Seed-Borne Endophytes and Their Host Effects
Abstract
1. Introduction
2. The Taxonomic Diversity of SBEs
2.1. Bacterial SBEs
2.2. Fungal SBEs
2.3. Actinomycetic SBEs
3. Factors Affect Plant SBEs
3.1. Plant Genotype and Its SBEs
3.2. Effects of Environmental Factors on SBEs
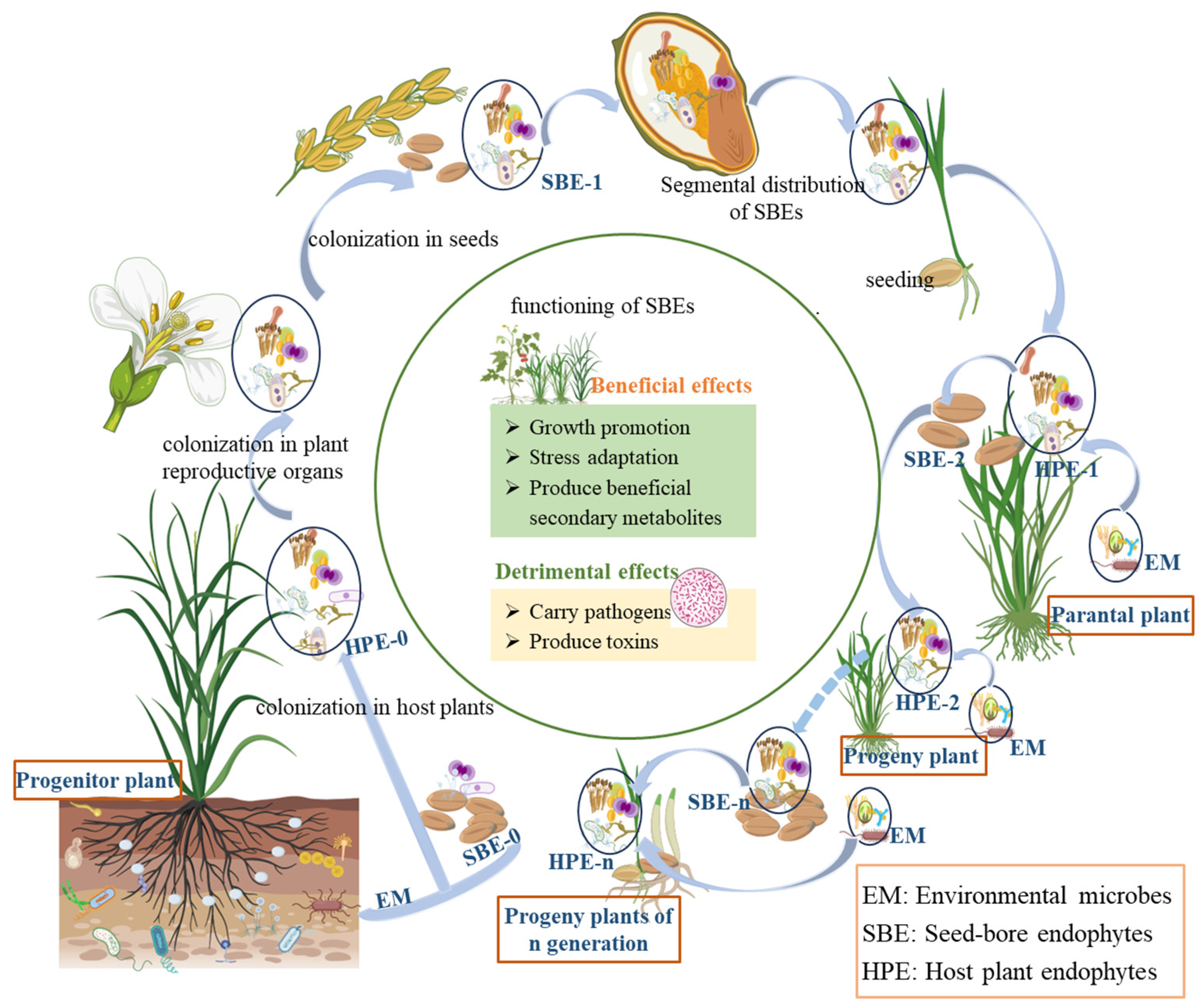
4. Acquisition and Transmission of SBEs
4.1. Colonization and Transmission of Plant SBEs
4.2. Characteristics of Seed-Borne Microorganisms
4.3. Distribution of SBEs in Seed Segments
5. Host Effects of SBEs
5.1. Beneficial Effects
5.1.1. Growth Promotion
5.1.2. Stress Adaptation
5.1.3. Produce Beneficial Secondary Metabolites
5.2. Detrimental Effects
5.2.1. Pathogenicity of Some SBEs
5.2.2. Produce Toxins
6. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EM | Environmental microbes |
| SBE | Seed-bore endophytes |
| HPE | Host plant endophytes |
| VTE | Vertically transmitted endophytes |
| PBM | Plant beneficial microorganisms |
References
- Stone, J.K.; Bacon, C.W.; White, J.F., Jr. (Eds.) An Overview of Endophytic Microbes: Endophytism Defined. In Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000; pp. 17–44. ISBN 978-0-429-17933-4. [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Llewellyn, T.; Downes, E.; Oddy, J.; MacIntosh, C.; Kallow, S.; Panis, B.; Dickie, J.B.; Gaya, E. Seed Banks as Incidental Fungi Banks: Fungal Endophyte Diversity in Stored Seeds of Banana Wild Relatives. Front. Microbiol. 2021, 12, 643731. [Google Scholar] [CrossRef]
- Zhai, K.-H.; Zhang, Y.-Y.; Gao, X.-Q. Research Progress on Mechanisms of Growth Promotion and Disease Resistance of Seed Endophytes. J. Agric. Biotechnol. 2023, 31, 1965–1979. (In Chinese) [Google Scholar]
- Xiang, Y.-Q.; Gan, Q.-X.; Li, N.; An, G.-Q.; Chen, L.-L.; Ma, Y.-T. Research Progress on Endophytes in Medicinal plant seeds. Pharm. Clin. Chin. Mater. Medica 2023, 14, 82–87. (In Chinese) [Google Scholar]
- Fan, Y.; Tang, X.-L.; Liu, Q.-H.; Yin, C.-Y. Advances in the Study of Endophytes in Woody Plant Seeds. Chin. J. Appl. Environ. Biol. 2022, 28, 1375–1383. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, K.-Y.; Liu, X.-L.; Dong, X.-Y.; Liu, R.-J.; He, L.-H.; Xie, Z.-H. Isolation of Endophytic Cultures from Sesbania cannabina Seeds and their Effects on Germination. J. Agric. Sci. Technol. 2020, 22, 40–48. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Wu, G.; Feng, H.; Zhang, G.; Shen, Q.; Zhang, R. Identification of Root-Secreted Compounds Involved in the Communication between Cucumber, the Beneficial Bacillus amyloliquefaciens, and the Soil-Borne Pathogen Fusarium oxysporum. Mol. Plant-Microbe Interact. 2017, 30, 53–62. [Google Scholar] [CrossRef]
- Xie, H.-L.; Wang, H.-C.; Cai, L.-T.; Zhou, H.; Liu, C.; Lu, N.; Shi, C.-H.; Wang, X.-P. Community Structure and Diversity of Endophytic Bacteria of Tobacco Seeds. Acta Microbiol. Sin. 2020, 60, 601–616. (In Chinese) [Google Scholar] [CrossRef]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of Grapevine Flowers, Berries, and Seeds: Identification of Cultivable Bacteria, Comparison with Other Plant Parts, and Visualization of Niches of Colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Zhu, K.-Y.; Han, S.-C.; Zhao, R.; Wen, Y.-J.; Hu, H.-C.; Qiao, Y.-M.; Lu, J.-F.; Cao, K.; Xu, Z.-H.; Bao, H.-Z. Isolation and Iientification of Endophytes from Sunflower Seeds. Crops 2023, 5, 280–284. (In Chinese) [Google Scholar] [CrossRef]
- Ferreira, A.; Quecine, M.C.; Lacava, P.T.; Oda, S.; Azevedo, J.L.; Araújo, W.L. Diversity of Endophytic Bacteria from Eucalyptus Species Seeds and Colonization of Seedlings by Pantoea Agglomerans. FEMS Microbiol. Lett. 2008, 287, 8–14. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Dong, C.-B.; Shao, Q.-Y.; Lu, Y.-X.; Dong, X.; Liang, Z.-Q.; Han, Y.-F. Community Composition and Ecological Functional analysis of the Endophytic Microorganisms in Eucommia ulmoides Seeds. For. Res. 2023, 36, 50–60. (In Chinese) [Google Scholar]
- Shi, X.-H.; Cheng, C.; Li, Y.-Q.; An, D.-D. Isolation and Characterization of Endophytic Colonizing Bacteria from the Seed of Caragana leucophloea Pojark. Microbiol. China 2016, 43, 164–171. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Zou, Y.; Wang, J.; Song, W. Investigation on Diversity and Population Succession Dynamics of Endophytic Bacteria from Seeds of Maize (Zea mays L., Nongda108) at Different Growth Stages. Ann. Microbiol. 2013, 63, 71–79. [Google Scholar] [CrossRef]
- Gong, W.-F.; Sun, Y.; Wang, R.-Q.; Wei, L.-P. Isolation of Endophytes from Rapeseeds in Tibet and Screening of Antagonistic Bacteria with Multiple Plant Growth Promoting Traits. J. Plant Prot. 2022, 49, 1053–1062. (In Chinese) [Google Scholar] [CrossRef]
