Pine Rhizosphere Soil Microorganisms Enhance the Growth and Resistance of Pinus massoniana Against Nematode Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Treatment
2.2. Microbial Strains
2.3. Pine Wood Nematode
2.4. Medium
2.5. Isolation and Screening of Pine Wood Nematode Fungi
2.6. Determination of the Nematicidal Activity of Fungal Fermentation Filtrates and Fungal Mycelia
2.7. Determination of Bacterial Fermentation Filtrates and Bacteriophage Nematicidal Activity
2.8. Molecular Biology Techniques for Identification Purposes
2.9. Determination of the Metabolites and Growth-Promoting Properties of Bacterial Strains
2.10. Screening of the Activity of Mixed Bacteria Against Pine Wood Nematodes
2.11. Evaluation of the Growth-Promotion Using Mixed Bacteria on P. massoniana Seedlings
2.12. Variation in Defense Enzyme Activities of P. massoniana Leaves After Different Treatments
2.13. Control of Pine Nematode Disease in P. massoniana Using Mixed Bacterial Agents
2.14. RNA Extraction and Real Time-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.15. Statistical Analysis
3. Results
3.1. Isolation and Screening of Pine Wood Nematode-Killing Fungi and Bacteria
3.1.1. Determination of Fungal Nematicidal Activity
3.1.2. Determination of Bacterial Fermentation Filtrate and Bacteriophage Nematicidal Activity
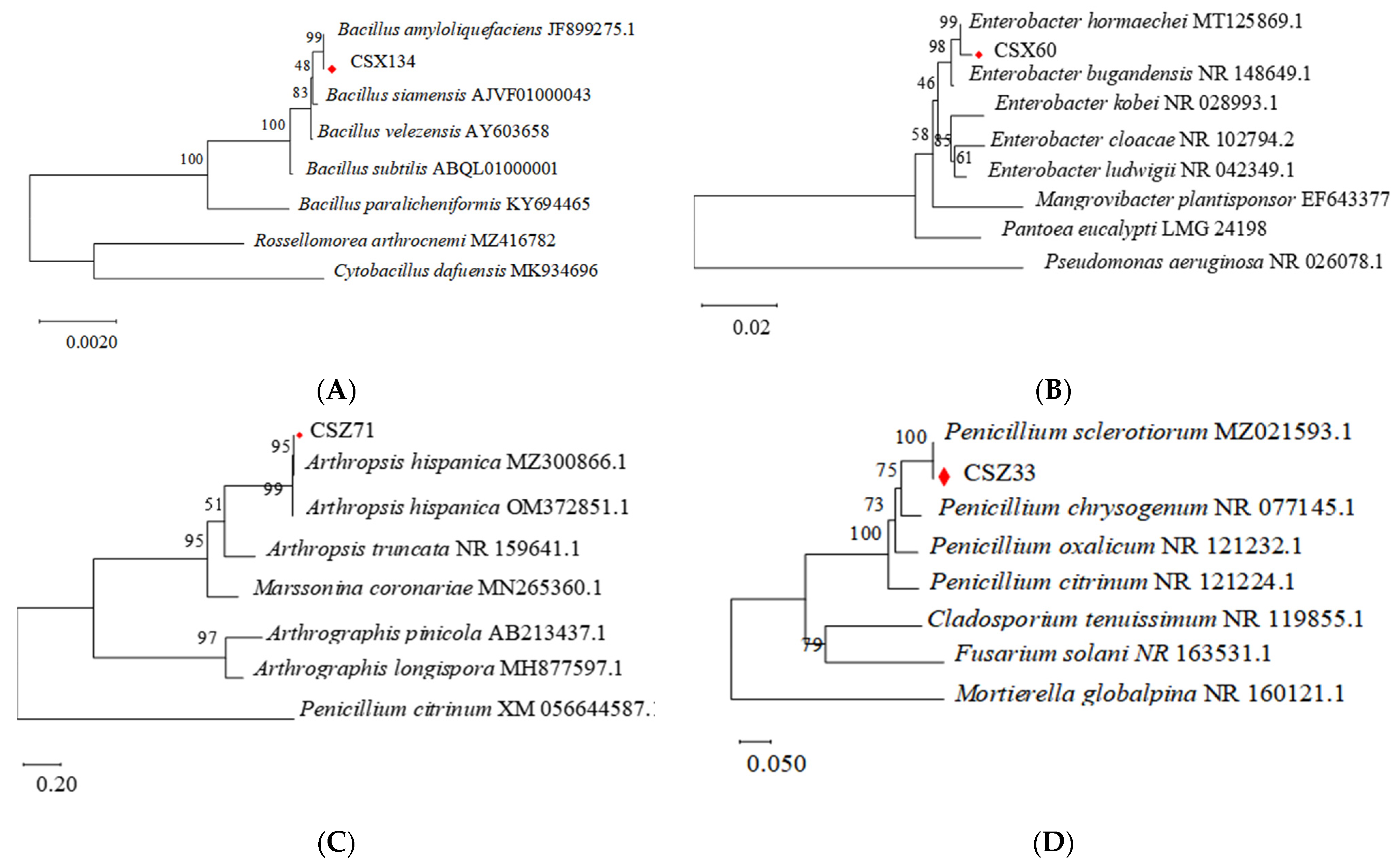
3.1.3. Molecular Biological Identification of Four Bacterial Strains
3.2. Determination of the Metabolites and Growth-Promoting Properties of Nematicidal Strains
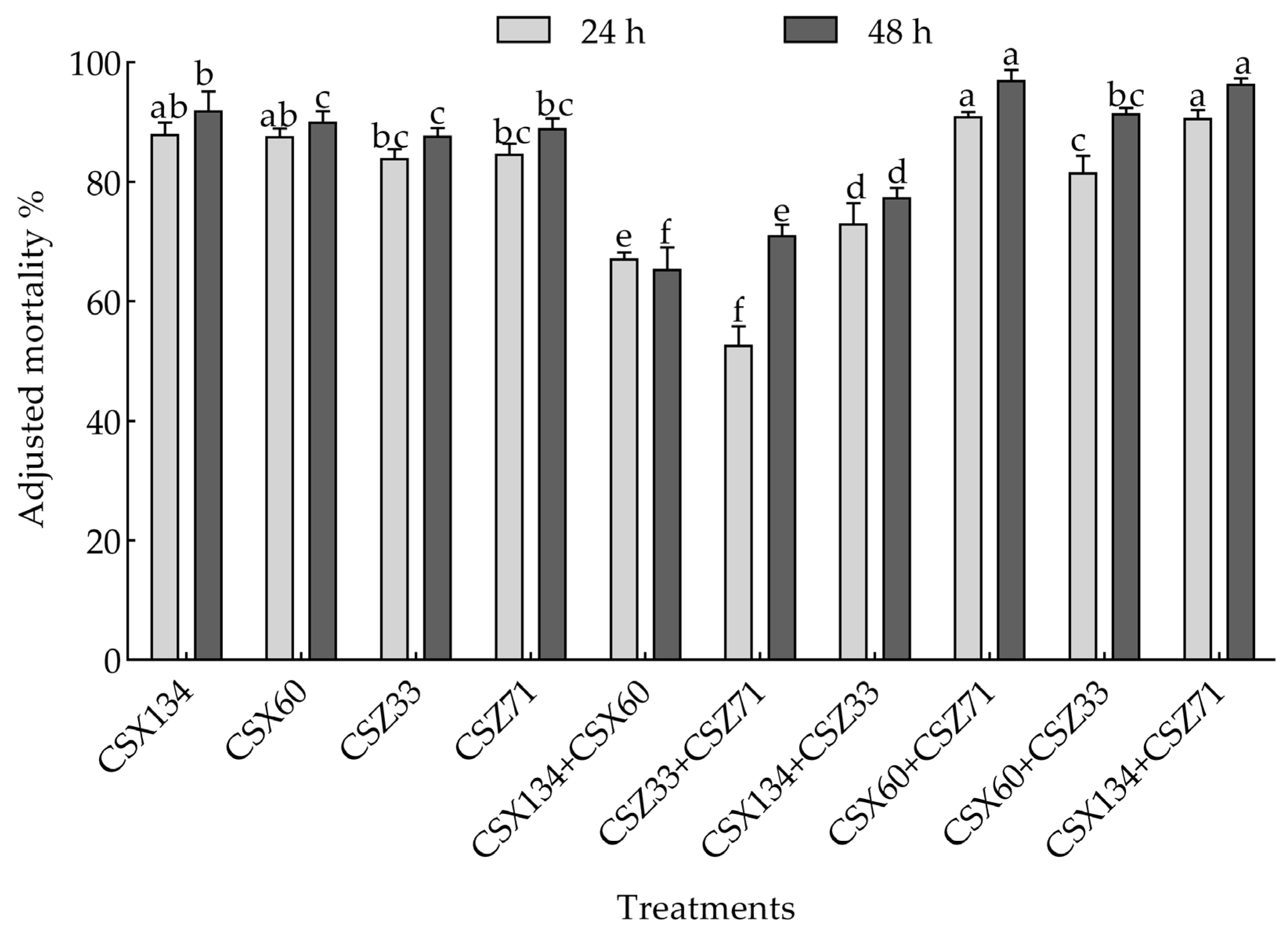
