Effects of Biochar on Cadmium Availability, Nitrification and Microbial Communities in Soils with Varied pH Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Construction and Sampling of Soil Microcosms
2.3. Soil and Biochar Sample Analysis
2.4. DNA Extraction and Illumina MiSeq Sequencing
| Target Genes | Primer Name | Primer Sequence (5′–3′) | Reaction Condition | Reference |
|---|---|---|---|---|
| Archaeal amoA | Arch-amoAF | STAATGGTCTGGCTTAGACG | 95 °C, 3 min; 38 × (95 °C, 20 s; 55 °C, 30 s; 72 °C, 45 s); Melt curve 60 °C to 95 °C, increment 0.5 °C | Francis et al. [29] (pp. 14683–14688) |
| Arch-amoAR | GCGGCCATCCATCTGTATGT | |||
| Bacterial amoA | amoA-1F | GGGGTTTCTACTGGTGGT | 95 °C, 3 min; 38 × (95 °C, 20 s; 56 °C, 30 s; 72 °C, 30 s); Melt curve 60 °C to 95 °C, increment 0.5 °C | Rotthauwe et al. [30] (pp. 4704–4712) |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC |
2.5. Statistical Analysis
3. Results
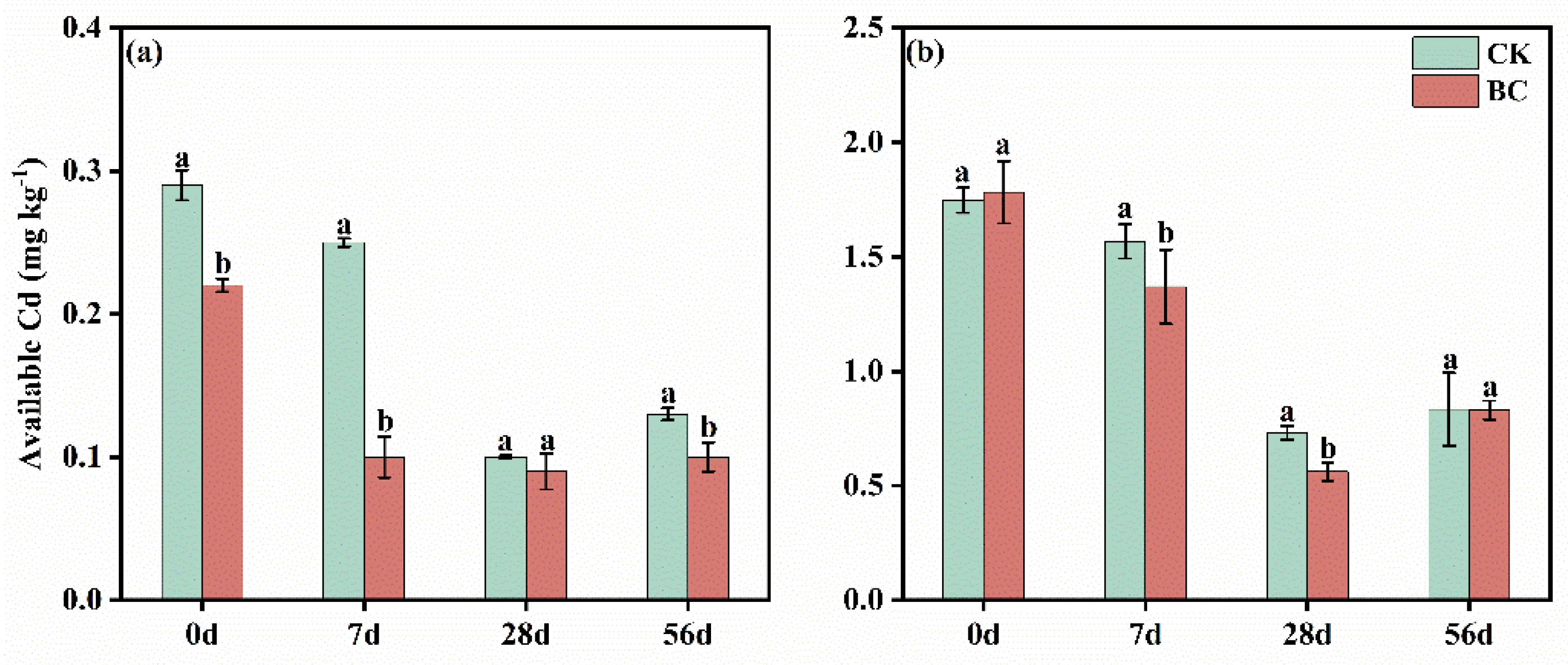
3.1. Effects of Biochar Addition on Soil Available Cd in Two Soils with Varied pH
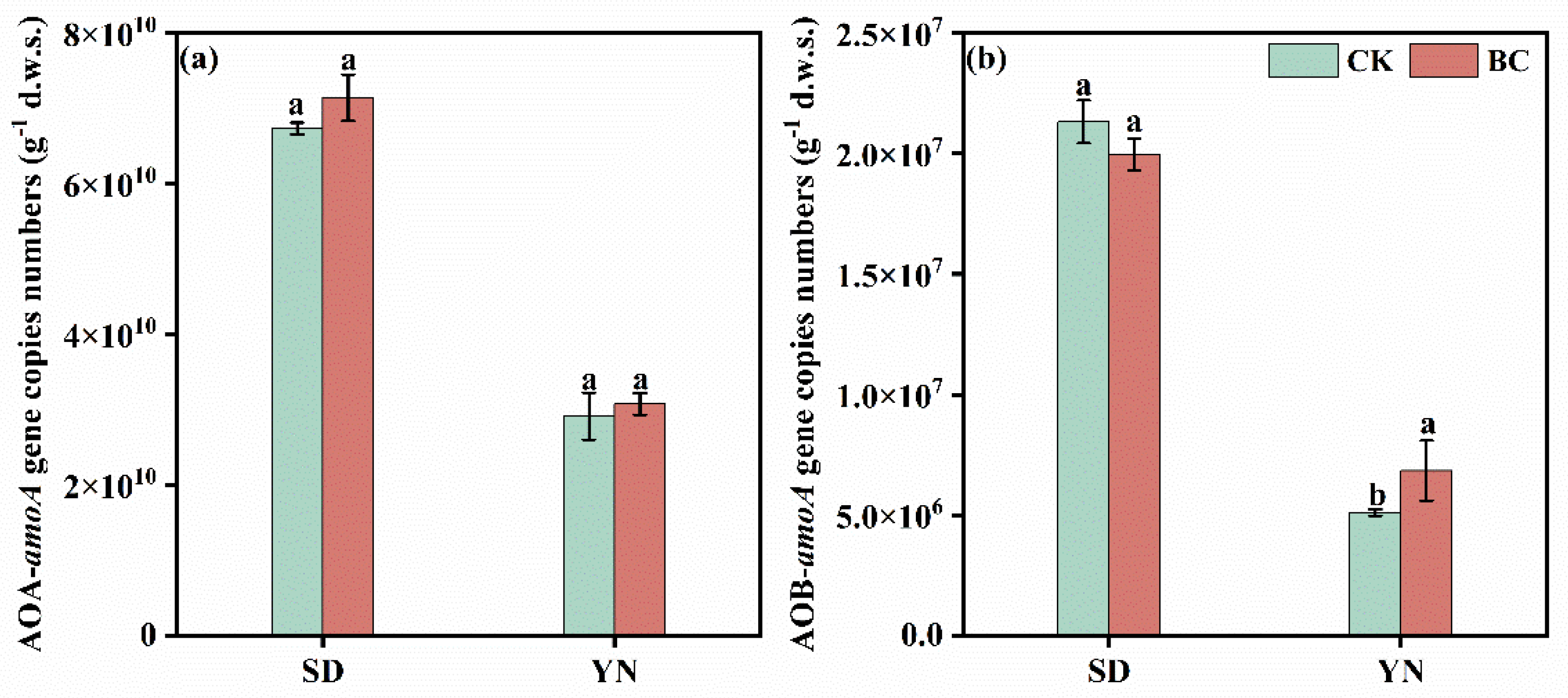
3.2. Effects of Biochar Addition on Nitrification Activity in Soils with Varied pH
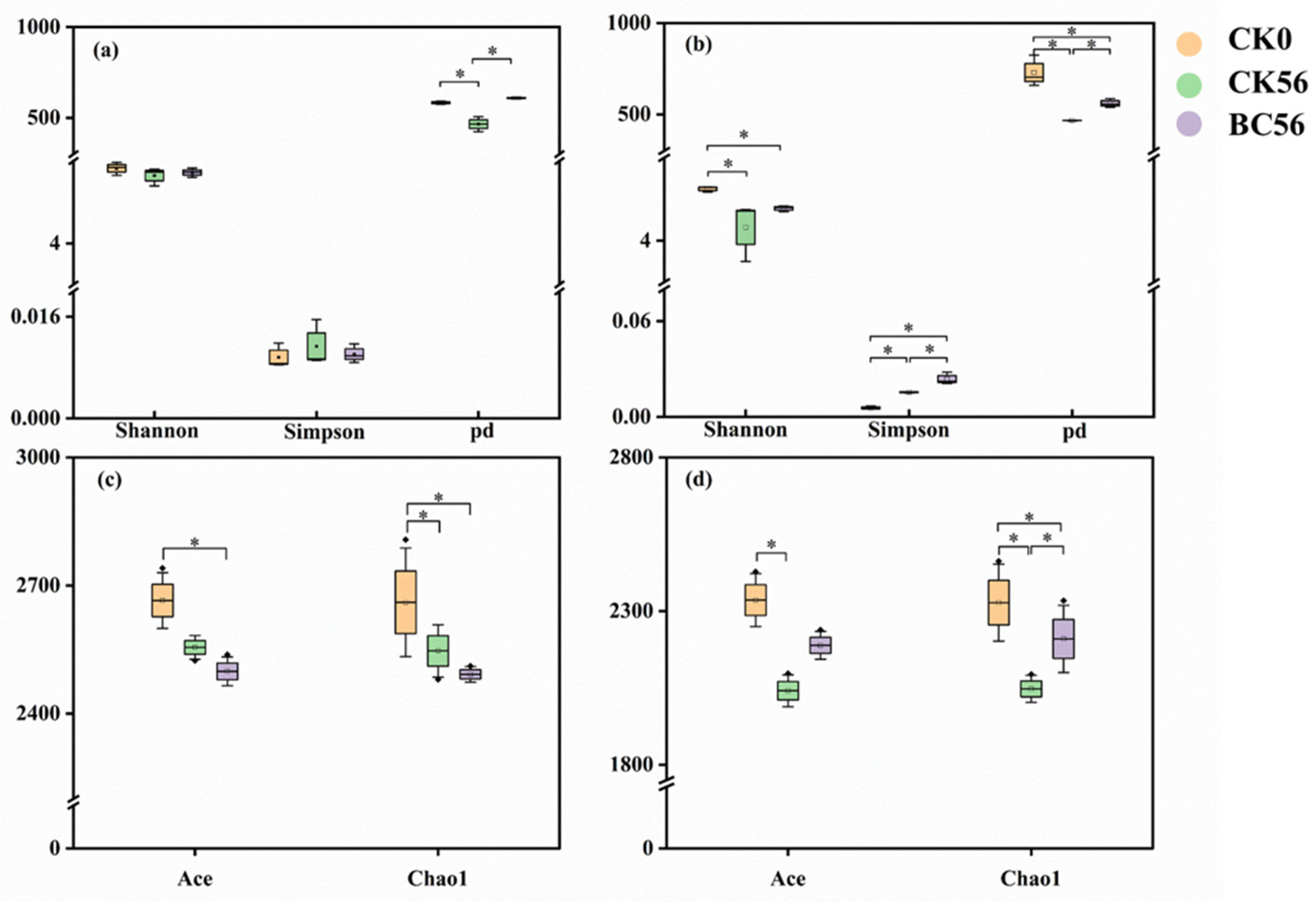
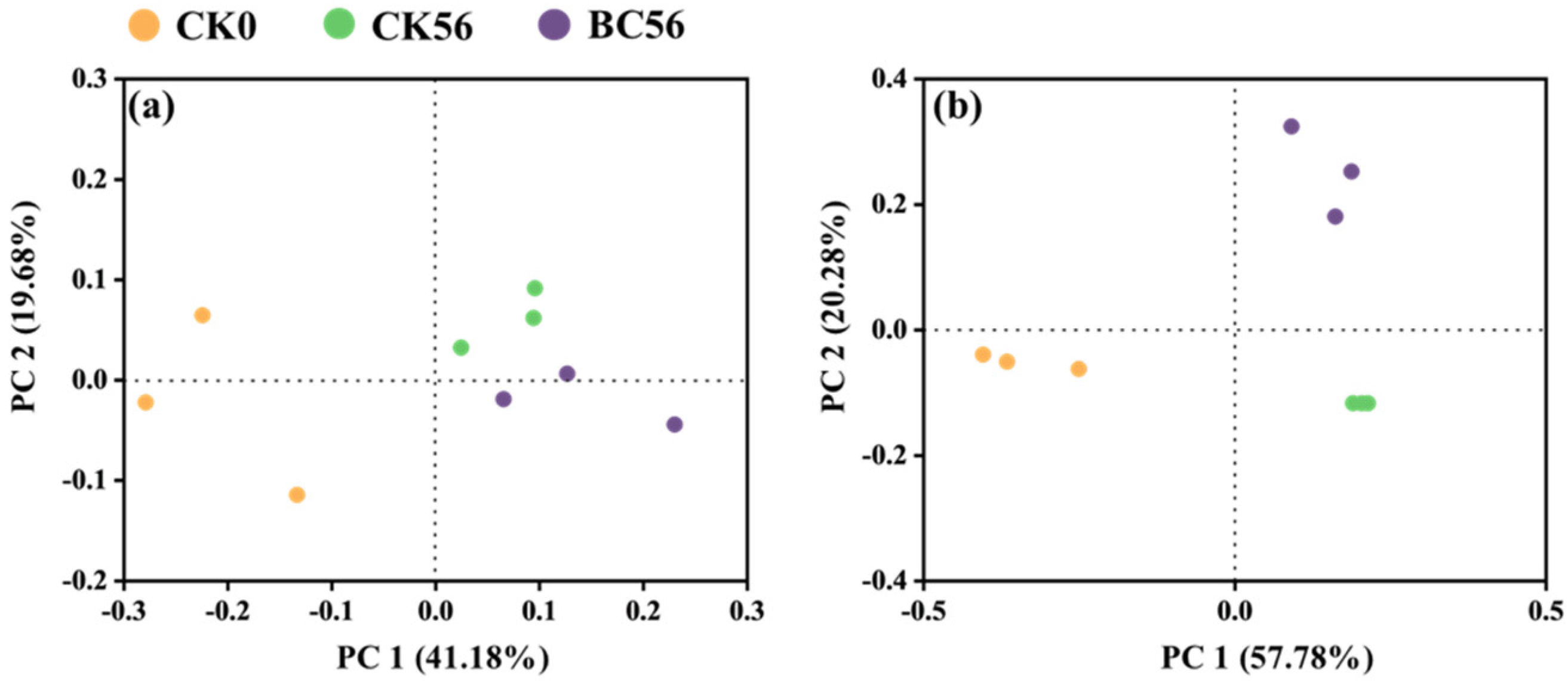
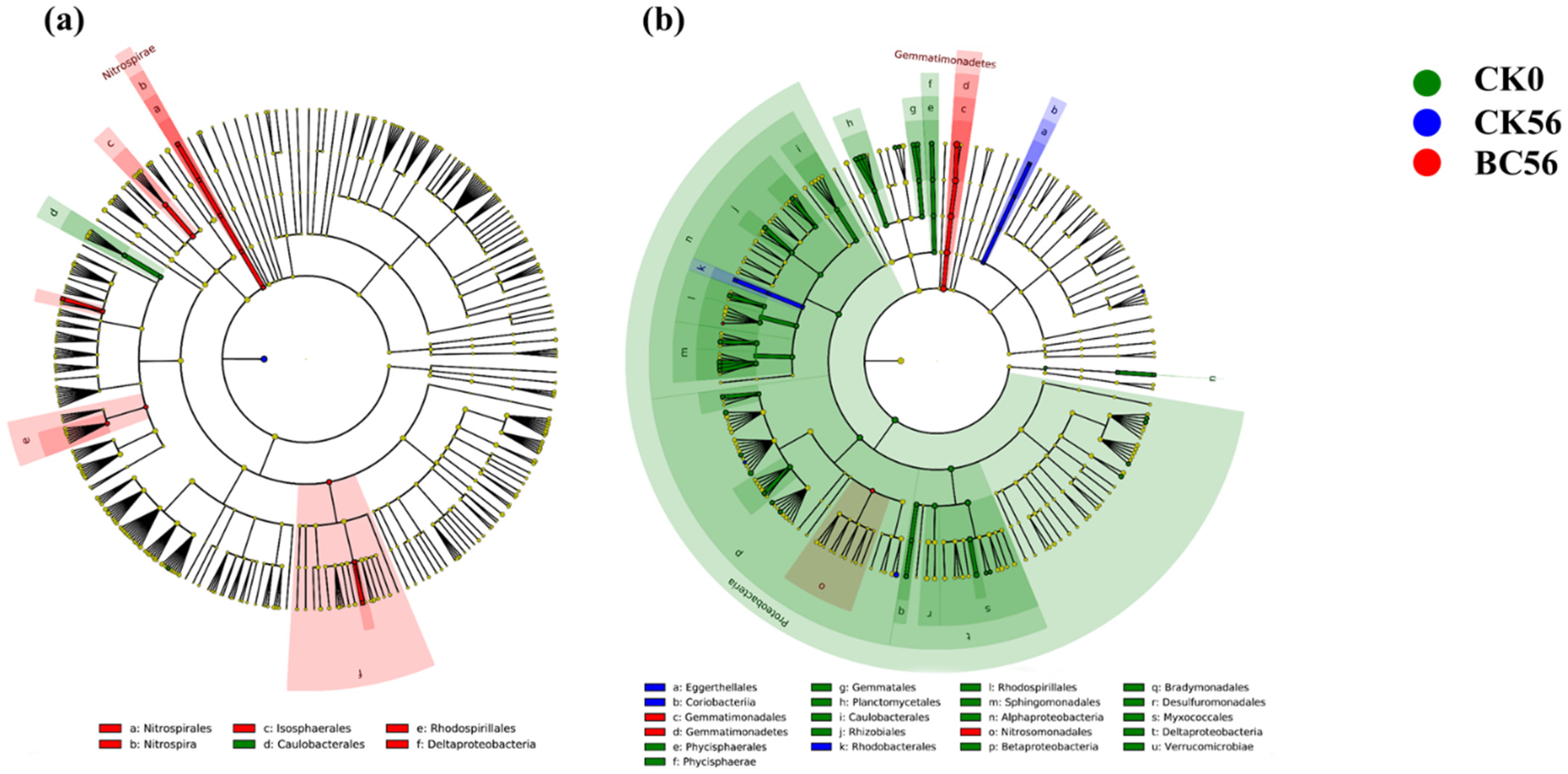
3.3. Effects of Biochar Addition on Soil Bacterial Community Diversity in Two Soils with Varied pH
3.4. Effects of Biochar Addition on Soil Bacterial Community Function in Two Soils with Varied pH
4. Discussion
4.1. Effects of Biochar Amendment on Cd Availability in Soils with Varied pH
4.2. Effects of Biochar Addition on Nitrification in Soils with Varied pH
4.3. Response of Soil Bacterial Communities to Biochar in Varied pH Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, Z.; Pan, H.; Sun, B.; Zeng, D.; He, L.; Yang, R.; Zhou, G. Cadmium contamination in soils and crops in four mining areas, China. J. Geochem. Explor. 