- Kuźniar, A.; Włodarczyk, K.; Grządziel, J.; Woźniak, M.; Furtak, K.; Gałązka, A.; Dziadczyk, E.; Skórzyńska-Polit, E.; Wolińska, A. New Insight into the Composition of Wheat Seed Microbiota. Int. J. Mol. Sci. 2020, 21, 4634. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Geng, Y.-N.; Liu, L.; Gao, D.-M. Community Structure and Diversity of Endophytes of Bupleurum chinense DC. seeds. Biot. Resour. 2022, 44, 36–44. (In Chinese) [Google Scholar] [CrossRef]
- Qi, J.; Shi, S.-L. Preliminary Study on the Ability of Phosphorus-solubilizing and IAA-secreting of Endogenous Rhizobia in seeds of different alfalfa varieties. Grassl. Turf 2006, 18–20, 25. (In Chinese) [Google Scholar] [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic Bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef]
- Cankar, K.; Kraigher, H.; Ravnikar, M.; Rupnik, M. Bacterial Endophytes from Seeds of Norway Spruce (Picea abies L. Karst). FEMS Microbiol. Lett. 2005, 244, 341–345. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Wisniewski, M.; Schena, L.; Tack, A.J.M. Experimental Evidence of Microbial Inheritance in Plants and Transmission Routes from Seed to Phyllosphere and Root. Environ. Microbiol. 2021, 23, 2199–2214. [Google Scholar] [CrossRef]
- Maehara, S.; Agusta, A.; Kitamura, C.; Ohashi, K.; Shibuya, H. Composition of the Endophytic Filamentous Fungi Associated with Cinchona Ledgeriana Seeds and Production of Cinchona Alkaloids. J. Nat. Med. 2016, 70, 271–275. [Google Scholar] [CrossRef]
- Donnarumma, F.; Capuana, M.; Vettori, C.; Petrini, G.; Giannini, R.; Indorato, C.; Mastromei, G. Isolation and Characterisation of Bacterial Colonies from Seeds and in Vitro Cultures of Fraxinus Spp. from Italian Sites. Plant Biol. Stuttg. Ger. 2011, 13, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-C.; Wu, Q.-S.; Gao, Y.; Chen, Q.-Q.; Wang, Y.-Q.; Niu, X.-J.; Weng, Q.-B. Analysis of Composition of Endophytic Community of Dendrobium nobile LindI. seeds Based on High-throughput Sequencing. Seed 2020, 39, 94–98. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, A.-M.; Guo, B.-M.; Han, X.-Y.; Li, X.-R. Diversity of Endophytic Bacteria in Seeds of Hippophae rhamnoides subsp. sinensis in Two Different Habitats. Acta Ecol. Sin. 2020, 40, 5247–5257. (In Chinese) [Google Scholar]
- Xiang, Y.-Q.; Liao, H.-L.; Li, N.; Guo, F.-R.; Peng, M.-Y.; Ma, Y.-T. Isolation, Identification and Functional Verification of Bacteria from Seeds of Coptis chinensis. Nat. Prod. Res. Dev. 2023, 35, 191–199. (In Chinese) [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial Seed Endophyte Shapes Disease Resistance in Rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-Y.; Gao, J.-S.; Xu, F.-H.; Cao, Y.-H.; Tang, X.; Zhang, X.-X. Diversity of Endophytic Bacteria in Rice Seeds and their Secretion of Indole Acetic Acid. Acta Microbiol. Sin. 2013, 53, 269–275. (In Chinese) [Google Scholar] [CrossRef]
- Liu, W. Study of the Development of Seed and Endophytes Isolated from Ginkgo biloba. Master’s Thesis, Jiangsu Normal University, Xuzhou, China, 2015. (In Chinese). [Google Scholar]
- Cheng, C.; Li, H.; Liu, Y.; Li, J.-X.; Yao, S.; Bai, F.-R.; Tan, W.-Q. Investigation on Diversity of Entophytic Fungus Community in Xisha Wild Noni (Morinda citrifolia L.) seed. Food Ferment. Ind. 2013, 39, 7–10. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Zheng, B.; Hu, R.; Liu, Y.; Jing, Y.; Xiao, Y.; Sun, M.; Chen, W.; Zhou, Q. Pseudomonas Species Isolated from Tobacco Seed Promote Root Growth and Reduce Lead Contents in Nicotiana Tobacum K326. Can. J. Microbiol. 2019, 65, 214–223. [Google Scholar] [CrossRef]
- Mukherjee, A.; Singh, B.K.; Verma, J.P. Harnessing Chickpea (Cicer arietinum L.) Seed Endophytes for Enhancing Plant Growth Attributes and Bio-Controlling against Fusarium sp. Microbiol. Res. 2020, 237, 126469. [Google Scholar] [CrossRef]
- Shaik, S.P.; Thomas, P. In Vitro Activation of Seed-Transmitted Cultivation-Recalcitrant Endophytic Bacteria in Tomato and Host−endophyte Mutualism. Microorganisms 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ming, X.-D.; Zhang, X.-Y.; Hao, J.-J.; Fu, L.-P.; Wang, Q.-K.; Lv, X.; Chen, W.; Liu, Q.-L. Diversity of Endophytic Bacteria in Faba bean Seeds by High-throughput Sequencing. J. Agric. Sci. Technol. 2021, 23, 73–80. (In Chinese) [Google Scholar] [CrossRef]
- Feng, W.-N.; Peng, P.-H. Microbial Composition Associated with the Rhizosphere and Seed Endosphere of Paeonia szechuanica. J. Northeast. For. Univ. 2020, 48, 88–94. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yan, B.; Zhao, H.; Liu, F.; Zhang, H.; Sheng, Q.; Miao, F.; Liang, Z. Core Microbiome of Medicinal Plant Salvia Miltiorrhiza Seed: A Rich Reservoir of Beneficial Microbes for Secondary Metabolism? Int. J. Mol. Sci. 2018, 19, 672. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, L.-L.; Ye, M.-D.; Huang, C.-S.; Zhu, Y.-D.; Wang, Q.R.; Li, S.-B. Diversity of Bacterial Endophytes Communities in the Seeds of Several Palmae plants via High throughput Sequencing Method. Acta Microbiol. Sin. 2019, 59, 554–565. (In Chinese) [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Cui, X.; Nian, H. Community Analysis of Endophytic Bacteria from the Seeds of the Medicinal Plant Panax notoginseng. J. Agric. Sci. 2017, 9, 37–45. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, J.; Yang, J.; Hou, X.; He, L.; Zhang, Z. Seed-Borne Bacterial Diversity of Fescue (Festuca Ovina L.) and Properties Study. Microorganisms 2024, 12, 329. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wen, X.-S.; Wang, Y.-M.; Ren, Y.-Y.; Li, B.-Q.; Leng, S.-H. Isolation and Identification of Endophytic Fungi from Schisandra chinensis Seeds and Bacteriostasis Study. J. Chin. Med. Mater. 2020, 43, 1087–1091. (In Chinese) [Google Scholar] [CrossRef]