3.3. Screening of the Activity of Mixed Bacteria Against Pine Wood Nematodes
3.4. Promotion of the Growth of P. massoniana Seedlings via Mixed Bacteria
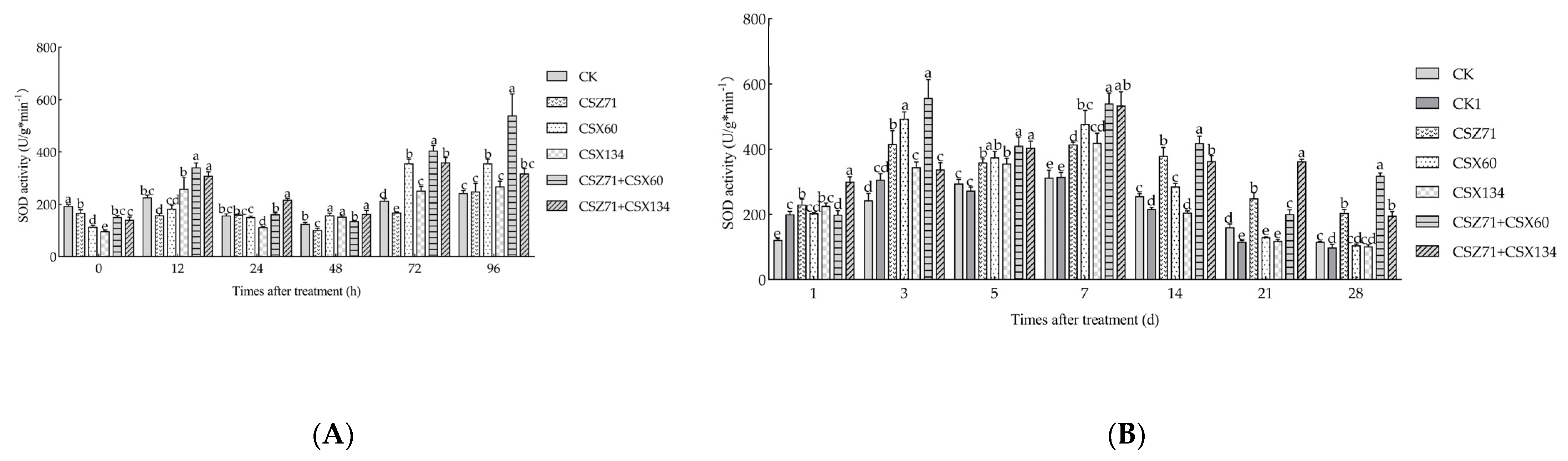
3.5. Influence of Fermentation Filtrate of Mixed Bacteria on the SOD Activity of P. massoniana
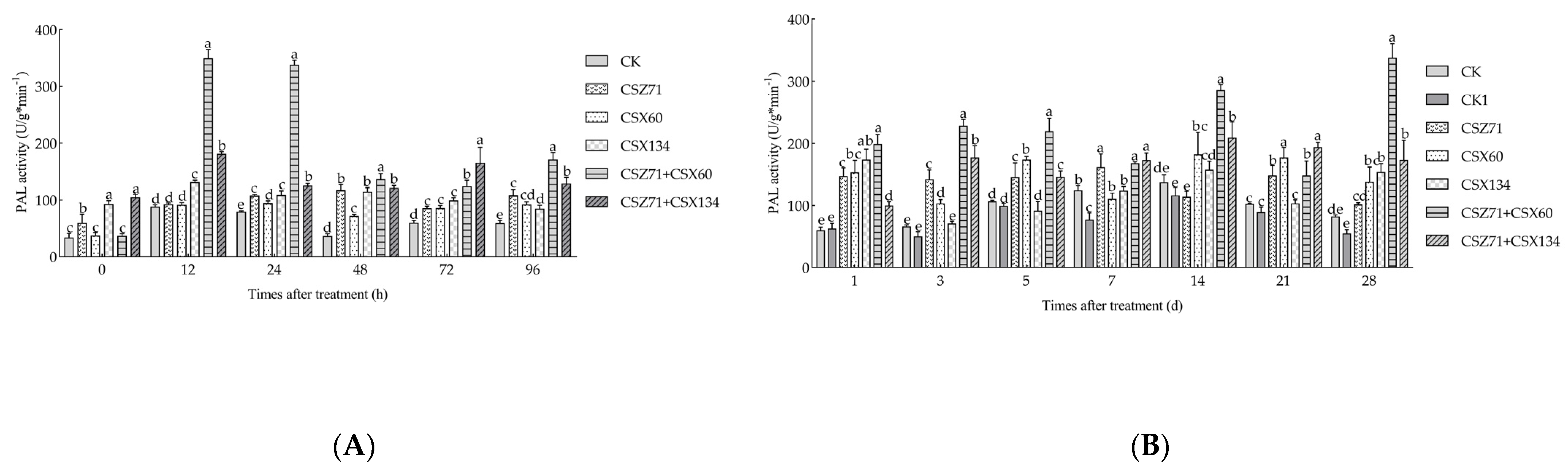
3.6. Influence of Fermentation Filtrate of Mixed Bacteria on PAL Activity of P. massoniana
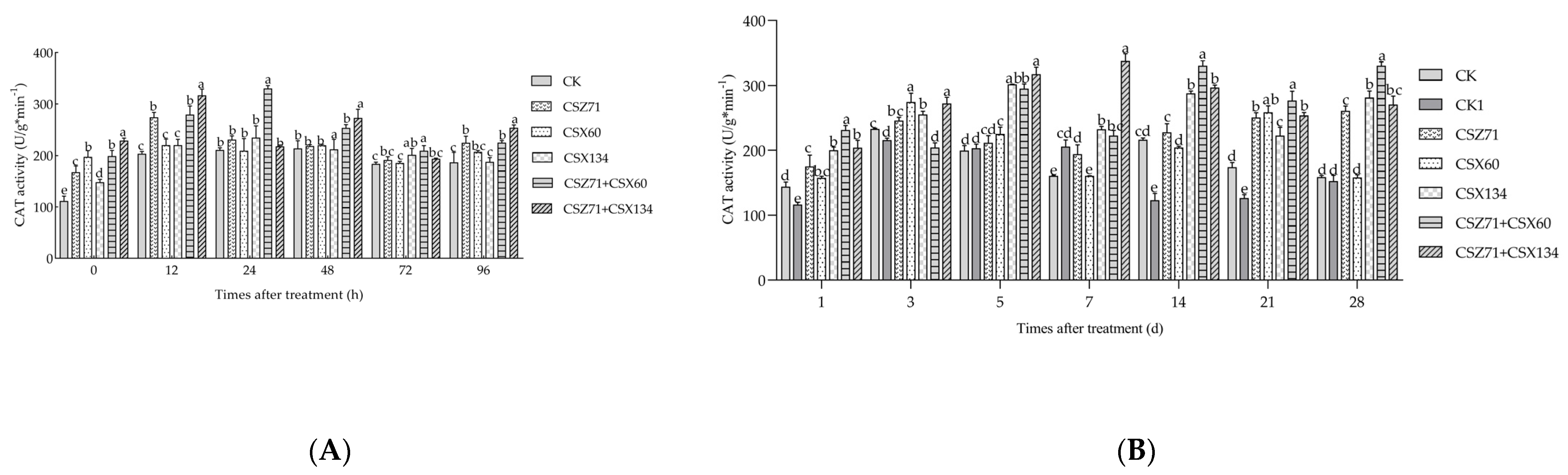
3.7. Influence of Fermentation Filtrate of Mixed Bacteria on the CAT Activity of P. massoniana
3.8. Effect of Fermentation Filtrate of Mixed Bacteria on the Control of Pine Wood Nematode Disease
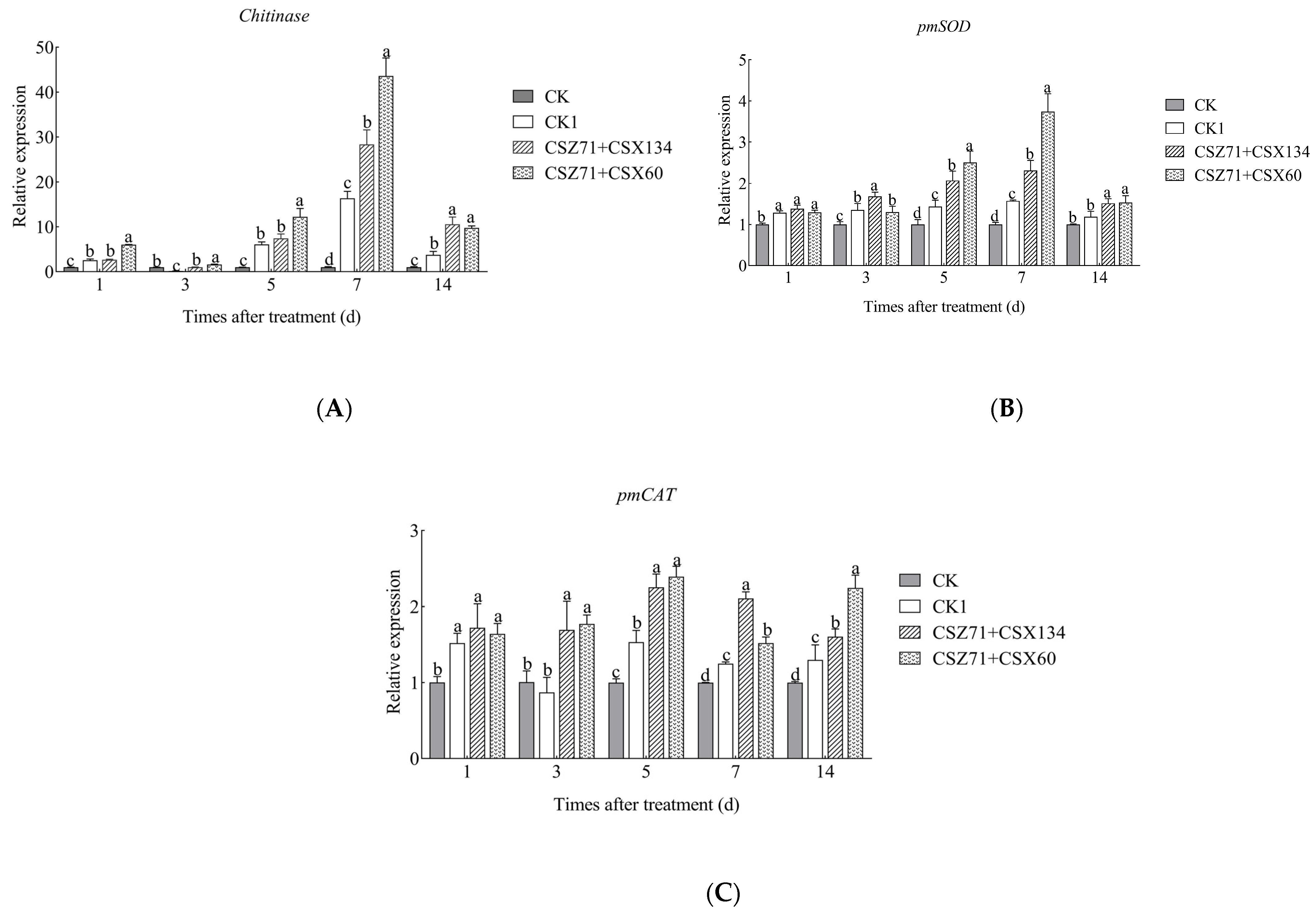
3.9. Effects of Fermentation Filtrate of Mixed Bacteria on the Expression Levels of Related Genes of P. massoniana
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nickle, W.R.; Golden, A.M.; Mamiya, Y.; Wergin, W. On the taxonomy and morphology of the pine wood nematode, Bursaphelenchus xylophilus (Steiner &Buhrer 1934) Nickle 1970. J. Nematol. 1981, 13, 385. [Google Scholar] [PubMed]
- Cheng, H.; Lin, M.; Li, W.; Fang, Z. The occurrence of a pine wilting disease caused by a nematode found in Nanjing. ResearchGate 1983, 4, 1–5. [Google Scholar]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Yi, C.K.; Byun, B.H.; Park, J.D.; Yang, S.I.; Chang, K.H. First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Res. Rep. For. Res. Inst. 1992, 141–149. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19920663349 (accessed on 15 March 2025).
