Physiology, Heavy Metal Resistance, and Genome Analysis of Two Cupriavidus gilardii Strains Isolated from the Naica Mine (Mexico)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. DNA Extraction, Genome Sequencing, Assembly and Annotation
2.3. Physiological Characterization
2.4. Evaluation of Heavy Metals Tolerance
2.5. Comparative Genomics Analyses
3. Results
3.1. General Properties of the NOV2-1 and OV2-1 Genomes
3.2. Phylogenetic Characterization
3.3. Physiological Characteristics of NOV2-1 and OV2-1
3.4. Tolerance to Heavy Metals
3.5. Genomic Analysis of HMR Gene Clusters
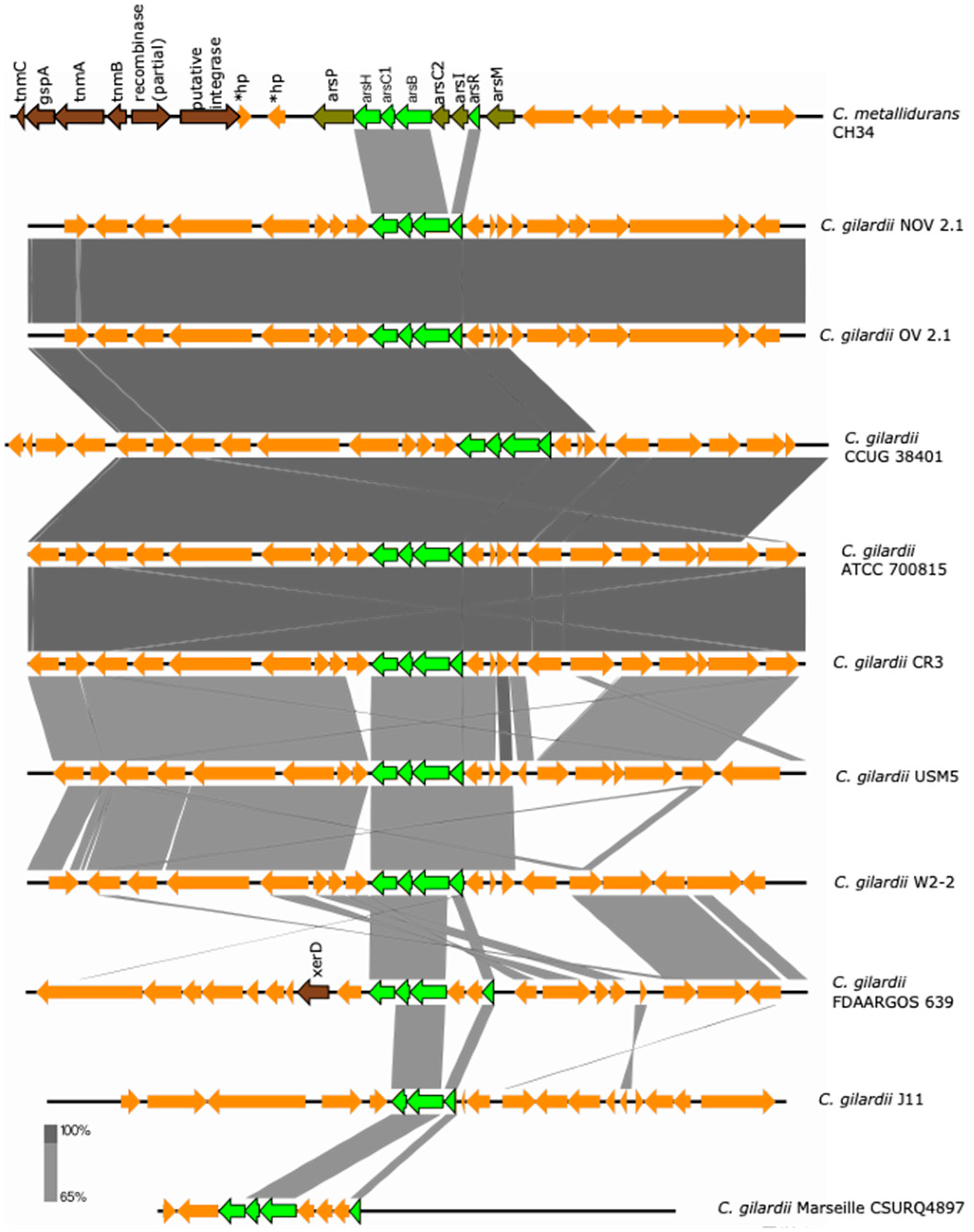
3.5.1. ars Cluster
3.5.2. czc Cluster
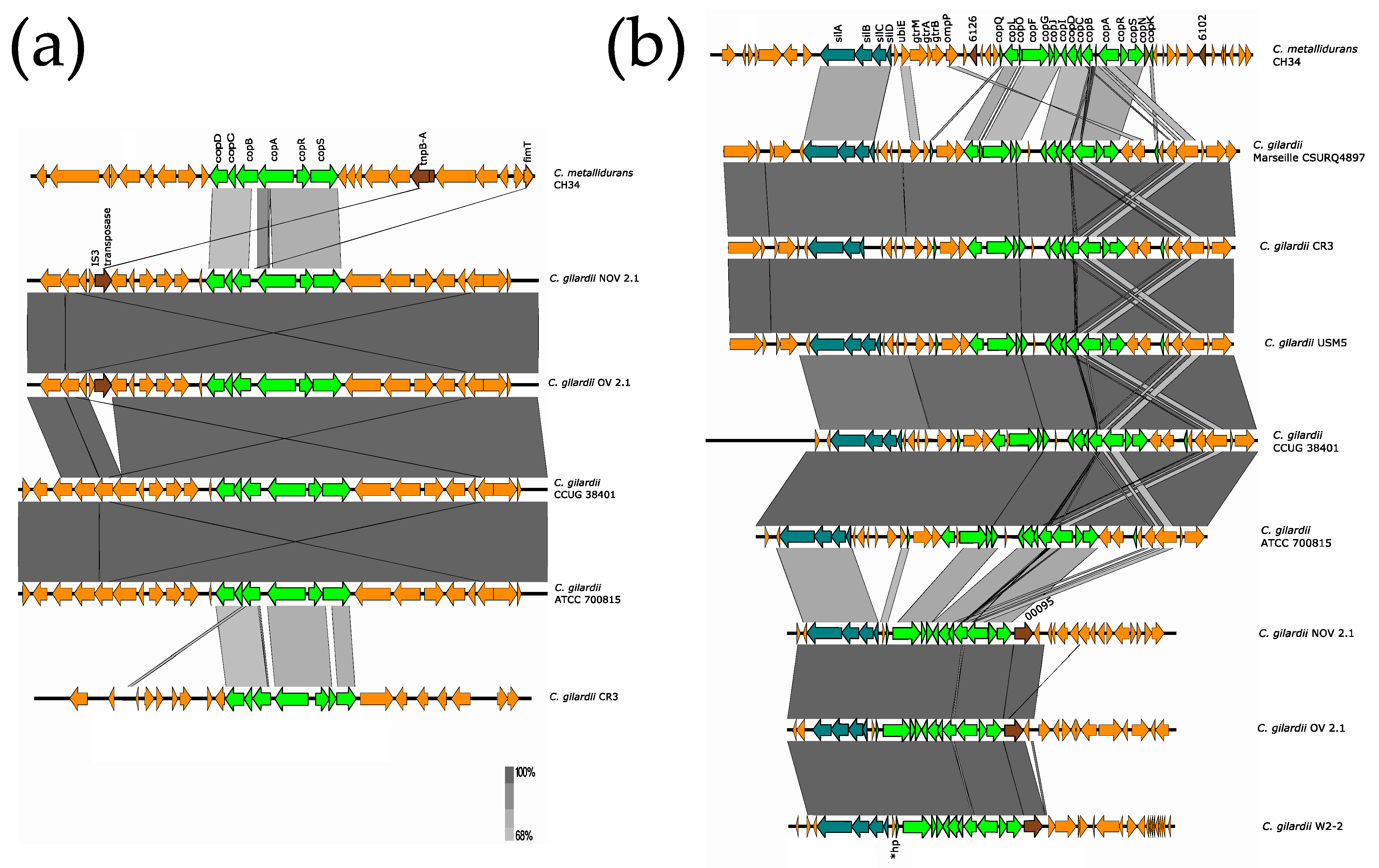
3.5.3. cop Cluster I
3.5.4. sil and cop Cluster 2
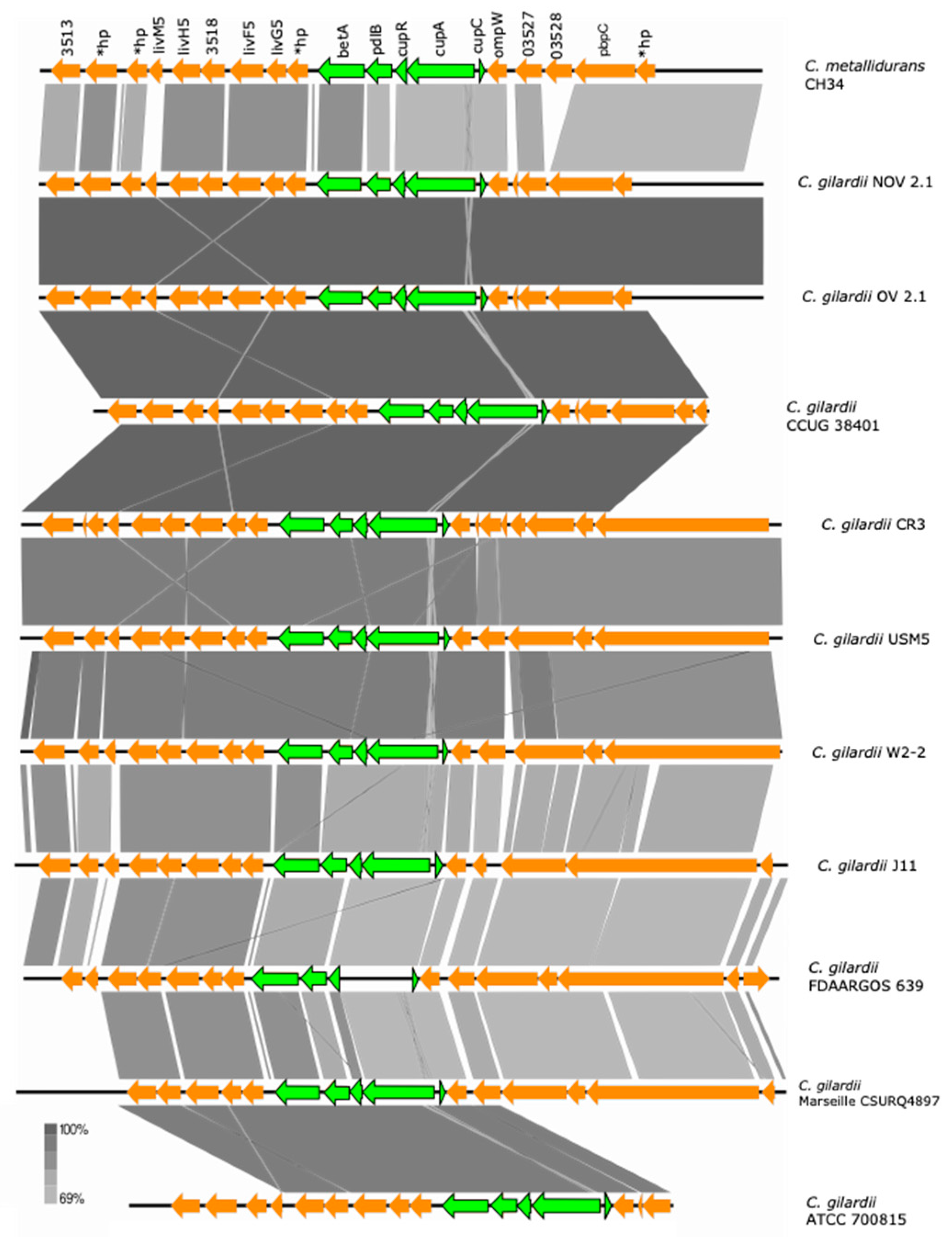
3.5.5. cup Cluster
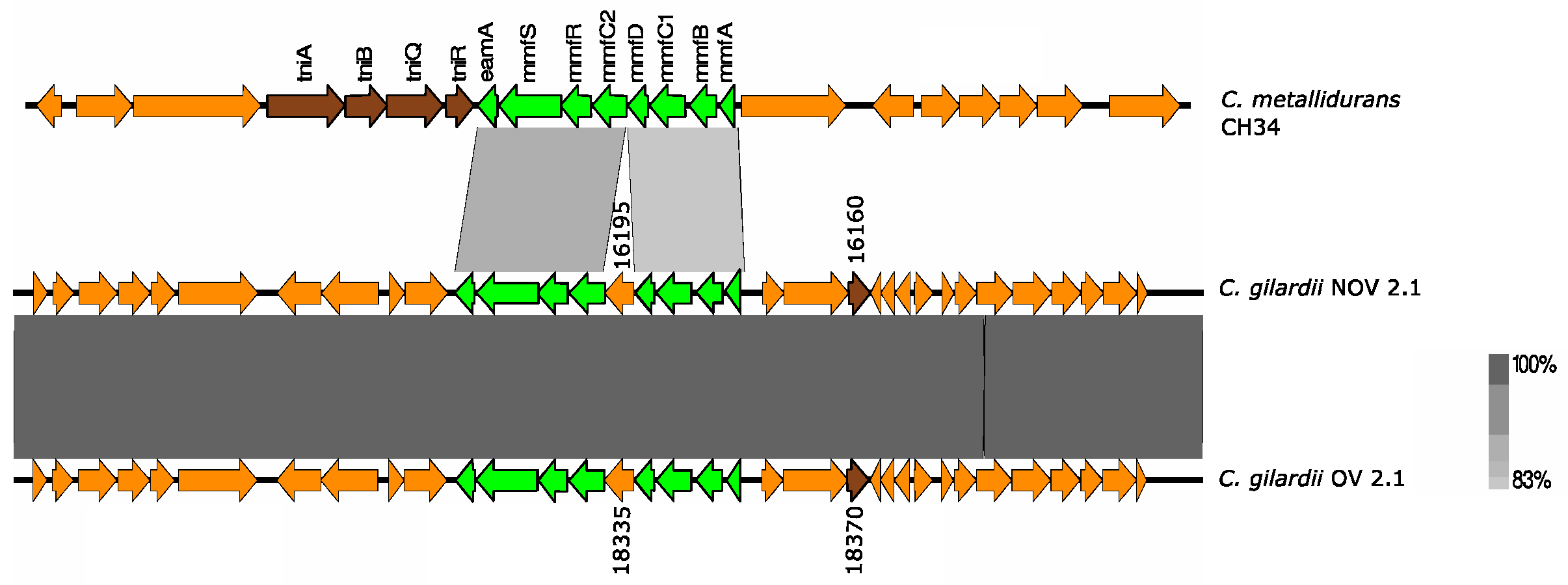
3.5.6. mmf Cluster
3.5.7. mer Cluster
4. Discussion
5. Major Implications and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gázquez, F.; Calaforra, J.M.; Forti, P.; Badino, G. The Caves of Naica: A Decade of Research. Bol. Geol. Y Min. 2016, 127, 147–163. [Google Scholar]
- Peñoles Naica. Available online: https://www.penoles.com.mx/nuestras-operaciones/unidades-mineras/naica.html (accessed on 12 February 2025).
