Gastrointestinal Microorganisms and Blood Metabolites in Holstein Calves with Different Heat Stress Responses in the Same Hot Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Experiment Design
2.2. DNA Extraction, PCR Amplification, and 16S rRNA Sequencing
2.3. Metabolomics Analysis of Serum Samples and Data Processing
2.4. Statistical Analysis
3. Results
3.1. Blood Indicators Analyses
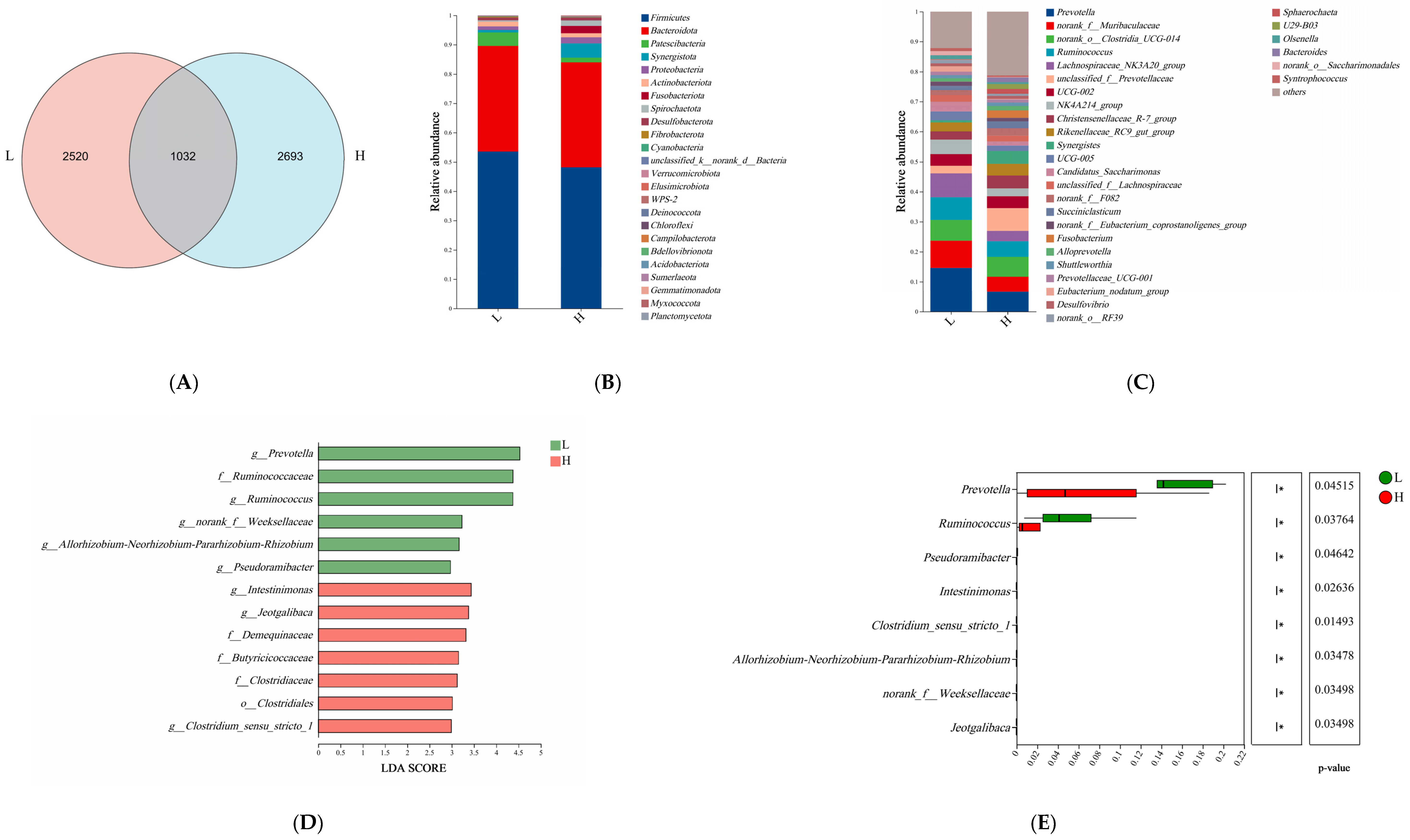
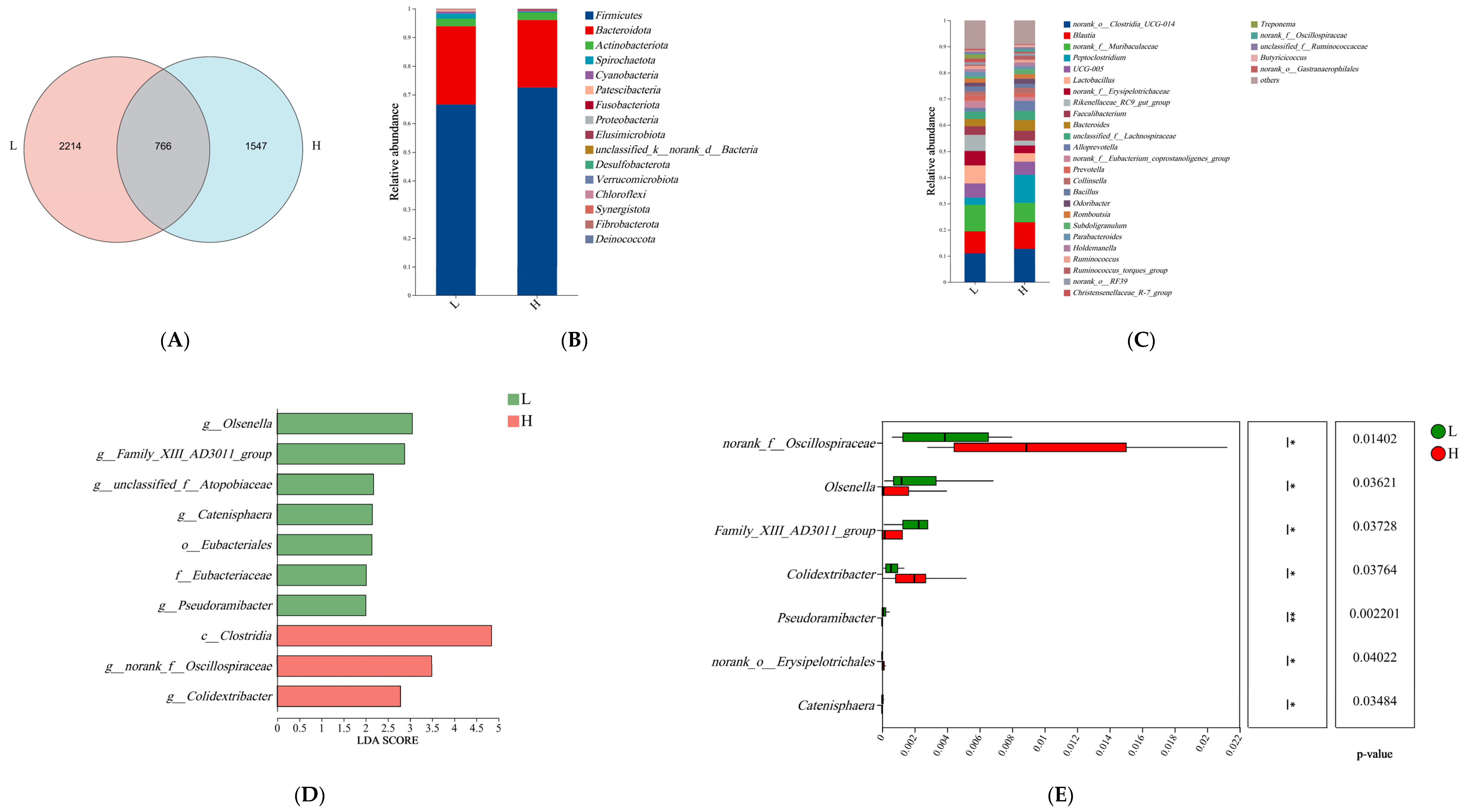
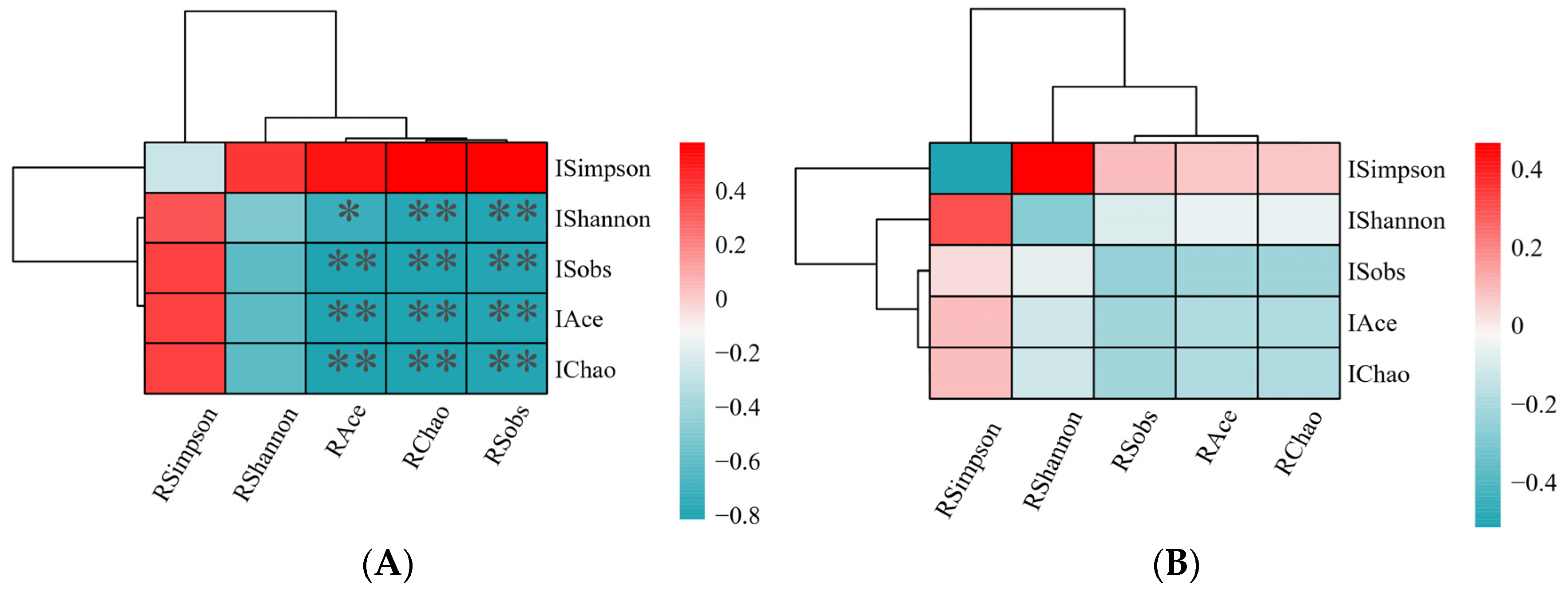
3.2. Microbiome Analyses
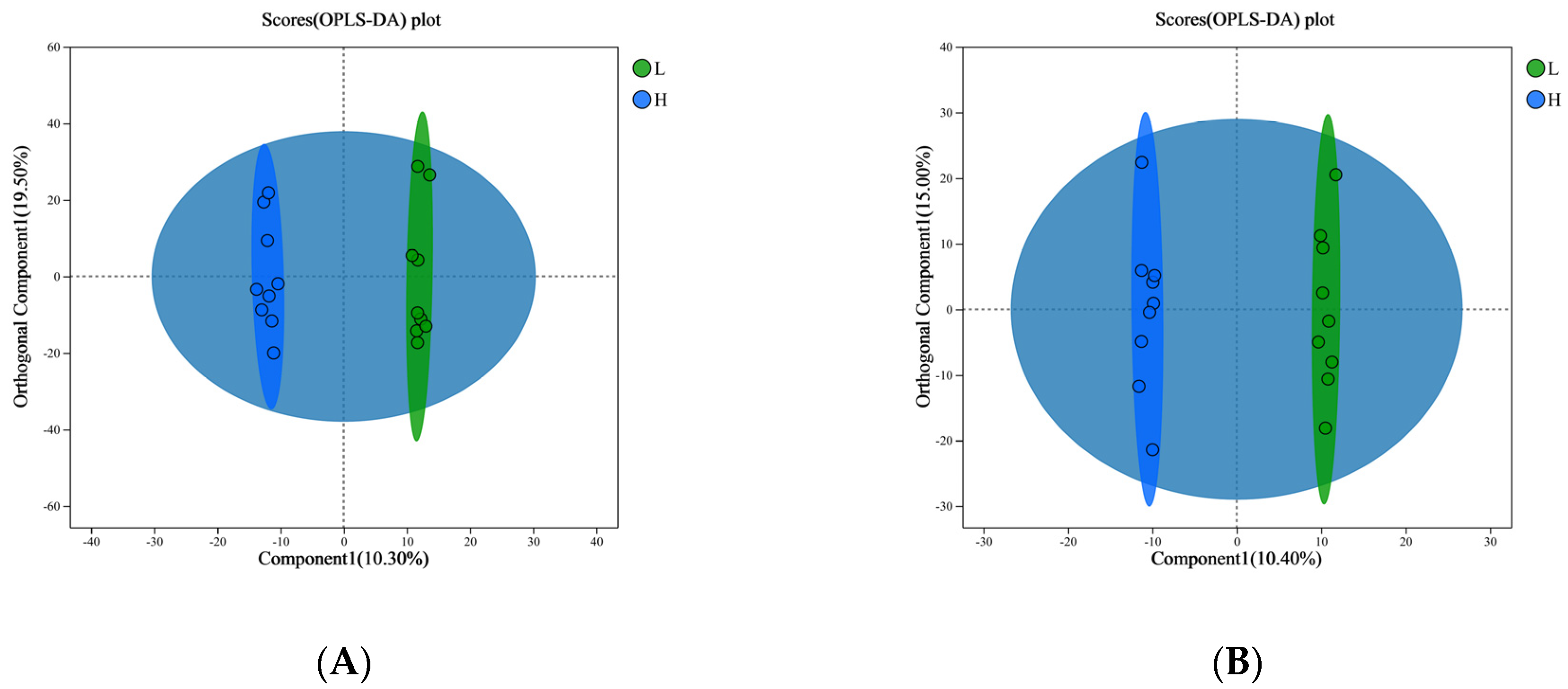
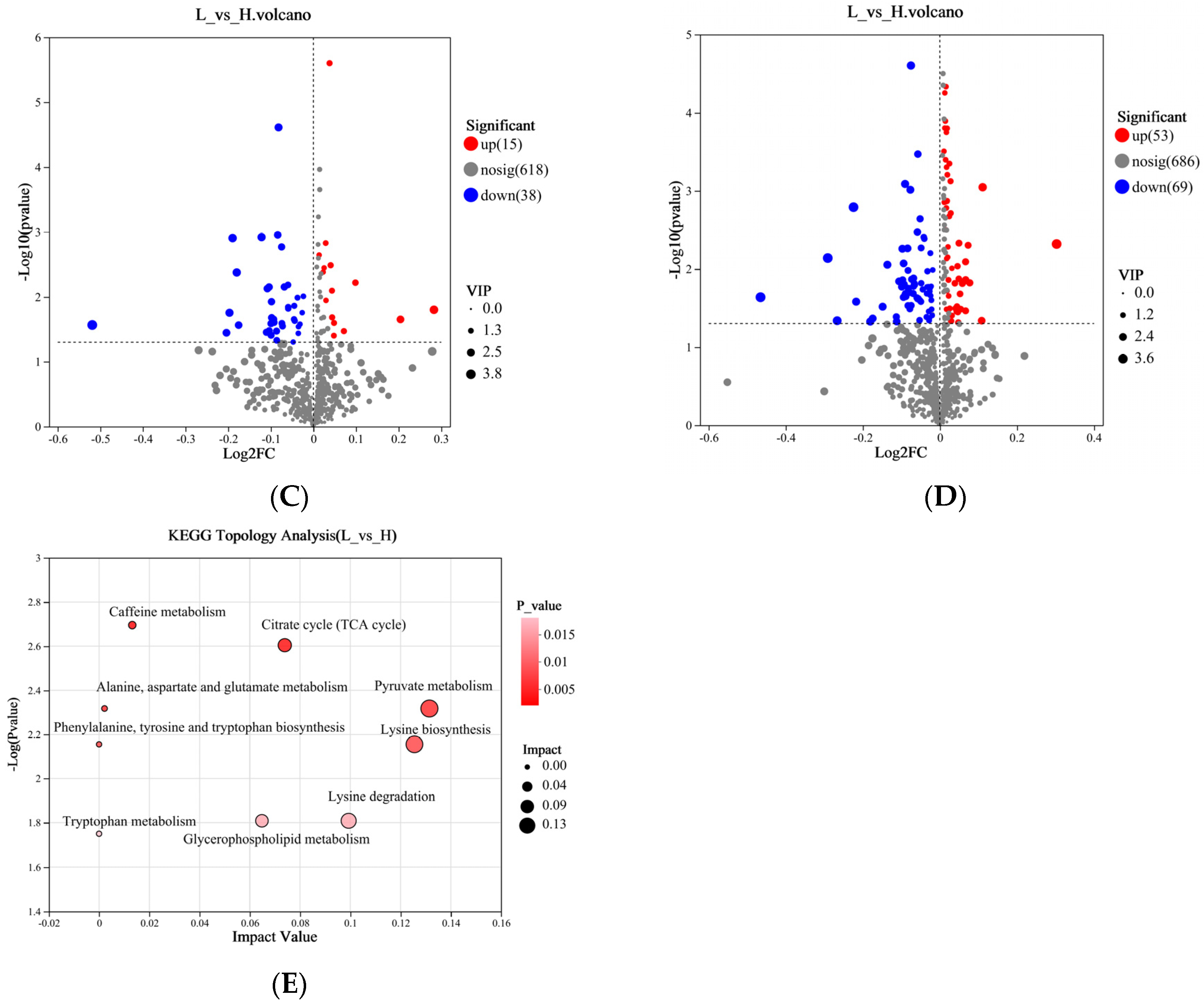
3.3. Differential Metabolites Screening and Analysis of Host Serum
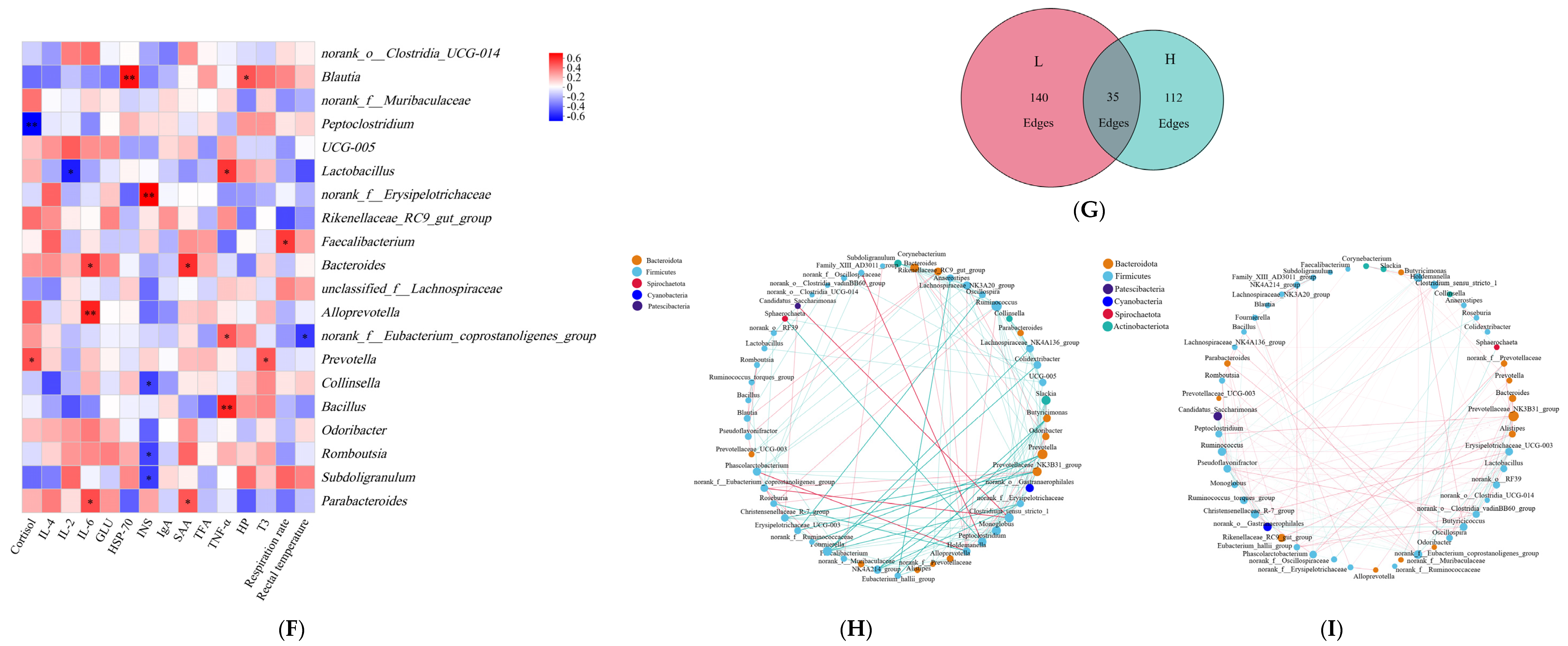
3.4. Microbe–Host Metabolite Interactions Associated with Cattle Heat Stress Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar]
- Polsky, L.; von Keyserlingk, M. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [PubMed]
- Wang, J.; Li, J.; Wang, F.; Xiao, J.; Wang, Y.; Yang, H.; Li, S.; Cao, Z. Heat stress on calves and heifers: A review. J. Anim. Sci. Biotechnol. 2020, 11, 79. [Google Scholar]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar]
- Morrison, S.R. Ruminant heat stress: Effect on production and means of alleviation. J. Anim. Sci. 1983, 57, 1594–1600. [Google Scholar] [CrossRef]
- Selvam, R.; Suresh, S.; Saravanakumar, M.; Chandrasekaran, C.V.; Prashanth, D. Alleviation of heat stress by a polyherbal formulation, phytocee: Impact on zootechnical parameters, cloacal temperature, and stress markers. Pharmacogn. Res. 2018, 10, 1–8. [Google Scholar]