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Martín, A.B. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Pan, J.L.; Yao, H.W.; Dong, Y.Q. Analysis of the epidemic situation of pine wilt disease in China in 2019. For. Pest Dis. 2021, 40, 32–37. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wu, H.; Huang, R.F.; Wang, J.; Zhang, R.X.; Song, Y.S. Separation and identification of Bursaphelenchus xylophilus from Pinus sylvestris var. mongolica in Fushun city. For. Pest Dis. 2020, 39, 6–10. [Google Scholar]
- Hu, L.J.; Wu, X.Q.; Li, H.Y.; Wang, Y.C.; Huang, X.; Wang, Y.; Li, Y. BxCDP1 from the pine wood nematode Bursaphelenchus xylophilus is recognized as a novel molecular pattern. Mol. Plant Pathol. 2020, 21, 923–935. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.; Li, J.; Ren, J.; Ren, L.; Luo, Y. Pine wilt disease in Northeast and Northwest China: A comprehensive risk review. Forests 2023, 14, 174. [Google Scholar] [CrossRef]
- Zhu, L.Z. Cloning and Analysis of PmDXR Gene in Pinus massoniana; Nanjing Forestry University: Nanjing, China, 2022. [Google Scholar]
- Kim, B.N.; Kim, J.H.; Ahn, J.Y.; Kim, S.; Cho, B.K.; Kim, Y.H.; Min, J. A short review of the pinewood nematode, Bursaphelenchus xylophilus. Toxicol. Environ. Health Sci. 2020, 12, 297–304. [Google Scholar] [CrossRef]
- Lee, S.M.; Chung, Y.J.; Moon, Y.S.; Lee, S.G.; Lee, D.W.; Choo, H.Y.; Lee, C.K. Insecticidal activity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus alternatus) larvae. J. Korean For. Soc. 2003, 92, 191–198. [Google Scholar]
- Seleim, M.; Abo--Elyousr, K.; Mohamed, A.; Saead, F. First report of potato bacterial ring rot caused by Clavibacter michiganensis subsp. sepedonicus in Africa. New Dis. Rep. 2014, 30, 15. [Google Scholar] [CrossRef]
- Shahid, M.; Hameed, S.; Tariq, M.; Zafar, M.; Ali, A.; Ahmad, N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann. Microbiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.H.; Zu, X.; Yu, X.Y.; Zhu, H.J.; Li, X.J.; Zhong, J.; Liu, E.M. Efficacy of Plant Growth-Promoting Bacteria Bacillus cereus YN917 for Biocontrol of Rice Blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Zhou, J.; Yu, H.; Zhang, C.; Lv, Y.; Sun, J. Enhancement of oxidative stress contributes to increased pathogenicity of the invasive pine wood nematode. Philos. Trans. R. Soc. B 2019, 374, 20180323. [Google Scholar] [CrossRef]
- Cao, Y.F.; Wang, L.F.; Wang, X.C.; Fan, J.H. Pathogenicity of Bursaphelenchus xylophilus to Larix olgensis Seedlings. Sci. Silv. Sin. 2022, 56, 108–115. Available online: http://www.linyekexue.net/EN/10.11707/j.1001-7488.20201111 (accessed on 15 March 2025).