- Carreño-Márquez, I.J.A.; Castillo-Sandoval, I.; Pérez-Cázares, B.E.; Fuentes-Cobas, L.E.; Esparza-Ponce, H.E.; Menéndez-Méndez, E.; Fuentes-Montero, M.E.; Montero-Cabrera, M.E. Evolution of the Astonishing Naica Giant Crystals in Chihuahua, Mexico. Minerals 2021, 11, 292. [Google Scholar] [CrossRef]
- Briceño Prieto, S.B. Caracterización Geoquímica de Los Megacristales de Yeso de Naica, Chihuahua y Su Relación Con Los Procesos de Interacción Agua-Roca. Master’s Thesis, Universidad Nacional Autónoma de México, Ciudad de Mexico, Mexico, 2011. [Google Scholar]
- Bencomo-Calderón, M.; Herrera-Peraza, E.F.; Villalobos-Aragón, A. As and Pb Presence within the Meoqui-Delicias Aquifer, Chihuahua, Mexico. Water 2024, 16, 2538. [Google Scholar] [CrossRef]
- Otálora, F.; García-Ruiz, J. Nucleation and Growth of the Naica Giant Gypsum Crystals. Chem. Soc. Rev. 2014, 43, 2013–2026. [Google Scholar]
- Forti, P.; Sanna, L. The Naica Project—A Multidisciplinary Study of the Largest Gypsum Crystals of the World. Episodes 2010, 33, 23–32. [Google Scholar] [CrossRef]
- Espino del Castillo, A.; Beraldi-Campesi, H.; Amador-Lemus, P.; Beltrán, H.I.; Le Borgne, S. Bacterial Diversity Associated with Mineral Substrates and Hot Springs from Caves and Tunnels of the Naica Underground System (Chihuahua, Mexico). Int. J. Speleol. 2018, 47, 213–227. [Google Scholar] [CrossRef]
- Quintana, E.T.; Badillo, R.F.; Maldonado, L.A. Characterisation of the First Actinobacterial Group Isolated from a Mexican Extremophile Environment. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2013, 104, 63–70. [Google Scholar] [CrossRef]
- Ragon, M.; Van Driessche, A.E.S.; García-Ruíz, J.M.; Moreira, D.; López-García, P. Microbial Diversity in the Deep-Subsurface Hydrothermal Aquifer Feeding the Giant Gypsum Crystal-Bearing Naica Mine, Mexico. Front. Microbiol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Espino del Castillo Rodríguez, A. Caracterización de La Comunidad Bacteriana Presente En Diferentes Superficies Minerales Del Yacimiento de La Mina de Naica (Chihuahua, México) y Su Participación Como Agente Biogeoquímico. Ph.D. Thesis, Universidad Autónoma Metropolitana, Ciudad de Mexico, Mexico, 2019. [Google Scholar]
- Vandamme, P.; Coenye, T. Taxonomy of the Genus Cupriavidus: A Tale of Lost and Found. Int. J. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef]
- Coenye, T. The Family Burkholderiaceae. In The Prokaryotes, 4th ed.; Section II Betaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 759–776. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Kämpfer, P.; De Baere, T.; Falsen, E.; Verschraegen, G. Wautersia Gen. Nov., a Novel Genus Accomodating the Phylogenetic Lineage Including Ralstonia eutropha and Related Species, and Proposal of Ralstonia [Pseudomonas] Syzygii (Roberts et al. 1990) Comb. Nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 317–327. [Google Scholar] [CrossRef]
- Parte, A.C. LPSN—List of Prokaryotic Names with Standing in Nomenclature. Nucleic Acids Res. 2014, 42, D613–D616. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M. The History of Cupriavidus metallidurans Strains Isolated from Anthropogenic Environments. In Metal Response in Cupriavidus metallidurans: Volume I: From Habitats to Genes and Proteins; Mergeay, M., Van Houdt, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–19. [Google Scholar] [CrossRef]