- Rauba, J.; Heins, B.J.; Chester-Jones, H.; Diaz, H.L.; Ziegler, D.; Linn, J.; Broadwater, N. Relationships between protein and energy consumed from milk replacer and starter and calf growth and first-lactation production of holstein dairy cows. J. Dairy Sci. 2019, 102, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Place, N.T.; Heinrichs, A.J.; Erb, H.N. The effects of disease, management, and nutrition on average daily gain of dairy heifers from birth to four months. J. Dairy Sci. 1998, 81, 1004–1009. [Google Scholar]
- Yazdi, M.H.; Mirzaei-Alamouti, H.R.; Amanlou, H.; Mahjoubi, E.; Nabipour, A.; Aghaziarati, N.; Baumgard, L.H. Effects of heat stress on metabolism, digestibility, and rumen epithelial characteristics in growing holstein calves. J. Anim. Sci. 2016, 94, 77–89. [Google Scholar]
- Broucek, J.; Kisac, P.; Uhrincat, M. Effect of hot temperatures on the hematological parameters, health and performance of calves. Int. J. Biometeorol. 2009, 53, 201–208. [Google Scholar]
- Baccari, F.J.; Johnson, H.D.; Hahn, G.L. Environmental heat effects on growth, plasma t3, and postheat compensatory effects on holstein calves. Proc. Soc. Exp. Biol. Med. 1983, 173, 312–318. [Google Scholar] [CrossRef]
- Yu, Z.; Cantet, J.M.; Paz, H.A.; Kaufman, J.D.; Orellano, M.S.; Ipharraguerre, I.R.; Rius, A.G. Heat stress-associated changes in the intestinal barrier, inflammatory signals, and microbiome communities in dairy calves. J. Dairy Sci. 2024, 107, 1175–1196. [Google Scholar] [CrossRef]
- Duffy, E.M.; Tietze, S.M.; Knoell, A.L.; Aluthge, N.D.; Fernando, S.C.; Schmidt, T.S.; Yates, D.T.; Petersen, J.L. Rumen bacterial composition in lambs is affected by beta-adrenergic agonist supplementation and heat stress at the phylum level. Transl. Anim. Sci. 2018, 2, S145–S148. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Nonaka, I.; Higuchi, K.; Takusari, N.; Kurihara, M.; Takenaka, A.; Mitsumori, M.; Kajikawa, H.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in holstein heifers. Anaerobe 2007, 13, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Guo, R.B.; Gu, T.T.; Zong, Y.B.; Xu, W.W.; Chen, L.; Tian, Y.; Li, G.Q.; Lu, L.Z.; Zeng, T. Investigating the correlation between phenotypes, adrenal transcriptome, and serum metabolism in laying ducks exhibiting varying behaviours under the same stressor. Animal 2024, 18, 101343. [Google Scholar] [CrossRef] [PubMed]
- NRC. A Guide to Environmental Research on Animals; National Academy of Sciences: Washington, DC, USA, 1971; p. 374. [Google Scholar]
- Wu, D.; Wang, C.; Simujide, H.; Liu, B.; Chen, Z.; Zhao, P.; Huangfu, M.; Liu, J.; Gao, X.; Wu, Y.; et al. Reproductive hormones mediate intestinal microbiota shifts during estrus synchronization in grazing simmental cows. Animals 2022, 12, 1751. [Google Scholar] [CrossRef]
- Edgar, R.C. Uparse: Highly accurate otu sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive bayesian classifier for rapid assignment of rrna sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Kim, H.S.; Whon, T.W.; Sung, H.; Jeong, Y.S.; Jung, E.S.; Shin, N.R.; Hyun, D.W.; Kim, P.S.; Lee, J.Y.; Lee, C.H.; et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 2021, 12, 161. [Google Scholar] [CrossRef]
- Alderete, T.L.; Jones, R.B.; Shaffer, J.P.; Holzhausen, E.A.; Patterson, W.B.; Kazemian, E.; Chatzi, L.; Knight, R.; Plows, J.F.; Berger, P.K.; et al. Early life gut microbiota is associated with rapid infant growth in hispanics from southern california. Gut Microbes 2021, 13, 1961203. [Google