2018, 192, 72–84. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.-M.; Wang, Q.; Liu, C. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.-M.; Gu, Y.; Kopittke, P.M.; Zhao, F.-J.; Wang, P. Iron–manganese (oxyhydro) oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Xie, M.; Jiang, Q.; Chen, W.; Ao, T. The impact of stabilizing amendments on the microbial community and metabolism in cadmium-contaminated paddy soils. Chem. Eng. J. 2020, 395, 125132. [Google Scholar] [CrossRef]
- Zhou, F.; Cui, J.; Zhou, J.; Yang, J.; Li, Y.; Leng, Q.; Wang, Y.; He, D.; Song, L.; Gao, M. Increasing atmospheric deposition nitrogen and ammonium reduced microbial activity and changed the bacterial community composition of red paddy soil. Sci. Total Environ. 2018, 633, 776–784. [Google Scholar] [CrossRef]
- Ghosh, U.; Luthy, R.G.; Cornelissen, G.; Werner, D.; Menzie, C.A. In-situ sorbent amendments: A new direction in contaminated sediment management. Environ. Sci. Technol. 2011, 45, 1163–1168. [Google Scholar] [CrossRef]
- Aransiola, S.A.; Josiah, I.U.J.; Abioye, O.P.; Bala, J.D.; Rivadeneira-Mendoza, B.F.; Prasad, R.; Luque, R.; Rodríguez-Díaz, J.M.; Maddela, N.R. Micro and vermicompost assisted remediation of heavy metal contaminated soils using phytoextractors. Case Stud. Chem. Environ. Eng. 2024, 9, 100755. [Google Scholar] [CrossRef]
- Wang, R.; Wei, S.; Jia, P.; Liu, T.; Hou, D.; Xie, R.; Lin, Z.; Ge, J.; Qiao, Y.; Chang, X. Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Sci. Total Environ. 2019, 676, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Qiao, S.; Wang, X.; Sheng, M.; Wei, M.; Chen, Q.; Xu, H.; Xu, F. Immobilization of cadmium by Burkholderia sp. QY14 through modified microbially induced phosphate precipitation. J. Hazard. Mater. 