- Ma, W.; Liu, Z.-P.; Sun, L.-Y.; Zhang, K.-X.; Xu, J.; Wen, D.; Zhao, R.; Liu, X.-B. Endophytic Fungi Diversity of Ginseng Seeds by Using 454 GS FLX. Inf. Tradit. Chin. Med. 2017, 34, 28–32. (In Chinese) [Google Scholar]
- Zhang, J.; Hu, H.; Li, S.; Shang, W.; Jiang, J.; Xu, X.; Liu, D.; Hu, X. Diversity of Fungal Endophytes in American Ginseng Seeds. Plant Dis. 2023, 107, 2784–2791. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Cheng, Y.-L.; Cai, C.-J.; Fan, L.; Gao, J.; Hou, C.-L. Diversity and Antimicrobial Activity of Culturable Endophytic Fungi Isolated from Moso Bamboo Seeds. PLoS ONE 2014, 9, e95838. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Ibrahim, A.M.; Yahia, R.; Shady, N.H.; Mahmoud, B.K.; Abdelmohsen, U.R.; Fouad, M.A. Evaluation of the Anti-Infective Potential of the Seed Endophytic Fungi of Corchorus olitorius through Metabolomics and Molecular Docking Approach. BMC Microbiol. 2023, 23, 355. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, K.-Q.; Peng, H.-Z.; Jin, Q.-Y.; Hua, X.-Q.; Zheng, Y.-Q. Isolation and Molecular Identification of Endophytic Fungi in Phyllostachys heterocycla cv. pubescens. Seed. J. Bamboo Res. 2015, 34, 8–11, 39. (In Chinese) [Google Scholar]
- Wang, S.-P.; Yang, G.-L.; Chen, J.; Jiang, X.-Z.; Wang, M. Culturable Endophytic Fungal Diversity of Quinoa seeds in Tibet, Southwest China. Mycosystema 2022, 41, 204–213. (In Chinese) [Google Scholar] [CrossRef]
- Qi, F.-H.; Jing, T.-Z.; Wang, Z.-X.; Zhan, Y.-G. Fungal Endophytes from Acer Ginnala Maxim: Isolation, Identification and Their Yield of Gallic Acid. Lett. Appl. Microbiol. 2009, 49, 98–104. [Google Scholar] [CrossRef]
- De La Bastide, P.Y.; LeBlanc, J.; Kong, L.; Finston, T.; May, E.M.; Reich, R.; Hintz, W.E.; Von Aderkas, P. Fungal Colonizers and Seed Loss in Lodgepole Pine Orchards of British Columbia. Botany 2019, 97, 23–33. [Google Scholar] [CrossRef]
- Fort, T.; Pauvert, C.; Zanne, A.E.; Ovaskainen, O.; Caignard, T.; Barret, M.; Compant, S.; Hampe, A.; Delzon, S.; Vacher, C. Maternal Effects Shape the Seed Mycobiome in Quercus petraea. New Phytol. 2021, 230, 1594–1608. [Google Scholar] [CrossRef]
- Franić, I.; Eschen, R.; Allan, E.; Hartmann, M.; Schneider, S.; Prospero, S. Drivers of Richness and Community Composition of Fungal Endophytes of Tree Seeds. FEMS Microbiol. Ecol. 2020, 96, fiaa166. [Google Scholar] [CrossRef]
- Luo, G.-M.; Dong, Y.-K.; Zhu, Y.-Y.; Zhu, J.-X.; Zeng, J.-X.; Wang, X.-Y.; Hu, Y.-Z.; Luo, Y.-J.; Gong, Y.-H. Detection of Endogenous Bacteria of Gardenia Seed and Comparative Study of Chemical Treatment. Chin. Pharm. J. 2015, 50, 1665–1669. (In Chinese) [Google Scholar]
- Li, X.-N.; Zhou, Z.-F.; Liu, F.-L.; Li, J. Isolation and Identification of Endophytes from Seeds of Torreya yunnanensis and its Antimicrobial Activity Screening. Biol. Disaster Sci. 2016, 39, 20–26. (In Chinese) [Google Scholar]
- Zhou, W.; Wei, Q.; Feng, R.; Liu, Y.; Liang, H.; Li, J.; Yan, K. Diversity and Spatial Distribution of Endophytic Fungi in Cinnamomum Longepaniculatum of Yibin, China. Arch. Microbiol. 2021, 203, 3361–3372. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. 2017, 70, 514–519. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, L.-B.; Wang, T. Research Progress of Endophytes in bamboo. J. Bamboo Res. 2020, 39, 34–39. (In Chinese) [Google Scholar] [CrossRef]
- Yao, X.-L.; Kang, Q.-J.; Xiong, S.-Z.; Li, F.; Wang, Y.; Lin, S.-J.; Bai, L.-Q.; Ma, W.; Deng, Z.-X. Isolation and Identification of Endophytic Actinomycetes from the Seeds of Camptotheca acuminata Decne. and Isolation of Antimicrobial Substances from those Endophytic Actinomycete. Microbiol. China 2014, 41, 1109–1120. (In Chinese) [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Lou, K. Isolation, Characterization, and Insecticidal Activity of an Endophyte of Drunken Horse Grass, Achnatherum Inebrians. J. Insect Sci. Online 2013, 13, 151. [Google Scholar] [CrossRef]
- Qi, H.-X.; Zhou, X.-C.; Hu, M.-J.; Gao, Y.; Chen, Y.-P.; Zhang, Q.-C.; Gu, P.-W. Diversity and Distribution of Endophytic Actinomycetes Strains in Sophora alopecuroides L. from Baijitan Nature Reserve of Ningxia. Microbiol. China 2015, 42, 990–1000. (In Chinese) [Google Scholar] [CrossRef]