Scholar]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihara, M.; Kamagata, Y. An rrna-based analysis for evaluating the effect of heat stress on the rumen microbial composition of holstein heifers. Anaerobe 2010, 16, 27–33. [Google Scholar] [PubMed]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar]
- Yin, T.; Halli, K.; Konig, S. Effects of prenatal heat stress on birth weight and birth weight genetic parameters in german holstein calves. JDS Commun. 2023, 4, 469–473. [Google Scholar] [PubMed]
- Ahmed, B.; Younas, U.; Asar, T.O.; Monteiro, A.; Hayen, M.J.; Tao, S.; Dahl, G.E. Maternal heat stress reduces body and organ growth in calves: Relationship to immune status. JDS Commun. 2021, 2, 295–299. [Google Scholar]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar]
- Koch, F.; Reyer, H.; Gors, S.; Hansen, C.; Wimmers, K.; Kuhla, B. Heat stress and feeding effects on the mucosa-associated and digesta microbiome and their relationship to plasma and digesta fluid metabolites in the jejunum of dairy cows. J. Dairy Sci. 2024, 107, 5162–5177. [Google Scholar] [PubMed]
- Kim, W.S.; Kim, J.; Lee, H.G. Identification of potential biomarkers and metabolic pathways of different levels of heat stress in beef calves. Int. J. Mol. Sci. 2022, 23, 10155. [Google Scholar] [CrossRef]
- Yu, Z.; Cantet, J.M.; Nair, M.; Rius, A.G. Dexamethasone administration restored growth in dairy calves exposed to heat stress. J. Dairy Sci. 2024, 107, 11669–11680. [Google Scholar]
- Chikkagoudara, K.P.; Singh, P.; Bhatt, N.; Barman, D.; Selvaraj, R.; Lathwal, S.S.; Singh, S.V.; Choudhary, S.; Uddin, J. Effect of heat stress mitigations on physiological, behavioural, and hormonal responses of buffalo calves. Int. J. Biometeorol. 2022, 66, 995–1003. [Google Scholar]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [PubMed]