- Bakker, P.A.; Berendsen, R.L.; Doornbos, R.F.; Wintermans, P.C.; Pieterse, C.M. The rhizosphere revisited: Root microbiomics. Front. Plant Sci. 2013, 4, 165. [Google Scholar] [CrossRef]
- Chemeltorit, P.P.; Mutaqin, K.H.; Widodo, W. Combining Trichoderma hamatum THSW13 and Pseudomonas aeruginosa BJ10–86: A synergistic chili pepper seed treatment for Phytophthora capsici infested soil. Eur. J. Plant Pathol. 2017, 147, 157–166. [Google Scholar] [CrossRef]
- Bunbury-Blanchette, A.L.; Walker, A.K. Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biol. Control. 2019, 130, 127–135. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Prakasam, V.; Jonathan, E.I.; Paramathma, M.; Samiyappan, R. Combining Pseudomonas, Bacillus and Trichoderma strains with organic amendments and micronutrient to enhance suppression of collar and root rot disease in physic nut. Appl. Soil Ecol. 2011, 49, 215–223. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhon, L.; Shen, D.; Zhu, L.; Fan, B. Inhibition of pine wood nematode Bursaphelenchus xylophilus by rhizobacterium Bacillus velezensis FZB42. Acta Microbiol. Sin. 2021, 61, 1287–1298. [Google Scholar] [CrossRef]
- Yang, H.L.; Sun, X.L.; Song, W. Current development on induced resistance by plant growth promoting and endophytic bacteria. Acta Phytopathol. Sin. 2000, 30, 106–110. [Google Scholar]
- Han, Z.M.; Hong, Y.D.; Zhao, B.G. A study on pathogenicity of bacteria carried by pine wood nematodes. J. Phytopathol. 2010, 151, 683–689. [Google Scholar] [CrossRef]
- Gabriel, P.; Diogo, N.P.; Romeu, F.; Paula, V.; Susana, S.S.; Luís, F.; Isabel, M.O.A.; Paula, V.M. Nematicidal Bacteria Associated to Pinewood Nematode Produce Extracellular Proteases. PLoS ONE 2013, 8, e79705. [Google Scholar] [CrossRef]
- Liu, M.J.; Hwang, B.S.; Jin, C.Z.; Li, W.J.; Park, D.J.; Seo, S.T.; Kim, C.J. Screening, isolation and evaluation of a nematicidal compound from actinomycetes against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2018, 75, 1585–1593. [Google Scholar] [CrossRef]
- Dong, J.Y.; Li, X.P.; Li, G.H.; Liu, Y.J.; Zhang, K.Q. Preliminary results on nematicidal activity from culture filtrates of Basidiomycetes against the pine wood nematode, Bursaphelenchus xylophilus (Aphelenchoididae). Ann. Microbiol. 2006, 56, 163–166. [Google Scholar] [CrossRef]
- Elamary, R.; Salem, W. Optimizing and purifying extracellular amylase from soil bacteria to inhibitclinical biofilm-forming bacteria. PeerJ 2020, 8, e10288. [Google Scholar] [CrossRef] [PubMed]
- Maehara, N.; Futai, K. Effect of fungal interactions on the numbers of the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), carried by the Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae). Fundam. Appl. Nematol. 1997, 20, 611–617. [Google Scholar] [CrossRef]
- Yu, J. Isolation and Identification of Active Components of Endophytic fungi of Melia azedarach Against Bursaphelenchus xylophilus and Its Nematicidal Mechanism; Zhejiang Sci-Tech University: HangZhou, China, 2023; p. 15. [Google Scholar]
- Li, L.; Tan, J.; Chen, F. The screening and identification of two bacterial strains with nematicidal activity against Bursaphelenchus xylophilus. J. Nanjing For. Univ. 2017, 41, 37–41. [Google Scholar] [CrossRef]
- Kakhki, A.M.; Amoozegar, M.A.; Khaledi, E.M. Diversity of hydrolytic enzymes in haloarchaeal strains isolated from salt lake. Int. J. Environ. Sci. Technol. 2021, 8, 705–714. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Ohkama-Ohtsu, N.; Sarhadi, W.A.; Kojima, K.; Rallos, R.V.; Yokoyama, T. Isolation and screening of indigenous plant growth-promoting rhizobacteria from different rice cultivars in Afghanistan soils. Microbes Environ. 2019, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Guo, T.; Lay, K.D.; Ou, K.; Cai, K.; Ding, Y.; Liu, J.; Cao, Y. Isolation of soybean-specific plant growth-promoting rhizobacteria using soybean agglutin and evaluation of their effects to improve soybean growth, yield, and soil nutritional status. Microbiol. Res. 2022, 261, 127076. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C.; Luo, Y.Q.; Zhang, Q. Changes in Water Content, Pigments and Antioxidant Enzyme Activities in Pine Needles of Pinus thunbergii and Pinus massoniana Affected by Pine Wood Nematode. Sci. Silv. Sin. 2012, 48, 140–143. [Google Scholar]
- Xie, W.F.; Li, H.M.; Huang, A.Z.; Feng, L.Z.; Zhang, F.P. The Dynamic Change of Gene Expression from Pinus massoniana in Response to Bursaphelenchus xylophius Infestation. J. Fujian Agric. For. Univ. 2017, 32, 403–409. [Google Scholar] [CrossRef]
- Xie, Y.N.; Liu, B.; Gao, K.; Zhao, Y.X.; Li, W.H.; Deng, L.L.; Zhou, Z.C.; Liu, Q.H. Comprehensive Analysis and Functional Verification of the Pinus massoniana NBS-LRR Gene Family Involved in the Resistance to Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2023, 24, 1812. [Google Scholar] [CrossRef]
- Xie, W.; Liang, G.; Zhang, F. Effect of Bursaphelenchus xylophilus infestation to the gene expression of Pinus massoniana. J. For. Environ. 2018, 38, 481–487. [Google Scholar] [CrossRef]
- Yu, C.; Rong, M.; Liu, Y.; Sun, P.; Xu, Y.; Wei, J. Genome-Wide Identification and Characterization of HSP70 Gene Family in Aquilaria sinensis (Lour.) Gilg. Genes 2021, 13, 8. [Google Scholar] [CrossRef]