- Arroyo-Herrera, I.; Maldonado-Hernández, J.; Rojas-Rojas, F.U.; Meza-Radilla, G.; Larios-Serrato, V.; Vásquez-Murrieta, M.S.; Whitman, W.B.; De Los Santos, P.E. Cupriavidus agavae Sp. Nov., a Species Isolated from Agave L. Rhizosphere in Northeast Mexico. Int. J. Syst. Evol. Microbiol. 2020, 70, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, M.; Xiao, J.; Hao, L.; Crowley, D.E.; Zhang, Z.; Yu, J.; Huang, N.; Huo, M.; Wu, J. Genome Sequence Analysis of the Naphthenic Acid Degrading and Metal Resistant Bacterium Cupriavidus gilardii CR3. PLoS ONE 2015, 10, e0132881. [Google Scholar] [CrossRef] [PubMed]
- von Rozycki, T.; Nies, D.H. Cupriavidus metallidurans: Evolution of a Metal-Resistant Bacterium. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2009, 96, 115–139. [Google Scholar] [CrossRef]
- De La Rosa- Acosta, M.; Jiménez-Collazo, J.; Maldonado-Román, M.; Malavé-Llamas, K.; Carlos Musa-Wasil, J. Bacteria as Potential Indicators of Heavy Metal Contamination in a Tropical Mangrove and the Implications on Environmental and Human Health. J. Trop. Life Sci. 2015, 5, 110–116. [Google Scholar] [CrossRef]
- Makkar, N.S.; Casipa, L.E. Cupriavidus necator gen. nov., sp. nov.; A Nonobligate Bacterial Predator of Bacteria in Soil. Int. J. Syst. Evol. Microbiol. 1987, 37, 323–326. [Google Scholar] [CrossRef]
- Chen, W.M.; Laevens, S.; Lee, T.M.; Coenye, T.; De Vos, P.; Mergeay, M.; Vandamme, P. Ralstonia taiwanensis Sp. Nov., Isolated from Root Nodules of Mimosa Species and Sputum of a Cystic Fibrosis Patient. Int. J. Syst. Evol. Microbiol. 2001, 51, 1729–1735. [Google Scholar] [CrossRef]
- Goris, J.; De Vos, P.; Coenye, T.; Hoste, B.; Jansses, D.; Brim, H.; Diels, L.; Mergeay, M.; Kersters, K.; Vandamme, P. Classification of Metal-Resistant Bacteria from Industrial Biotopes as Ralstonia campinensis Sp. Nov., Ralstonia metallidurans Sp. Nov. and Ralstonia Basilensis Steinle et al. 1998 Emend. Int. J. Syst. Evol. Microbiol. 2001, 51, 1773–1782. [Google Scholar] [CrossRef]
- Andrews, S. FastQC—A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 August 2022).
- Krueger, F. A Wrapper Tool around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 15 February 2025).
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Wences, A.H.; Schatz, M.C. Metassembler: Merging and Optimizing de Novo Genome Assemblies. Genome Biol. 2015, 16, 207. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Tatusova, T.; Dicuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and Taxonomy in Diagnostics for Food Security: Soft-Rotting Enterobacterial Plant Pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
- Contreras-Moreira, B.; Vinuesa, P. GET_HOMOLOGUES, a Versatile Software Package for Scalable and Robust Microbial Pangenome Analysis. Appl. Environ. Microbiol. 2013, 79, 7696–7701. [Google Scholar] [CrossRef]
- Vinuesa, P.; Ochoa-Sánchez, L.E.; Contreras-Moreira, B. GET_PHYLOMARKERS, a Software Package to Select Optimal Orthologous Clusters for Phylogenomics and Inferring Pan-Genome Phylogenies, Used for a Critical Geno-Taxonomic Revision of the Genus Stenotrophomonas. Front. Microbiol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Monsieurs, P.; Moors, H.; Van Houdt, R.; Janssen, P.J.; Janssen, A.; Coninx, I.; Mergeay, M.; Leys, N. Heavy Metal Resistance in Cupriavidus metallidurans CH34 Is Governed by an Intricate Transcriptional Network. BioMetals 2011, 24, 1133–1151. [Google Scholar] [CrossRef]
- Monsieurs, P.; Hobman, J.; Vandenbussche, G.; Mergeay, M.; Van Houdt, R. Response of Cupriavidus metallidurans CH34 to Metals. In Metal Response in Cupriavidus metallidurans: Volume I: From Habitats to Genes and Proteins; Mergeay, M., Van Houdt, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 21–44. [Google Scholar] [CrossRef]