- Aggarwal, A.; Ashutosh; Chandra, G.; Singh, A.K. Heat shock protein 70, oxidative stress, and antioxidant status in periparturient crossbred cows supplemented with alpha-tocopherol acetate. Trop. Anim. Health Prod. 2013, 45, 239–245. [Google Scholar] [PubMed]
- Rakib, M.; Messina, V.; Gargiulo, J.I.; Lyons, N.A.; Garcia, S.C. Graduate student literature review: Potential use of hsp70 as an indicator of heat stress in dairy cows—A review. J. Dairy Sci. 2024, 107, 11597–11610. [Google Scholar]
- Kidd, P. Th1/th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.J. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [PubMed]
- Fontoura, A.; Sainz, D.L.M.V.; Richards, A.T.; Tate, B.N.; Van Amburgh, M.E.; Grilli, E.; McFadden, J.W. Effects of dietary organic acid and pure botanical supplementation on growth performance and circulating measures of metabolic health in holstein calves challenged by heat stress. J. Dairy Sci. 2023, 106, 2904–2918. [Google Scholar]
- Ma, L.; Yang, Y.; Liu, W.; Bu, D. Sodium butyrate supplementation impacts the gastrointestinal bacteria of dairy calves before weaning. Appl. Microbiol. Biotechnol. 2023, 107, 3291–3304. [Google Scholar]
- Park, T.; Ma, L.; Gao, S.; Bu, D.; Yu, Z. Heat stress impacts the multi-domain ruminal microbiota and some of the functional features independent of its effect on feed intake in lactating dairy cows. J. Anim. Sci. Biotechnol. 2022, 13, 71. [Google Scholar]
- Cangiano, L.R.; Yohe, T.T.; Steele, M.A.; Renaud, D.L. Invited review: Strategic use of microbial-based probiotics and prebiotics in dairy calf rearing. Appl. Anim. Sci. 2020, 36, 630–651. [Google Scholar]