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of Bacterial Endophytes from Germinated Maize Kernels. Can. J. Microbiol. 2007, 53, 802–808. [Google Scholar] [CrossRef]
- Kaga, H.; Mano, H.; Tanaka, F.; Watanabe, A.; Kaneko, S.; Morisaki, H. Rice Seeds as Sources of Endophytic Bacteria. Microbes Environ. 2009, 24, 154–162. [Google Scholar] [CrossRef]
- Ruiz, D.; Agaras, B.; de Werra, P.; Wall, L.G.; Valverde, C. Characterization and Screening of Plant Probiotic Traits of Bacteria Isolated from Rice Seeds Cultivated in Argentina. J. Microbiol. 2011, 49, 902–912. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, C.-G.; Kim, K.; Kang, Y.; Kim, Y.K.; Sa, T. The Influence of Host Genotype and Salt Stress on the Seed Endophytic Community of Salt-Sensitive and Salt-Tolerant Rice Cultivars. BMC Plant Biol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Chen, X.; Krug, L.; Yang, H.; Li, H.; Yang, M.; Berg, G.; Cernava, T. Nicotiana Tabacum Seed Endophytic Communities Share a Common Core Structure and Genotype-Specific Signatures in Diverging Cultivars. Comput. Struct. Biotechnol. J. 2020, 18, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial Seed Endophytes: Genera, Vertical Transmission and Interaction with Plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Dutta, S.; Choi, S.Y.; Lee, Y.H. Temporal Dynamics of Endogenous Bacterial Composition in Rice Seeds during Maturation and Storage, and Spatial Dynamics of the Bacteria during Seedling Growth. Front. Microbiol. 2022, 13, 877781. [Google Scholar] [CrossRef]
- Liang, D.; Guo, J.; Hou, F.; Bowatte, S. High Level of Conservation and Diversity among the Endophytic Seed Bacteriome in Eight Alpine Grassland Species Growing at the Qinghai Tibetan Plateau. FEMS Microbiol. Ecol. 2021, 97, fiab060. [Google Scholar] [CrossRef]
- Yang, J.; Xie, J.; Chen, H.; Zhu, S.; Hou, X.; Zhang, Z. Diversity and Biological Characteristics of Seed-Borne Bacteria of Achnatherum Splendens. Microorganisms 2024, 12, 339. [Google Scholar] [CrossRef]
- Chandel, A.; Mann, R.; Kaur, J.; Norton, S.; Auer, D.; Edwards, J.; Spangenberg, G.; Sawbridge, T. The Role of Soil Microbial Diversity in the Conservation of Native Seed Bacterial Microbiomes. Microorganisms 2022, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Li, S.-F.; Yang, M.-H.; Zhang, S.-Q.; Zhang, B.-Y. Effects of Over-winter Conditions on Viability of Seed-infecting Endophytic fungi and Seed Germination of Ambrosia artemisiifolia. Acta Agric. Univ. Jiangxiensi 2018, 40, 1256–1263. (In Chinese) [Google Scholar] [CrossRef]
- Aleynova, O.A.; Nityagovsky, N.N.; Suprun, A.R.; Ananev, A.A.; Dubrovina, A.S.; Kiselev, K.V. The Diversity of Fungal Endophytes from Wild Grape Vitis amurensis Rupr. Plants 2022, 11, 2897. [Google Scholar] [CrossRef]
- Duo, L.-A.; Yang, C.-J.; Ma, M.-C.; Song, D.-Y.; Zhao, S.-L. Effects of Graphene Oxide on the Structure and Diversity of Endophytic Fungal Community in the Seeds of Lolium perenne L. Acta Ecol. Sin. 2024, 44, 3550–3559. (In Chinese) [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.-A.; Barret, M. Ecological Patterns of Seed Microbiome Diversity, Transmission, and Assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Xun, W.; Shao, J.; Shen, Q.; Zhang, R. Rhizosphere Microbiome: Functional Compensatory Assembly for Plant Fitness. Comput. Struct. Biotechnol. J. 2021, 19, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Xie, X.; Guo, S.; Zhou, Y.; Zhang, X.; Yu, N.; Wang, E. An Amplification-Selection Model for Quantified Rhizosphere Microbiota Assembly. Sci. Bull. 2020, 65, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Kost, T.; Stopnisek, N.; Agnoli, K.; Eberl, L.; Weisskopf, L. Oxalotrophy, a Widespread Trait of Plant-Associated Burkholderia Species, Is Involved in Successful Root Colonization of Lupin and Maize by Burkholderia phytofirmans. Front. Microbiol. 2014, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Matušinsky, P.; Florová, V.; Sedláková, B.; Mlčoch, P.; Bleša, D. Colonization Dynamic and Distribution of the Endophytic Fungus Microdochium Bolleyi in Plants Measured by qPCR. PLoS ONE 2024, 19, e0297633. [Google Scholar] [CrossRef]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clément, C. Endophytic Colonization of Vitis Vinifera L. by Burkholderia Phytofirmans Strain PsJN: From the Rhizosphere to Inflorescence Tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef]
- Rodríguez, C.E.; Mitter, B.; Barret, M.; Sessitsch, A.; Compant, S. Commentary: Seed Bacterial Inhabitants and Their Routes of Colonization. Plant Soil 2018, 422, 129–134. [Google Scholar] [CrossRef]