- Utami, U.; Harianie, L.; Dunyana, N.R. Romaidi Lead-Resistant Bacteria Isolated from Oil Wastewater Sample for Bioremediation of Lead. Water Sci. Technol. 2020, 81, 2244–2249. [Google Scholar] [CrossRef]
- Maertens, L.; Leys, N.; Matroule, J.Y.; Van Houdt, R. The Transcriptomic Landscape of Cupriavidus metallidurans CH34 Acutely Exposed to Copper. Genes. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Abbaszade, G.; Szabó, A.; Vajna, B.; Farkas, R.; Szabó, C.; Tóth, E. Whole Genome Sequence Analysis of Cupriavidus Campinensis S14E4C, a Heavy Metal Resistant Bacterium. Mol. Biol. Rep. 2020, 47, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Falsent, E.; Hostef, B.; W Govant, J.R.; Kersters, K.; Vandamme, P. Classification of Alcaligenes faecalis-like Isolates from the Environment and Human Clinical Samples as Ralstonia gilardii Sp. Nov. Int. J. Syst. Evol. Microbiol. 1999, 49, 405–413. [Google Scholar]
- Sichtig, H.; Minogue, T.; Yan, Y.; Stefan, C.; Hall, A.; Tallon, L.; Sadzewicz, L.; Nadendla, S.; Klimke, W.; Hatcher, E.; et al. FDA-ARGOS Is a Database with Public Quality-Controlled Reference Genomes for Diagnostic Use and Regulatory Science. Nat. Commun. 2019, 10, 3313. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, W.; Wang, S.; Xu, L.; Yan, L.; Liao, P. First Case Report of Infection Caused by Cupriavidus gilardii in a Non-Immunocompromised Chinese Patient. IDCases 2017, 10, 127–129. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, M.; Zhou, D.; Fan, W.; Wang, X.; Huo, M. Bioremoval of Cu2+ from CMP Wastewater by a Novel Copper-Resistant Bacterium Cupriavidus gilardii CR3: Characteristics and Mechanisms. RSC Adv. 2017, 7, 18793–18802. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Liu, J.; Pan, D.; Tu, X.; Lv, P.; Wang, Y.; Cao, H.; Wang, Y.; Hua, R. Rapid Biodegradation of the Herbicide 2,4-Dichlorophenoxyacetic Acid by Cupriavidus gilardii T-1. J. Agric. Food Chem. 2017, 65, 3711–3720. [Google Scholar] [CrossRef]
- Pérez-Pantoj, D.; Leiva-Novoa, P.; Donoso, R.A.; Little, C.; Godoy, M.; Pieper, D.H.; Gonzáleza, B. Hierarchy of Carbon Source Utilization in Soil Bacteria: Hegemonic Preference for Benzoate in Complex Aromatic Compound Mixtures Degraded by Cupriavidus pinatubonensis Strain JMP134. Appl. Environ. Microbiol. 2015, 81, 3914–3924. [Google Scholar] [CrossRef]
- Ruiz, C.; McCarley, A.; Espejo, M.L.; Cooper, K.K.; Harmon, D.E. Comparative Genomics Reveals a Well-Conserved Intrinsic Resistome in the Emerging Multidrug-Resistant Pathogen Cupriavidus gilardii. mSphere 2019, 4, e00631-19. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Moriuchi, R.; Dohra, H.; Kanesaki, Y.; Ogawa, N. Complete Genome Sequence of 3-Chlorobenzoate-Degrading Bacterium Cupriavidus necator NH9 and Reclassification of the Strains of the Genera Cupriavidus and Ralstonia Based on Phylogenetic and Whole-Genome Sequence Analyses. Front. Microbiol. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Fournes, F.; Val, M.E.; Skovgaard, O.; Mazel, D. Replicate Once per Cell Cycle: Replication Control of Secondary Chromosomes. Front. Microbiol. 2018, 9, 1833. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.W.; Lower, R.P.J.; Kim, N.K.D.; Young, J.P.W. Introducing the Bacterial “Chromid”: Not a Chromosome, Not a Plasmid. Trends Microbiol. 2010, 18, 141–148. [Google Scholar] [CrossRef]
- Janssen, P.J.; van Houdt, R.; Moors, H.; Monsieurs, P.; Morin, N.; Michaux, A.; Benotmane, M.A.; Leys, N.; Vallaeys, T.; Lapidus, A.; et al. The Complete Genome Sequence of Cupriavidus metallidurans Strain CH34, a Master Survivalist in Harsh and Anthropogenic Environments. PLoS ONE 2010, 5, e10433. [Google Scholar] [CrossRef]