- Guo, Y.; Li, Z.; Deng, M.; Li, Y.; Liu, G.; Liu, D.; Liu, Q.; Liu, Q.; Sun, B. Effects of a multi-strain probiotic on growth, health, and fecal bacterial flora of neonatal dairy calves. Anim. Biosci. 2022, 35, 204–216. [Google Scholar]
- Zhang, J.; Xu, C.; Huo, D.; Hu, Q.; Peng, Q. Comparative study of the gut microbiome potentially related to milk protein in murrah buffaloes (Bubalus bubalis) and chinese holstein cattle. Sci. Rep. 2017, 7, 42189. [Google Scholar] [CrossRef]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Rs5 produced more butyric acid through regulating the microbial community of human gut microbiota. J. Agric. Food. Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, F.; Zhang, C.; Zhang, Q.; Su, X.; Zhu, X.; Liu, A.; Shi, W.; Lin, W.; Jin, Z.; et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/gpr109a signaling pathway. J. Nanobiotechnol. 2021, 19, 170. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class i histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e201073. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum beta-lactamase-producing enteroaggregative escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Bongers, K.S.; Chanderraj, R.; Woods, R.J.; McDonald, R.A.; Adame, M.D.; Falkowski, N.R.; Brown, C.A.; Baker, J.M.; Winner, K.M.; Fergle, D.J.; et al. The gut microbiome modulates body temperature both in sepsis and health. Am. J. Respir. Crit. Care Med. 2023, 207, 1030–1041. [Google Scholar] [CrossRef]
- Liu, J.; Bian, G.; Sun, D.; Zhu, W.; Mao, S. Starter feeding supplementation alters colonic mucosal bacterial communities and modulates mucosal immune homeostasis in newborn lambs. Front. Microbiol. 2017, 8, 429. [Google Scholar] [CrossRef]