2021, 412, 125156. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.A.; Li, X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J. Influence of biochar and soil properties on soil and plant tissue concentrations of Cd and Pb: A meta-analysis. Sci. Total Environ. 2021, 755, 142582. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, R.; Zhang, Y.; Si, T.; Li, H.; Duan, H.; Li, L.; Pan, G. Biochar decreases Cd mobility and rice (Oryza sativa L.) uptake by affecting soil iron and sulfur cycling. Sci. Total Environ. 2022, 836, 155547. [Google Scholar] [CrossRef]
- Schofield, H.K.; Pettitt, T.R.; Tappin, A.D.; Rollinson, G.K.; Fitzsimons, M.F. Biochar incorporation increased nitrogen and carbon retention in a waste-derived soil. Sci. Total Environ. 2019, 690, 1228–1236. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fertil. Soils 2014, 50, 321–332. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef]
- Shidan, B. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- GB/T 42485-2023; Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Soils—Extraction with Potassium Chloride Solution and Determination with Manual method. State Administration for Market Regulation and National Standardization Administration of the People’s Republic of China: Beijing, China, 2023.
- Kettler, T.; Doran, J.W. Gilbert. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil. Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- GB/T 23739-2009; Soil Quality—Analysis of Available Lead and Cadmium Contents in Soils—Atomic Absorption Spectrometry. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2019.
- HJ 803-2016; Soil and Sediment—Determination of Twelve Metal Elements in Aqua Regia Extracts—Inductively Coupled Plasma Mass Spectrometry. Ministry of Ecological Environment of the People’s Republic of China: Beijing, China, 2016.