- Lykidis, A.; Pérez-Pantoja, D.; Ledger, T.; Mavromatis, K.; Anderson, I.J.; Ivanova, N.N.; Hooper, S.D.; Lapidus, A.; Lucas, S.; González, B.; et al. The Complete Multipartite Genome Sequence of Cupriavidus necator JMP134, a Versatile Pollutant Degrader. PLoS ONE 2010, 5, e9729. [Google Scholar] [CrossRef]
- Mergeay, M.; Van Houdt, R. Plasmids as Secondary Chromosomes. In Molecular Life Sciences an Encyclopedic Reference; Wells, R.D., Bond, J.S., Klinman, J., Masters, B.S.S., Eds.; Springer: New York, NY, USA, 2018; pp. 961–964. [Google Scholar] [CrossRef]
- Dicenzo, G.C.; Mengoni, A.; Perrin, E. Chromids Aid Genome Expansion and Functional Diversification in the Family Burkholderiaceae. Mol. Biol. Evol. 2019, 36, 562–574. [Google Scholar] [CrossRef]
- Van Houdt, R.; Vandecraen, J.; Heylen, W.; Leys, N.; Monsieurs, P.; Provoost, A.; Aertsen, A. Phenotypic and Genetic Characterization of Temperature-Induced Mutagenesis and Mortality in Cupriavidus metallidurans. Front. Microbiol. 2021, 12, 698330. [Google Scholar] [CrossRef]
- Arroua, B.; Bellanger, X.; Guilloteau, H.; Mathieu, L.; Merlin, C. Atypical Stress Response to Temperature and NaOCl Exposure Leading to Septation Defect during Cell Division in Cupriavidus metallidurans CH34. FEMS Microbiol. Lett. 2014, 353, 32–39. [Google Scholar] [CrossRef]
- Florentino, L.A.; Jaramillo, P.M.D.; Silva, K.B.; da Silva, J.S.; de Oliveira, S.M.; de Souza Moreira, F.M. Physiological and Symbiotic Diversity of Cupriavidus necator Strains Isolated from Nodules of Leguminosae Species. Sci. Agric. 2012, 69, 247–258. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C. Microbial Detoxification of Lignocellulosic Biomass Hydrolysates: Biochemical and Molecular Aspects, Challenges, Exploits and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1061667. [Google Scholar] [CrossRef]
- Agu, C.V.; Ujor, V.; Gopalan, V.; Ezeji, T.C. Use of Cupriavidus Basilensis-Aided Bioabatement to Enhance Fermentation of Acid-Pretreated Biomass Hydrolysates by Clostridium beijerinckii. J. Ind. Microbiol. Biotechnol. 2016, 43, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Van Houdt, R. Cupriavidus metallidurans CH34, a Historical Perspective on Its Discovery, Characterization and Metal Resistance. FEMS Microbiol. Ecol. 2021, 97, fiaa247. [Google Scholar] [CrossRef] [PubMed]
- Scherer, J.; Nies, D.H. CzcP Is a Novel Efflux System Contributing to Transition Metal Resistance in Cupriavidus metallidurans CH34. Mol. Microbiol. 2009, 73, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; Ross, M.O.; Hoffman, B.M.; Rosenzweig, A.C. Metal Selectivity of a Cd-, Co-, and Zn-Transporting P1B-Type ATPase. Biochemistry 2017, 56, 85–95. [Google Scholar] [CrossRef]
- Schulz, V.; Schmidt-Vogler, C.; Strohmeyer, P.; Weber, S.; Kleemann, D.; Nies, D.H.; Herzberg, M. Behind the Shield of Czc: ZntR Controls Expression of the Gene for the Zinc-Exporting P-Type ATPase ZntA in Cupriavidus metallidurans. J. Bacteriol. 2021, 203, e00052-21. [Google Scholar] [CrossRef]
- Legatzki, A.; Grass, G.; Anton, A.; Rensing, C.; Nies, D.H. Interplay of the Czc System and Two P-Type ATPases in Conferring Metal Resistance to Ralstonia metallidurans. J. Bacteriol. 2003, 185, 4354–4361. [Google Scholar] [CrossRef]
- Ali, M.M.; Provoost, A.; Maertens, L.; Leys, N.; Monsieurs, P.; Charlier, D.; Van Houdt, R. Genomic and Transcriptomic Changes That Mediate Increased Platinum Resistance in Cupriavidus metallidurans. Genes 2019, 10, 63. [Google Scholar] [CrossRef]
- Van Houdt, R.; Mergeay, M. Genomic Context of Metal Response Genes in Cupriavidus metallidurans with a Focus on Strain CH34. In Metal Response in Cupriavidus metallidurans: Volume I: From Habitats to Genes and Proteins; Mergeay, M., Van Houdt, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–89. [Google Scholar] [CrossRef]