- Xue Q, Wu X Q, Wu F; et al. Transcriptome analysis of Bursaphelenchus xylophilus uncovers the impact of Stenotrophomonas maltophilia on nematode and pine wilt disease. Forests 2020, 11, 908. [Google Scholar] [CrossRef]
- Deshmukh, S.; Hückelhoven, R.; Schäfer, P.; Imani, J.; Sharma, M.; Weiss, M.; Kogel, K.H. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc. Natl. Acad. Sci. USA 2006, 103, 18450–18457. [Google Scholar] [CrossRef]
- Franken, P. The plant strengthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biot. 2012, 96, 1455–1464. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Friman, V.P.; Gu, S.H.; Wang, X.F.; Eisenhauer, N.; Yang, T.J.; Ma, J.; Shen, Q.R.; Xu, Y.C.; et al. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. MBio. 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.S.; Wathelet, B.; Ongena, M.; Thonat, P.; Gancel, F.; Cholletimbeert, M.; Jacques, P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef]
- Oku, H.; Shiraishi, T.; Ouchi, S.; Kurozumi, S.; Ohta, H. Pine wilt toxin, the metabolite of a bacterium associated with a nematode. Naturwissenschaften 1980, 67, 198–199. [Google Scholar] [CrossRef]
- Le Dang, Q.; Son, S.W.; Cheon, H.M.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Pyochelin isolated from Burkholderia arboris KRICT1 carried by pine wood nematodes exhibits phytotoxicity in pine callus. Nematology 2011, 13, 521–528. [Google Scholar] [CrossRef]
- Xia, Y.D.; Liu, J.N.; Chen, C.; Mo, X.L.; Tan, Q.; He, Y.; Wang, Z.K.; Yin, J.; Zhou, G.Y. The Multifunctions and Future Prospects of Endophytes and Their Metabolites in Plant Disease Management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Mesapogu, S.; Kumar, M.; Yandigeri, M.S.; Singh, G.; Saxena, A.K. Co-inoculation of the endophytic fungus Piriformospora indica with the phosphate solubilising bacterium Pseudomonas striata affects population dynamics and plant growth in chickpea. Biol. Fertil. Soil. 2010, 46, 169–174. [Google Scholar] [CrossRef]
- Zhao, B.G.; Wang, H.L.; Han, S.F.; Han, Z.M. Distribution and pathogenicity of bacteria species carried by Bursaphelenchus xylophilus in China. Nematology 2003, 5, 899–906. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Z.; Wang, S.; Wang, H.; Chao, Y.; Sederoff, R.R.; Sederoff, H.; Yan, H.; Pan, J.; Peng, M.; et al. Gene sdaB Is Involved in the Nematocidal Activity of Enterobacter ludwigii AA4 Against the Pine Wood Nematode Bursaphelenchus xylophilus. Front. Microbiol. 2022, l13, 870519. [Google Scholar] [CrossRef]
- Han, G.; Mannaa, M.; Kim, N.; Jeon, H.W.; Jung, H.; Lee, H.H.; Kim, J.; Park, J.; Park, A.R.; Kim, J.C.; et al. Response of Pine Rhizosphere Microbiota to Foliar Treatment with Resistance-Inducing Bacteria against Pine Wilt Disease. Microorganisms 2021, 9, 688. [Google Scholar] [CrossRef]
- Bailey, B.A.; Lumsden, R.D. Direct effects of Trichoderma and Gliocladium on plant growth and. Trichoderma And Gliocladium, Volume 2: Enzymes. Biol. Control. Commer. Appl. 1998, 2, 185. [Google Scholar]
- Martinuz, A.; Schouten, A.; Menjivar, R.D.; Sikora, R.A. Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control. 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Peng, S.; Yang, R.; Yan, S.Z.; Chen, S.L. Control effect of the antinematode endophytic bacteria and rhizosphere actinomycetes to root-knot nematodes of tomato plant. Acta Phytophyl. Sin. 2012, 39, 63–69. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Yin, Y.N.; Tan, J.J.; Hao, D.J. A preliminary study on resistance of Pinus massoniana induced by Bacillus cereus NJSZ-13 strain to Bursaphelenchus xylophilus. J. Nanjing For. Univ. 2022, 46, 4. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ye, J.R.; Wei, C.J. Effect of Pine Wood Nematode (PWN) Infection on Phenylpropanes Metabolism in Masson Pine Seedlings. Sci. Silvae Sin. 2006, 12, 37. [Google Scholar]
- Wang, J.G.; Jiang, X.; Luan, Q.S.; Feng, J.; Wang, J.J.; Liu, M. Effects of Bursaphelenchus xylophilus Infestation on Physiological Indexes of Larix kaempferi. J. Southwest For. Univ. 2023, 43, 6. [Google Scholar] [CrossRef]
- Custers, J.H.; Harrison, S.J.; Sela-Buurlage, M.B.; Van Deventer, E.; Lageweg, W.; Howe, P.W.; Van Der Meijs, P.J.; Ponstein, A.S.; Simons, B.H.; Melchers, L.S.; et al. Isolation and characterisation of a class of carbohydrate oxidases from higher plants, with a role in active defence. Plant J. 2004, 39, 147–160. [Google Scholar] [CrossRef]
- Zheng, H.J.; Xu, L.L.; Xie, C.X.; Liu, Y.P.; Ye, J.R. Analysis of Resistance Genes in Pinus massoniana provenances against pine wood nematode disease. J. Anhui Agric. Univ. 2023, 50, 379–388. [Google Scholar] [CrossRef]
- Zeynadini-Riseh, A.; Mahdikhani-Moghadam, E.; Rouhani, H.; Moradi, M.; Saberi-Riseh, R.; Mohammadi, A. Effect of some probiotic bacteria as biocontrol agents of Meloidogyne incognita and evaluation of biochemical changes of plant defense enzymes on two cultivars of Pistachio. J. Agr. Sci. Tech. 2018, 20, 179–191. Available online: https://jast.modares.ac.ir/browse.php?a_id=9389&sid=23&slc_lang=en (accessed on 15 March 2025).