- Dunnigan, L.; Ashman, P.J.; Zhang, X.; Kwong, C.W. Production of biochar from rice husk: Particulate emissions from the combustion of raw pyrolysis volatiles. J. Clean. Prod. 2018, 172, 1639–1645. [Google Scholar] [CrossRef]
- Manter, D.K.; Reardon, C.L.; Ashworth, A.J.; Ibekwe, A.M.; Lehman, R.M.; Maul, J.E.; Miller, D.N.; Creed, T.; Ewing, P.M.; Park, S. Unveiling errors in soil microbial community sequencing: A case for reference soils and improved diagnostics for nanopore sequencing. Commun. Biol. 2024, 7, 913. [Google Scholar] [CrossRef]
- Pan, H.; Feng, H.; Liu, Y.; Lai, C.-Y.; Zhuge, Y.; Zhang, Q.; Tang, C.; Di, H.; Jia, Z.; Gubry-Rangin, C. Grazing weakens competitive interactions between active methanotrophs and nitrifiers modulating greenhouse-gas emissions in grassland soils. ISME Commun. 2021, 1, 74. [Google Scholar] [CrossRef] [PubMed]
- Uparse, R.E. Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 2604, 996–998. [Google Scholar] [CrossRef]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef]
- Rotthauwe, J.-H.; Witzel, K.-P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Li, Z.; Jia, M.; Christie, P.; Ali, S.; Wu, L. Use of a hyperaccumulator and biochar to remediate an acid soil highly contaminated with trace metals and/or oxytetracycline. Chemosphere 2018, 204, 390–397. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; He, Y.; Zhang, Y. The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci. Total Environ. 2020, 717, 137014. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, A.; Li, T.; Wu, X.; Liu, Y.; Naidu, R. Immobilization of Cd and Pb in a contaminated acidic soil amended with hydroxyapatite, bentonite, and biochar. J. Soils Sediments 2021, 21, 2262–2272. [Google Scholar] [CrossRef]
- Li, Y.; Ge, Y.; Zhang, C.; Zhou, Q. Mechanisms for high Cd activity in a red soil from southern China undergoing gradual reduction. Soil. Res. 2010, 48, 371–384. [Google Scholar] [CrossRef]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef]
- Chen, Q.; Dong, J.; Yi, Q.; Liu, X.; Zhang, J.; Zeng, Z. Proper mode of using rice straw biochar to treat Cd-contaminated irrigation water in mining regions based on a multiyear in situ experiment. ACS Sustain. Chem. Eng. 2019, 7, 9928–9936. [Google Scholar] [CrossRef]
- Ramtahal, G.; Umaharan, P.; Hanuman, A.; Davis, C.; Ali, L. The effectiveness of soil amendments, biochar and lime, in mitigating cadmium bioaccumulation in Theobroma cacao L. Sci. Total Environ. 2019, 693, 133563. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, W.; Zhang, H.; Lin, J.; Hu, J.; Lou, Y.; Wang, H.; Yang, Q.; Pan, H.; Zhuge, Y. Individual and combined contamination of oxytetracycline and cadmium inhibited nitrification by inhibiting ammonia oxidizers. Front. Microbiol. 2022, 13, 1062703. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, L.; Yu, M.; Afzal, M.; Dai, Z.; Brookes, P.; Xu, J. Nitrogen combined with biochar changed the feedback mechanism between soil nitrification and Cd availability in an acidic soil. J. Hazard. Mater. 2020, 390, 121631. [Google Scholar] [CrossRef]
- Chandran, K.; Love, N.G. Physiological state, growth mode, and oxidative stress play a role in Cd (II)-mediated inhibition of Nitrosomonas europaea 19718. Appl. Environ. Microbiol. 2008, 74, 2447–2453. [Google Scholar] [CrossRef]
- Jiang, X.; Hou, X.; Zhou, X.; Xin, X.; Wright, A.; Jia, Z. pH regulates key players of nitrification in paddy soils. Soil. Biol. Biochem. 2015, 81, 9–16. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Uchiyama, I.; Toyoda, A.; Wang, Y.; Shimomura, Y.; Okubo, T.; Kurisu, F.; Hirono, Y.; Nonaka, K. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil. ISME J. 2017, 11, 1130–1141. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, M.; Bi, R.; Bello, A.; Egbeagu, U.U.; Zhang, J.; Li, S.; Chen, Y.; Han, Y.; Sun, Y. Insight into the influence of biochar on nitrification based on multi-level and multi-aspect analyses of ammonia-oxidizing microorganisms during cattle manure composting. Bioresour. Technol. 