- James, E.K.; Gyaneshwar, P.; Mathan, N.; Barraquio, W.L.; Reddy, P.M.; Iannetta, P.P.M.; Olivares, F.L.; Ladha, J.K. Infection and Colonization of Rice Seedlings by the Plant Growth-Promoting Bacterium Herbaspirillum seropedicae Z67. Mol. Plant-Microbe Interact. 2002, 15, 894–906. [Google Scholar] [CrossRef]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Ait Barka, E. Endophytic Colonization of Vitis Vinifera L. by Plant Growth-Promoting Bacterium Burkholderia sp. Strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef]
- Tong, Y.-N. How Pine Trees “Blossom” and Set Seeds. Plant J. 1981, 16–17, 21. (In Chinese) [Google Scholar]
- Johnston-Monje, D.; Raizada, M.N. Conservation and Diversity of Seed Associated Endophytes in Zea across Boundaries of Evolution, Ethnography and Ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, C.-G.; Jeon, S.; Kang, Y.; Sa, T. Conservation and Transmission of Seed Bacterial Endophytes across Generations Following Crossbreeding and Repeated Inbreeding of Rice at Different Geographic Locations. Microbiol. Open 2019, 8, e00662. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased Drought Stress Resilience of Maize through Endophytic Colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The Plant Endosphere World—Bacterial Life within Plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Palmieri, D.; Vitale, S.; Lima, G.; Di Pietro, A.; Turrà, D. A Bacterial Endophyte Exploits Chemotropism of a Fungal Pathogen for Plant Colonization. Nat. Commun. 2020, 11, 5264. [Google Scholar] [CrossRef]
- Lucero, C.T.; Lorda, G.S.; Ludueña, L.M.; Anzuay, M.S.; Taurian, T. Motility and Biofilm Production Involved in the Interaction of Phosphate Solubilizing Endophytic Strains with Peanut, Maize and Soybean Plants. Rhizosphere 2020, 15, 100228. [Google Scholar] [CrossRef]
- Wang, H.-W.; Ma, C.-Y.; Xu, F.-J.; Lu, F.; Zhang, W.; Dai, C.-C. Root Endophyte-Enhanced Peanut-Rhizobia Interaction Is Associated with Regulation of Root Exudates. Microbiol. Res. 2021, 250, 126765. [Google Scholar] [CrossRef]
- Del Cerro, P.; Ayala-García, P.; Jiménez-Guerrero, I.; López-Baena, F.J.; Vinardell, J.M.; Megías, M.; Hungria, M.; Gil-Serrano, A.M.; Pérez-Montaño, F.; Ollero, F.J. The Non-Flavonoid Inducible nodA3 and the Flavonoid Regulated nodA1 Genes of Rhizobium Tropici CIAT 899 Guarantee Nod Factor Production and Nodulation of Different Host Legumes. Plant Soil 2019, 440, 185–200. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Ormeño-Orrillo, E.; López-López, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martínez-Romero, J.C.; Reddy, P.M.; Martínez-Romero, E. Nitrogen Fixation in Cereals. Front. Microbiol. 2018, 9, 1794. [Google Scholar] [CrossRef]
- Pucciariello, C.; Boscari, A.; Tagliani, A.; Brouquisse, R.; Perata, P. Exploring Legume-Rhizobia Symbiotic Models for Waterlogging Tolerance. Front. Plant Sci. 2019, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Mosquito, S.; Meng, X.; Devescovi, G.; Bertani, I.; Geller, A.M.; Levy, A.; Myers, M.P.; Bez, C.; Covaceuszach, S.; Venturi, V. LuxR Solos in the Plant Endophyte Kosakonia sp. Strain KO348. Appl. Environ. Microbiol. 2020, 86, e00622-20. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ou, F.; Staehelin, C.; Dai, W. Bradyrhizobium Sp. Strain ORS278 Promotes Rice Growth and Its Quorum Sensing System Is Required for Optimal Root Colonization. Environ. Microbiol. Rep. 2020, 12, 656–666. [Google Scholar] [CrossRef]
- Díaz Herrera, S.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat Seeds Harbour Bacterial Endophytes with Potential as Plant Growth Promoters and Biocontrol Agents of Fusarium graminearum. Microbiol. Res. 2016, 186–187, 37–43. [Google Scholar] [CrossRef]
- Eyre, A.W.; Wang, M.; Oh, Y.; Dean, R.A. Identification and Characterization of the Core Rice Seed Microbiome. Phytobiomes J. 2019, 3, 148–157. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, Y.; Li, L.; Qi, Y.; Gao, P.; Guo, J.; Liu, J.; Chen, Z.; Zhao, J.; Yu, L. Functional Dynamics Analysis of Endophytic Microbial Communities during Amorphophallus muelleri Seed Maturation. Sci. Rep. 2024, 14, 28432. [Google Scholar] [CrossRef]
- Barret, M.; Briand, M.; Bonneau, S.; Préveaux, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacquesa, M.-A. Emergence Shapes the Structure of the Seed Microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-Borne Endophytic Bacillus Amyloliquefaciens RWL-1 Produces Gibberellins and Regulates Endogenous Phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of Abscisic Acid and Gibberellins Produced by Endophytic Azospirillum in the Alleviation of Drought Effects in Maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Agarwal, T.; Kotamreddy, J.N.R.; Bhattacharya, R.; Mitra, A.; Maiti, T.K.; Maiti, M.K. Impact of Seed-Transmitted Endophytic Bacteria on Intra- and Inter-Cultivar Plant Growth Promotion Modulated by Certain Sets of Metabolites in Rice Crop. Microbiol. Res. 2020, 241, 126582. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An In Vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Department of Botany, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka; Wijesooriya, W.A.D.K.; Deshappriya, N. An Inoculum of Endophytic Fungi for Improved Growth of a Traditional Rice variety in Sri Lanka. Trop. Plant Res. 2016, 3, 470–480. [Google Scholar] [CrossRef]