- Warinner, C.; Rodrigues, J.F.; Vyas, R.; Trachsel, C.; Shved, N.; Grossmann, J.; Radini, A.; Hancock, Y.; Tito, R.Y.; Fiddyment, S.; et al. Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 2014, 46, 336–344. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Z.; Song, Q.; Jia, Y.; Qi, J.; Xu, M. Modulating gastrointestinal microbiota in preweaning dairy calves: Dose-dependent effects of milk-based sodium butyrate supplementation. Microorganisms 2024, 12, 333. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, B.B.; Li, J.; Shan, J.J.; Xu, J.; Zhang, C.Y.; Wei, X.T.; Zhu, R.R.; Wang, J.Y. Tricarboxylic acid cycle metabolites: New players in macrophage. Inflamm. Res. 2024, 73, 531–539. [Google Scholar] [PubMed]
- Li, G.; Huang, P.; Cui, S.S.; Tan, Y.Y.; He, Y.C.; Shen, X.; Jiang, Q.Y.; Huang, P.; He, G.Y.; Li, B.Y.; et al. Mechanisms of motor symptom improvement by long-term tai chi training in parkinson’s disease patients. Transl. Neurodegener. 2022, 11, 6. [Google Scholar]
- Khalaf, M.M.; Mahmoud, H.M.; Kandeil, M.A.; Mahmoud, H.A.; Salama, A.A. Fumaric acid protects rats from ciprofloxacin-provoked depression through modulating tlr4, nrf-2, and p190-rho gtp. Drug Chem. Toxicol. 2024, 47, 897–908. [Google Scholar]
- Reda, F.M.; Ismail, I.E.; Attia, A.I.; Fikry, A.M.; Khalifa, E.; Alagawany, M. Use of fumaric acid as a feed additive in quail’s nutrition: Its effect on growth rate, carcass, nutrient digestibility, digestive enzymes, blood metabolites, and intestinal microbiota. Poult. Sci. 2021, 100, 101493. [Google Scholar] [CrossRef]




| 6:00 | 14:00 | SEM | p-Value | |
|---|---|---|---|---|
| Temperature, °C | 20.01 | 29.77 | 1.36 | <0.0001 |
| Relative humidity, % | 87.41 | 58.08 | 13.38 | <0.0001 |