- Fekih, I.B.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of Arsenic Resistance Genes in Prokaryotes. Front. Microbiol. 2018, 9, 2473. [Google Scholar]
- Dunivin, T.K.; Yeh, S.Y.; Shade, A. A Global Survey of Arsenic-Related Genes in Soil Microbiomes. BMC Biol. 2019, 17, 45. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Mikušková, N.; Faggio, C. Assessing Metal Toxicity on Crustaceans in Aquatic Ecosystems: A Comprehensive Review. Biol. Trace Elem. Res. 2024, 202, 5743–5761. [Google Scholar]




| Feature | NOV2-1 | OV2-1 |
|---|---|---|
| Genome size (bp) | 5,687,442 | 5,647,760 |
| CDS | 5024 | 5011 |
| rRNA | 12 | 12 |
| tRNA | 59 | 59 |
| GC content (%) | 67.53 | 67.58 |
| Contigs number | 2 | 2 |
| Chromosomes number | 2 | 2 |
| Hypothetical proteins | 1186 | 1186 |
| Proteins with functional assignments | 4095 | 4064 |
| Characteristics | C. necator | C. metalidurans | C. taiwanensis | C. gilardii | ||
|---|---|---|---|---|---|---|
| LMG8453 | CH34 | LMG19424 | CCUG 38401 | NOV2-1 | OV2-1 | |
| Range for growth: | ||||||
| Temperature (°C) | 30–37 | 30–35 | 30–37 | 30–48 | ||
| pH | 5.0–9.0 | 7.0–9.0 | 7.0–9.9 | 5.5–9.0 | ||
| NaCl (% w/v) | >1 | <1 | ||||
| Heavy metal tolerance (mM): | ||||||
| Cd | 1.5 | 5 | 0.75 | 1.5 | 5 | 0.75 |
| Co | 0.5 | 0.8 | 0.8 | 0.7 | 0.7 | 0.7 |
| Cu | 3 | 3 | 1.5 | 3 | 1 | 3 |
| Pb | 8.5 | 9 | 8.5 | 9 | 8.5 | 8.5 |
| Ni | 3 | 7 | 3 | 7 | 3 | 3 |
| Zn | 2 | 7.5 | 7.5 | 7.5 | 7.5 | 1.5 |
| Assimilation of: | ||||||
| L-Lactate | + | − | + | − | + | + |
| Sodium citrate | + | + | + | − | − | + |
| L-Malate | - | + | - | - | + | + |
| Enzymatic activities: | ||||||
| L-Proline Arylamidase | + | + | + | − | − | − |
| L-Pyrrolydonyl-Arylamidase | + | + | + | − | − | − |
| Gamma-Glutamyl-Transferase | + | − | + | − | − | − |
| Urease | − | + | + | − | − | − |
| Alpha-Glucosidase | − | − | − | − | − | − |
| Glutamyl Arylamidase pNA | + | − | − | + | − | + |
| Other tests: | ||||||
| ELLMAN | + | + | + | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Sánchez, A.; Lozano-Aguirre, L.; Jiménez-Flores, G.; López-Sámano, M.; García-de Los Santos, A.; Cevallos, M.A.; Le Borgne, S. Physiology, Heavy Metal Resistance, and Genome Analysis of Two Cupriavidus gilardii Strains Isolated from the Naica Mine (Mexico). Microorganisms 2025, 13, 809. https://doi.org/10.3390/microorganisms13040809
González-Sánchez A, Lozano-Aguirre L, Jiménez-Flores G, López-Sámano M, García-de Los Santos A, Cevallos MA, Le Borgne S. Physiology, Heavy Metal Resistance, and Genome Analysis of Two Cupriavidus gilardii Strains Isolated from the Naica Mine (Mexico). Microorganisms. 2025; 13(4):809. https://doi.org/10.3390/microorganisms13040809
Chicago/Turabian StyleGonzález-Sánchez, Antonio, Luis Lozano-Aguirre, Guadalupe Jiménez-Flores, Mariana López-Sámano, Alejandro García-de Los Santos, Miguel A. Cevallos, and Sylvie Le Borgne. 2025. "Physiology, Heavy Metal Resistance, and Genome Analysis of Two Cupriavidus gilardii Strains Isolated from the Naica Mine (Mexico)" Microorganisms 13, no. 4: 809. https://doi.org/10.3390/microorganisms13040809
APA StyleGonzález-Sánchez, A., Lozano-Aguirre, L., Jiménez-Flores, G., López-Sámano, M., García-de Los Santos, A., Cevallos, M. A., & Le Borgne, S. (2025). Physiology, Heavy Metal Resistance, and Genome Analysis of Two Cupriavidus gilardii Strains Isolated from the Naica Mine (Mexico). Microorganisms, 13(4), 809. https://doi.org/10.3390/microorganisms13040809