- Xu, M.; Sheng, J.; Chen, L.; Men, Y.; Gan, L.; Guo, S.; Shen, L. Bacterial Community Compositions of Tomato (Lycopersicum esculentum Mill.) Seeds and Plant Growth Promoting Activity of ACC Deaminase Producing Bacillus Subtilis (HYT-12-1) on Tomato Seedlings. World J. Microbiol. Biotechnol. 2014, 30, 835–845. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Qin, Y.; Xie, X.-Q.; Khan, Q.; Wei, J.-L.; Sun, A.-N.; Su, Y.-M.; Guo, D.-J.; Li, Y.-R.; Xing, Y.-X. Endophytic Nitrogen-Fixing Bacteria DX120E Inoculation Altered the Carbon and Nitrogen Metabolism in Sugarcane. Front. Microbiol. 2022, 13, 1000033. [Google Scholar] [CrossRef]
- Fang, L.; Zheng, X.; Sun, Z.; Li, Y.; Deng, J.; Zhou, Y.I. Characterization of a Plant Growth-Promoting Endohyphal Bacillus Subtilis in Fusarium acuminatum from Spiranthes sinensis. Pol. J. Microbiol. 2023, 72, 29–37. [Google Scholar] [CrossRef]
- Zhao, D.-K.; Selosse, M.-A.; Wu, L.; Luo, Y.; Shao, S.-C.; Ruan, Y.-L. Orchid Reintroduction Based on Seed Germination-Promoting Mycorrhizal Fungi Derived from Protocorms or Seedlings. Front. Plant Sci. 2021, 12, 701152. [Google Scholar] [CrossRef]
- Wippel, K.; Tao, K.; Niu, Y.; Zgadzaj, R.; Kiel, N.; Guan, R.; Dahms, E.; Zhang, P.; Jensen, D.B.; Logemann, E.; et al. Host Preference and Invasiveness of Commensal Bacteria in the Lotus and Arabidopsis Root Microbiota. Nat. Microbiol. 2021, 6, 1150–1162. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.-M.; Cai, L. Disease-Induced Changes in Plant Microbiome Assembly and Functional Adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.L.; Bergen, M.S.; Kowalski, K.P.; White, J.F. Fungal Disease Prevention in Seedlings of Rice (Oryza sativa) and Other Grasses by Growth-Promoting Seed-Associated Endophytic Bacteria from Invasive Phragmites Aust. Microorg. 2018, 6, 21. [Google Scholar] [CrossRef]
- Saikia, K.; Bora, L.C. Exploring Actinomycetes and Endophytes of Rice Ecosystem for Induction of Disease Resistance against Bacterial Blight of Rice. Eur. J. Plant Pathol. 2021, 159, 67–79. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.P.; Mahfooz, S.; Singh, S.P.; Bhattacharya, A.; Mishra, N.; Nautiyal, C.S. Endophyte-Mediated Modulation of Defense-Related Genes and Systemic Resistance in Withania somnifera (L.) Dunal under Alternaria alternata Stress. Appl. Environ. Microbiol. 2018, 84, e02845-17. [Google Scholar] [CrossRef]
- Roane, T.M.; Rensing, C.; Pepper, I.L.; Maier, R.M. Chapter 21—Microorganisms and Metal Pollutants. In Environmental Microbiology, 2nd ed.; Maier, R.M., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 421–441. ISBN 978-0-12-370519-8. [Google Scholar]
- Parmar, S.; Sharma, V.K.; Li, T.; Tang, W.; Li, H. Fungal Seed Endophyte FZT214 Improves Dysphania Ambrosioides Cd Tolerance throughout Different Developmental Stages. Front. Microbiol. 2021, 12, 783475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, C.; Nan, Z. Effects of Cadmium Stress on Seed Germination and Seedling Growth of Elymus Dahuricus Infected with the Neotyphodium Endophyte. Sci. China Life Sci. 2012, 55, 793–799. [Google Scholar] [CrossRef]
- Li, X.; He, C.; He, X.; Su, F.; Hou, L.; Ren, Y.; Hou, Y. Dark Septate Endophytes Improve the Growth of Host and Non-Host Plants under Drought Stress through Altered Root Development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing Rhizosphere Microbiomes for Drought-Resilient Crop Production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Qian, Y.-W.; Diao, Q.; Luo, J.-J.; Ji, C.; Wang, Y.-J.; Jiang, X.-T.; He, L.-Y. Drought-tolerant Microorganisms: Changes in Research Hotspots and Mechanisms of Improving Plant Drought Tolerance. Microbiol. China 2024, 51, 4370–4382. (In Chinese) [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.-Y.; Wang, Y.; Li, C.-X.; He, Y.; Lin, H.-H.; Wang, T.; Ma, X.-R. The Differences and Overlaps in the Seed-Resident Microbiome of Four Leguminous and Three Gramineous Forages. Microb. Biotechnol. 2020, 13, 1461–1476. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing Endophytic Competence and Plant Growth Promotion of Bacterial Endophytes Inhabiting the Seed Endosphere of Rice. BMC Microbiol. 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, X.; Liu, B.; Zou, L.; Peng, K.; Zhao, G. Study on the Stability of Red Pigments Produced by a Seedborne Endnophytic Fungus from Tartary buckwheat. Guangdong Agric. Sci. 2014, 41, 126–129. [Google Scholar]