2021, 339, 125515. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, Q.; Dzakpasu, M.; He, Y.; Zhou, P.; Chen, R.; Li, Y.-Y. Biochar’s role to achieve multi-pathway nitrogen removal in anammox systems: Insights from electron donation and selective microbial enrichment. Chem. Eng. J. 2024, 482, 148824. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Wang, Y.; Zhang, Y.; Hu, C.; Liu, B. Long-term nitrogen fertilization elevates the activity and abundance of nitrifying and denitrifying microbial communities in an upland soil: Implications for nitrogen loss from intensive agricultural systems. Front. Microbiol. 2018, 9, 2424. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Ling, L.; Luo, Y.; Jiang, B.; Lv, J.; Meng, C.; Liao, Y.; Reid, B.J.; Ding, F.; Lu, Z.; Kuzyakov, Y. Biochar induces mineralization of soil recalcitrant components by activation of biochar responsive bacteria groups. Soil. Biol. Biochem. 2022, 172, 108778. [Google Scholar] [CrossRef]
- Zhu, Y.; Ge, X.; Wang, L.; You, Y.; Cheng, Y.; Ma, J.; Chen, F. Biochar rebuilds the network complexity of rare and abundant microbial taxa in reclaimed soil of mining areas to cooperatively avert cadmium stress. Front. Microbiol. 2022, 13, 972300. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, S.; Pan, P.; Chen, D.; Tang, S.; Chen, L.; Wang, X.; Gu, M.; Tang, F.; He, J. Microbial driving mechanism of soil conditioner on reducing cadmium uptake by rice and improving soil environment. Agric. Ecosyst. Environ. 2023, 349, 108452. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, S.; Fang, H.; Geng, J.; Li, Y.; Shi, F.; Wang, H.; Chen, L.; Zhou, Y. Copper and cadmium co-contamination increases the risk of nitrogen loss in red paddy soils. J. Hazard. Mater. 2024, 479, 135626. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; You, Y.; Cheng, Y.; Ma, J.; Chen, F. Enhancing network complexity and function of soil bacteria by thiourea-modified biochar under cadmium stress in post-mining area. Chemosphere 2022, 302, 134811. [Google Scholar] [CrossRef]



| Soil Physicochemical Properties | Neutral Shandong Soil | Acidic Yunnan Soil |
|---|---|---|
| Clay content (%) | 7.70 | 1.04 |
| Sand content (%) | 16.05 | 77.05 |
| Silt content (%) | 76.25 | 21.91 |
| NO3−-N content (mg kg−1) | 106.68 | 213.49 |
| NH4+-N content (mg kg−1) | 53.17 | 24.02 |
| Available phosphorus (mg kg−1) | 34.02 | 45.87 |
| Organic matter (g kg−1) | 28.15 | 109.01 |
| pH | 7.46 | 5.88 |
| Moisture content (%) | 2.66 | 13.11 |
| Electrical conductivity (μS cm−1) | 428 | 342 |
| Total nitrogen (g kg−1) | 1.00 | 4.07 |
| Available Cd (mg kg−1) | 0.37 | 1.57 |
| Cottonseed hull biochar (BC) | Volatile matter (%) | Ash content (%) | pH | C (%) | H (%) | N (%) | S (%) | O (%) | Total Cd (mg kg−1) |
| 52.90 | 15.30 | 7.53 | 65.66 | 3.85 | 3.15 | 0.25 | 27.09 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Cao, X.; Pan, H.; Lou, Y.; Wang, H.; Yang, Q.; Zhuge, Y. Effects of Biochar on Cadmium Availability, Nitrification and Microbial Communities in Soils with Varied pH Levels. Microorganisms 2025, 13, 839. https://doi.org/10.3390/microorganisms13040839
Zhao W, Cao X, Pan H, Lou Y, Wang H, Yang Q, Zhuge Y. Effects of Biochar on Cadmium Availability, Nitrification and Microbial Communities in Soils with Varied pH Levels. Microorganisms. 2025; 13(4):839. https://doi.org/10.3390/microorganisms13040839
Chicago/Turabian StyleZhao, Wei, Xiaoxu Cao, Hong Pan, Yanhong Lou, Hui Wang, Quangang Yang, and Yuping Zhuge. 2025. "Effects of Biochar on Cadmium Availability, Nitrification and Microbial Communities in Soils with Varied pH Levels" Microorganisms 13, no. 4: 839. https://doi.org/10.3390/microorganisms13040839
APA StyleZhao, W., Cao, X., Pan, H., Lou, Y., Wang, H., Yang, Q., & Zhuge, Y. (2025). Effects of Biochar on Cadmium Availability, Nitrification and Microbial Communities in Soils with Varied pH Levels. Microorganisms, 13(4), 839. https://doi.org/10.3390/microorganisms13040839