| Treatment | Processing Details/Pot |
|---|---|
| Treatment CK | CK 100 mL sterile water; root irrigation every 30 days. |
| Treatment 3: CSX134 | CSX134 bacteria were added to 100 mL of fermentation filtrate, and roots were irrigated every 30 days. |
| Treatment 4: CSX60 | CSX60 bacteria were added to 100 mL of fermentation filtrate, and roots were irrigated every 30 days. |
| Treatment 5: CSZ71 | Add 100 mL of fermentation filtrate of CSZ71 fungus, and irrigate the roots every 30 days. |
| Treatment 6: CSZ71+CSX134 | Add 100 mL of CSZ71+CSX134 mixed bacteria in a 1:1 mixture with fermentation filtrate, rooting every 30 days. |
| Treatment 7: CSZ71+CSX60 | Add 100 mL of CSZ71+CSX60 mixed bacteria in a 1:1 mixture with fermentation filtrate, rooting every 30 days. |
| Item | Substances | CSX134 | CSX60 | CSZ71 | CSZ33 |
|---|---|---|---|---|---|
| PGP traits | IAA | + | + | + | − |
| Hyperkalosis | − | + | − | + | |
| Organic phosphate | + | + | + | + | |
| Nitrogen fixation | + | + | + | + | |
| Extracellular enzymes | Cellulase | + | − | − | + |
| Chitinase | − | − | − | − | |
| Protease | + | + | + | + | |
| Amylase | + | − | − | + |
| Treatments | Shoot Fresh Weight (gt) | Shoot Dry Weight (g) | Root Resh Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|
| CK | 2.81 ± 0.15 d | 0.92 ± 0.10 c | 0.93 ± 0.06 c | 0.23 ± 0.01 b |
| CSZ71 | 3.89 ± 0.27 c | 1.19 ± 0.05 b | 1.14 ± 0.09 ab | 0.31 ± 0.05 b |
| CSX60 | 4.97 ± 01.7 b | 1.52 ± 0.03 a | 2.08 ± 0.16 a | 0.46 ± 0.03 a |
| CSX134 | 3.77 ± 0.10 c | 0.96 ± 0.04 bc | 1.38 ± 0.10 b | 0.30 ± 0.01 b |
| CSZ71+CSX60 | 5.89 ± 0.06 a | 1.63 ± 0.06 a | 2.28 ± 0.03 a | 0.45 ± 0.03 a |
| CSZ71+CSX134 | 5.74 ± 0.25 a | 1.47 ± 0.13 a | 2.14 ± 0.10 a | 0.55 ± 0.02 a |
| Treatments | Disease Index (%) | Biocontrol Efficacy (%) |
|---|---|---|
| CK | 0 | – |
| CK1 | 77.50 ± 3.82 a | – |
| CSX60 | 30.83 ± 1.39 bc | 60.06 ± 2.80 bc |
| CSX134 | 35.00 ± 2.89 b | 54.53 ± 5.36 d |
| CSZ71 | 36.67 ± 3.00 b | 52.77 ± 2.37 d |
| CSX134+CSZ71 | 23.33 ± 2.20 cd | 69.65 ± 4.00 ab |
| CSX60+CSZ71 | 19.17 ± 1.67 d | 75.07 ± 3.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Deng, C.; Zhang, Y.; Liu, M.; Zhou, G.; Liu, J. Pine Rhizosphere Soil Microorganisms Enhance the Growth and Resistance of Pinus massoniana Against Nematode Infection. Microorganisms 2025, 13, 790. https://doi.org/10.3390/microorganisms13040790
Zhu J, Deng C, Zhang Y, Liu M, Zhou G, Liu J. Pine Rhizosphere Soil Microorganisms Enhance the Growth and Resistance of Pinus massoniana Against Nematode Infection. Microorganisms. 2025; 13(4):790. https://doi.org/10.3390/microorganisms13040790
Chicago/Turabian StyleZhu, Jiacheng, Chenxi Deng, Yichi Zhang, Manman Liu, Guoying Zhou, and Junang Liu. 2025. "Pine Rhizosphere Soil Microorganisms Enhance the Growth and Resistance of Pinus massoniana Against Nematode Infection" Microorganisms 13, no. 4: 790. https://doi.org/10.3390/microorganisms13040790
APA StyleZhu, J., Deng, C., Zhang, Y., Liu, M., Zhou, G., & Liu, J. (2025). Pine Rhizosphere Soil Microorganisms Enhance the Growth and Resistance of Pinus massoniana Against Nematode Infection. Microorganisms, 13(4), 790. https://doi.org/10.3390/microorganisms13040790