| THI | 67.43 | 79.08 | 1.74 | <0.0001 |
| L | H | SEM | p-Value | |
|---|---|---|---|---|
| Initial BW, kg | 39.70 | 38.80 | 4.42 | 0.65 |
| Final BW, kg | 100.87 | 103.37 | 2.59 | 0.56 |
| ADG, kg/d | 0.87 | 0.92 | 0.03 | 0.35 |
| L | H | SEM | p-Value | |
|---|---|---|---|---|
| 6:00 | ||||
| Respiration rate, breath/min | 56.11 | 58.51 | 2.92 | 0.568 |
| Rectal temperature, °C | 39.01 | 39.08 | 0.04 | 0.164 |
| 14:00 | ||||
| Respiration rate, breath/min | 62.68 | 79.78 | 2.90 | 0.001 |
| Rectal temperature, °C | 39.10 | 39.36 | 0.04 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhao, G.; Niu, S.; Jia, Y.; Wu, D.; Xu, M. Gastrointestinal Microorganisms and Blood Metabolites in Holstein Calves with Different Heat Stress Responses in the Same Hot Environment. Microorganisms 2025, 13, 801. https://doi.org/10.3390/microorganisms13040801
Zhang Z, Zhao G, Niu S, Jia Y, Wu D, Xu M. Gastrointestinal Microorganisms and Blood Metabolites in Holstein Calves with Different Heat Stress Responses in the Same Hot Environment. Microorganisms. 2025; 13(4):801. https://doi.org/10.3390/microorganisms13040801
Chicago/Turabian StyleZhang, Zhanhe, Guangrui Zhao, Song Niu, Yang Jia, Donglin Wu, and Ming Xu. 2025. "Gastrointestinal Microorganisms and Blood Metabolites in Holstein Calves with Different Heat Stress Responses in the Same Hot Environment" Microorganisms 13, no. 4: 801. https://doi.org/10.3390/microorganisms13040801
APA StyleZhang, Z., Zhao, G., Niu, S., Jia, Y., Wu, D., & Xu, M. (2025). Gastrointestinal Microorganisms and Blood Metabolites in Holstein Calves with Different Heat Stress Responses in the Same Hot Environment. Microorganisms, 13(4), 801. https://doi.org/10.3390/microorganisms13040801