- Pan, Y.; Zheng, W.; Yang, S. Chemical and Activity Investigation on Metabolites Produced by an Endophytic Fungi Psathyrella Candolleana from the Seed of Ginkgo Biloba. Nat. Prod. Res. 2020, 34, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Shady, N.H.; Sobhy, S.K.; Mostafa, Y.A.; Yahia, R.; Glaeser, S.P.; Kämpfer, P.; El-Katatny, M.H.; Abdelmohsen, U.R. Phytochemical Analysis and Anti-Infective Potential of Fungal Endophytes Isolated from Nigella sativa Seeds. BMC Microbiol. 2023, 23, 343. [Google Scholar] [CrossRef]
- Zhong, L.; Niu, B.; Xiang, D.; Wu, Q.; Peng, L.; Zou, L.; Zhao, J. Endophytic Fungi in Buckwheat Seeds: Exploring Links with Flavonoid Accumulation. Front. Microbiol. 2024, 15, 1353763. [Google Scholar] [CrossRef]
- Pan, X.; Li, T.; Liao, C.; Zhu, Y.; Yang, M. The Influences of Fungal Endophytes Inoculation on the Biochemical Status of Grape Cells of Different Varieties in Vitro. Plant Biotechnol. 2022, 39, 335–343. [Google Scholar] [CrossRef]
- Qu, J.-Z.; Liu, F.; Pan, X.-X.; Liao, C.-M.; Li, T.; Zhang, H.-B.; Yang, M.-Z. Coordinative Changes in Metabolites in Grape Cells Exposed to Endophytic Fungi and Their Extracts. Molecules 2022, 27, 5566. [Google Scholar] [CrossRef]
- Nan, Z.B. Incidence and Distribution of Endophytic Fungi in Seeds of Some Native and Introduced Grasses in China. Acta Prataculturae Sin. 1996, 2, 1–8. [Google Scholar]
- Joost, R.E. Acremonium in Fescue and Ryegrass: Boon or Bane? A Review. J. Anim. Sci. 1995, 73, 881–888. [Google Scholar] [CrossRef]
- Nan, Z.-B.; Li, C.-J. Roles of the Grass-Neotyphodium Association in Pastoral Agriculture Systems. Acta Ecol. Sin. 2004, 3, 605–616. (In Chinese) [Google Scholar]
- Wang, Q.; Song, R.; Fan, S.; Coleman, J.J.; Xu, X.; Hu, X. Diversity of Fusarium Community Assembly Shapes Mycotoxin Accumulation of Diseased Wheat Heads. Mol. Ecol. 2023, 32, 2504–2518. [Google Scholar] [CrossRef] [PubMed]
- Rétif, F.; Kunz, C.; Calabro, K.; Duval, C.; Prado, S.; Bailly, C.; Baudouin, E. Seed Fungal Endophytes as Biostimulants and Biocontrol Agents to Improve Seed Performance. Front. Plant Sci. 2023, 14, 1260292. [Google Scholar] [CrossRef]
- Chen, C.-X.; Guo, L.-R.; Wang, Y.-T.; Wen, Y.; Li, Y.; Lu, C.-X.; Zhou, P.; Huang, S.-Y.; Li, Y.-Q.; Pan, X.-X.; et al. Targeted Manipulation of Vertically Transmitted Endophytes to Confer Beneficial Traits in Grapevines. Horticulturae 2024, 10, 607. [Google Scholar] [CrossRef]
- Kong, H.G.; Song, G.C.; Ryu, C.-M. Inheritance of Seed and Rhizosphere Microbial Communities through Plant-Soil Feedback and Soil Memory. Environ. Microbiol. Rep. 2019, 11, 479–486. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root Exudate Metabolites Drive Plant-Soil Feedbacks on Growth and Defense by Shaping the Rhizosphere Microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Hema, P.; Murali, M.; Shilpa, N.; Nataraj, K.; Basavaraj, G.L.; Singh, S.B.; Aiyaz, M.; Udayashankar, A.C.; Amruthesh, K.N. Fungal Endophytes as Mitigators against Biotic and Abiotic Stresses in Crop Plants. J. Fungi 2024, 10, 116. [Google Scholar] [CrossRef] [PubMed]
| Host Plant | Detected Bacterial SBEs |
|---|---|
| Pinus virginiana Mill. [6] | Erwinia, Sphingomonas, Enterobacter, Gammaproteobacteria, Pantoea, Bacillus, Staphylococcus |
| Pinus ponderosa Douglas ex C. Lawson. [6] | Lysinibacillus, Psychrobacillus, Bacillus, Pseudomonas |
| Sesbania cannabina (Retz.) Pers. [7] | Bacillus, Rhizobium, Cellulomonas |
| Vitis vinifera L. [10] | Bacillus altitudinis, Bacillus simplex, Bacillus thuringiensis, Paenibacillus amylolyticus, Staphylococcus aureus |
| Helianthus annuus L. [11] | Bacillus subtilis, Enterobacter |
| Eucalyptus spp. [12] | Bacillus megaterium, Enterococcus mundtii, Methylobacterium variabile, Methylobacterium gregens, Paenibacillus humicus, Sphingomonas phyllosphaerae |
| Eucommia ulmoides Oliv. [13] | Peribacillus, Cytobacillus, Metabacillus, Solibacillus, Bacillus proteolyticus |
| Caragana leucophloea Pojark. [14] | Sphingomonas, Bacillus, Bacillus licheniformis, Bacillus cereus, Bacillus subtilis |
| Zea mays L. [15] | Pseudomonas, Pantoea, Sphingomonas, Enterobacter, Stenotrophomonas, Serratia |
| Brassica napus L. [16] | Bacillus, Ochrobactrum, Alcaligenes, Brevundimonas, Stenotrophomonas, Vitreoscilla, Achromobacte, Pseudomonas, Pseudochrobactrum, Sphingomonas, Serratia, Providencia |
| Triticum aestivum L. [17] | Paenibacillus, Acidovorax, Pantoea, Burkholderia, Serratia, Lactobacillus, Massilia, Neisseria, Methanobacterium, Chryseobacterium |
| Bupleurum chinense DC. [18] | Pseudomonas, Rhizobium, Sphingomonas |
| Medicago sativa spp. [19] | Rhizobium |
| Coffea arabica L. [20] | Burkholderia, Stenotrophomonas, Bacillus, Yersinia frederiksenii |
| Picea abies (L.) H. Karst. [21] | Pseudomonas, Rahnella |
| Quercus palustris Münchh [22] | Pseudomonas, Propionibacterium, Erwinia |
| Cinchona ledgeriana (Rubiaceae) Moens exTrim. [23] | Diaporthe |
| Fraxinus spp. [24] | Pantoea, Rhodococcus, Exiguobacterium, Staphylococcus, Bacillus |
| Dendrobium nobile Lindl. [25] | Pantoea, Pseudomonas, Acinetobacter, Dechloromonas, Vibrio |
