q.T.; Du, X.; Yang, R.; Huang, S.Y.; Sang, H.; Liu, W.D. Chronically recurrent and widespread Tinea corporis due to Trichophyton rubrum in an immunocompetent patient. Mycopathologia 2015, 179, 293–297. [Google Scholar] [CrossRef]
- Kick, G.; Korting, H.C. The definition of Trichophyton rubrum syndrome. Mycoses 2001, 44, 167–171. [Google Scholar] [CrossRef]
- Ilkit, M.; Durdu, M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit. Rev. Microbiol. 2015, 41, 374–388. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Reich, A.; Garlowska, E.; Kulig, M.; Baran, E. Onychomycosis Epidemiology Study Group. Factors influencing coexistence of toenail onychomycosis with tinea pedis and other dermatomycoses: A survey of 2761 patients. Arch Dermatol. 2006, 142, 1279–1284. [Google Scholar] [CrossRef]
- Kawai, M.; Suzuki, T.; Hiruma, M.; Ikeda, S. A retrospective cohort study of Tinea pedis and Tinea unguium in patients in a psychiatric hospital. Med. Mycol. J. 2014, 55, E35–E41. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cooper, E.A. Update in antifungal therapy of dermatophytosis. Mycopathologia 2008, 166, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.H.; Arendrup, M.C. Molecular diagnosis of dermatophyte infections. Curr. Opin. Infect. Dis. 2012, 25, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Mir, N.A.; Lingaraju, M.C.; Buyamayum, B.; Dev, K. Recent advances in the diagnosis of dermatophytosis. J. Basic Microbiol. 2020, 60, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Atzori, L.; Pau, M.; Aste, N. Dermatophyte infections mimicking other skin diseases: A 154-person case survey of Tinea atypica in the district of Cagliari (Italy). Int. J. Dermatol. 2012, 51, 410–415. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New antifungal agents and new formulations against dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef]
- Roana, J.; Mandras, N.; Scalas, D.; Campagna, P.; Tullio, V. Antifungal Activity of Melaleuca alternifolia essential oil (TTO) and its synergy with itraconazole or ketoconazole against Trichophyton rubrum. Molecules 2021, 26, 461. [Google Scholar] [CrossRef]







| KOH Fungi | KOH Fungi | KOH Fungi | KOH Fungi | KOH Fungi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body Site | 1st Patient | 2nd Patient | 3rd Patient | 4th Patient | 5th Patient | |||||
| Soles/dorsum | + | T. rubrum | + | T. rubrum | + | T. rubrum | + | T. rubrum | + | T. rubrum |
| Toenails | + | T. rubrum | + | T. rubrum | + | T. rubrum | + | T. rubrum | + | T. rubrum |
| Legs/knee | -- | + | T. rubrum | + | T. rubrum | + | T. rubrum | + | T. rubrum | |
| Inguinal site | + | T. rubrum | + | T. rubrum | -- | + | T. rubrum | -- | ||
| Buttocks/thighs | + | T. rubrum | + | T. rubrum | -- | + | T. rubrum | -- | ||
| Palms | -- | -- | + | T. rubrum | + | T. rubrum | -- | |||
| Fingernails | + | T. rubrum | -- | -- | + | T. rubrum | -- | |||
| Face | -- | -- | -- | + | T. rubrum | + | T. rubrum | |||
| Scalp | -- | -- | + | T. rubrum | -- | -- | ||||
| Neckline/Abdomen/Back | -- | -- | -- | + | T. rubrum | + | T. rubrum | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tullio, V.; Panzone, M.; Cervetti, O.; Roana, J.; Mandras, N. Case Report: From Misdiagnosis to Accurate Identification: Managing a Case Series of Trichophyton rubrum Infections. Microorganisms 2025, 13, 895. https://doi.org/10.3390/microorganisms13040895
Tullio V, Panzone M, Cervetti O, Roana J, Mandras N. Case Report: From Misdiagnosis to Accurate Identification: Managing a Case Series of Trichophyton rubrum Infections. Microorganisms. 2025; 13(4):895. https://doi.org/10.3390/microorganisms13040895
Chicago/Turabian StyleTullio, Vivian, Michele Panzone, Ornella Cervetti, Janira Roana, and Narcisa Mandras. 2025. "Case Report: From Misdiagnosis to Accurate Identification: Managing a Case Series of Trichophyton rubrum Infections" Microorganisms 13, no. 4: 895. https://doi.org/10.3390/microorganisms13040895
APA StyleTullio, V., Panzone, M., Cervetti, O., Roana, J., & Mandras, N. (2025). Case Report: From Misdiagnosis to Accurate Identification: Managing a Case Series of Trichophyton rubrum Infections. Microorganisms, 13(4), 895. https://doi.org/10.3390/microorganisms13040895