| Hippophae rhamnoides subsp. Sinensis [26] | Stenotrophomonas, Phyllobacterium, Variovorax, Cyanobacteria, Bacillus, Staphylococcus, Pseudomonas, Acinetobacter |
| Coptis chinensis Franch. [27] | Bacillus, Stenotrophomonas, Achromobacte, |
| Oryza sativa L. [28,29] | Bacillus, Pseudomonas, Paenibacillus, Acidovorax, Pantoea, Sphingomonas, Burkholderia, Rhizobium |
| Ginkgo biloba L. [30] | Bacillus, Pseudomonas |
| Morinda citrifolia L. [31] | Bacillus, Acinetobacter |
| Nicotiana tabacum L. [32] | Bacillus, Pseudomonas, Paenibacillus, Pantoea, Stenotrophomonas |
| Cicer arietinum L. [33] | Bacillus, Pseudomonas, Staphylococcus, Pantoea, Enterobacter |
| Solanum lycopersicum L. [34] | Bacillus |
| Vicia faba L. [35] | Bacillus, Bacteroides, Lactobacillus |
| Paeonia szechuanica [36] | Leptospirillum, Lactobacillus, Helicobacter, Acidiphilium, Renibacterium |
| Salvia miltiorrhiza Bunge [37] | Pseudomonas, Pantoea, Sphingomonas |
| Trachycarpus fortune (Hook.) H. Wendl. [38] | Enterococcus, Paenibacillus |
| Areca triandra Roxb. exBuch.-Ham. [38] | Enterococcus, Paenibacillus |
| Caryota mitis Lour. [38] | Enterococcus, Sphingomonas |
| Phoenix roebelenii O. Brien [38] | Enterococcus, Cellulomonas, Methylobacterium, Sphingomonas |
| Arenga engleri Becc. [38] | Enterococcus, Paenibacillus |
| Livistona chinensis (Jacq.) R. Br. exMart. [38] | Lactococcus, Oceanobacillus |
| Phoenix canariensis Chabaud [38] | Saccharopolyspora, Kosakonia, Enterobacter, Goodfellowiella |
| Panax notoginseng (Burk.) F. H. Chen [39] | R-proteobacteria, Pseudomonas, Enterobacter, Stenotrophomonas |
| Festuca ovina L. [40] | Bacillus, Paenibacillus, Pseudomonas |
| Host Plant | Detected Fungal SBEs |
|---|---|
| Eucalyptus spp. [12] | Alternaria |
| Eucommia ulmoides Oliver [13] | Cunninghamella, Aspergillus, Penicillium, Talaromyces, Cladophialophora, Alternaria, Periconia, Cladosporium, Arthrinium, Trichoderma, Bjerkandera |
| Bupleurum chinense DC. [18] | Papiliotrema, Filobasidium, Aspergillus |
| Quercus palustris Münchh [22] | Alternaria, Debaryomyces, Apiognomonia, Mycosphaerella, Malassezia, Aureobasidium, Epicoccum |
| Dendrobium nobile Lindl. [25] | Alternaria, Debaryomyces, Ruistroemia, Entoloma, Psathyrella |
| Morinda citrifolia L. [31] | Eremothecium coryli, Pseudozyma aphidis, Pseudozyma hubeiensis, Cryptococcus flavescens, Kodamaea ohmeri, Cladosporium sphaerospermum, Phaeoacremonium, Gibberella, Penicillium |
| Paeonia szechuanica Fang [36] | Penicillium, Geomyces, Aspergillus, Gibberella, Rhizopus, Lichtheimia |
| Salvia miltiorrhiza Bunge [37] | Talaromyces |
| Schisandra chinensis (Turcz.) Baill. [41] | Penicillium, Penicillium thomii, Penicillium expansum |
| Panax ginseng C. A. Mey. [42] | Fusarium, Cephalotheca, Podospora, Wardomyces, Haematonectria |
| Panax quinquefolius L. [43] | Acremonium, Fusarium, Cladosporium, Gibberella |
| Phyllostachys edulis (Carr.) H. De Lehaie [44] | Leptosphaerulina, Simplicillium, Sebacina |
| Corchorus olitorius L. [45] | Penicillium, Fusarium, Aspergillus |
| Phyllostachys heterocycla cv. Pubescens [46] | Colletotrichum, Cladosporium cladosporioides, Shiraia bambusicola |
| Chenopodium quinoa Willd. [47] | Alternaria, Fusarium, Phoma, Cladosporium, Peyronellaea, Epicoccum, Didymella |
| Acer ginnala Maxim [48] | Alternaria, Epicoccum |
| Lodgepole pine Parl. [49] | Alternaria |
| Quercus petraea Liebl [50] | Taphrina carpini, Cladosporium delicatulum, Epicoccum nigrum, Curvibasidium cygneicollum |
| Acer palmatum Thunb. [51] | Colletotrichum, Septoria |
| Larix gmelinii (Rupr.) Kuzen. [51] | Rhodotorula, Didymella, Cystobasidium |
| Pinus spp. [51] | Kabatina |
| Fagus spp. [51] | Parastaganospora |
| Gardenia jasminoides Ellis. [52] | Aspergillus, Penicillium, Mucor, Rhizopus |
| Torreya yunnanensis Cheng et L. K. Fu. [53] | Alternaria, Trichoderma, Phomopsis, Fusarium, Colletotrichum, Paecilomyces |
| Cinnamomum longepaniculatum (Gamble) N. Chao [54] | Pestalotiopsis, Parastaganospora, Aspergillus, Paraconiothyrium, Peniophora, Cryptodiscus, Penicillium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Geng, S.; Zhu, Y.; He, X.; Pan, X.; Yang, M. Seed-Borne Endophytes and Their Host Effects. Microorganisms 2025, 13, 842. https://doi.org/10.3390/microorganisms13040842
Hu H, Geng S, Zhu Y, He X, Pan X, Yang M. Seed-Borne Endophytes and Their Host Effects. Microorganisms. 2025; 13(4):842. https://doi.org/10.3390/microorganisms13040842
Chicago/Turabian StyleHu, Hongyan, Shucun Geng, Youyong Zhu, Xiahong He, Xiaoxia Pan, and Mingzhi Yang. 2025. "Seed-Borne Endophytes and Their Host Effects" Microorganisms 13, no. 4: 842. https://doi.org/10.3390/microorganisms13040842
APA StyleHu, H., Geng, S., Zhu, Y., He, X., Pan, X., & Yang, M. (2025). Seed-Borne Endophytes and Their Host Effects. Microorganisms, 13(4), 842. https://doi.org/10.3390/microorganisms13040842






