A Narrative Review on the Impact of Probiotic Supplementation on Muscle Development, Metabolic Regulation, and Fiber Traits Related to Meat Quality in Broiler Chickens
Abstract
1. Introduction
2. Muscle Fibers and Meat Quality
3. Characteristics of Probiotics
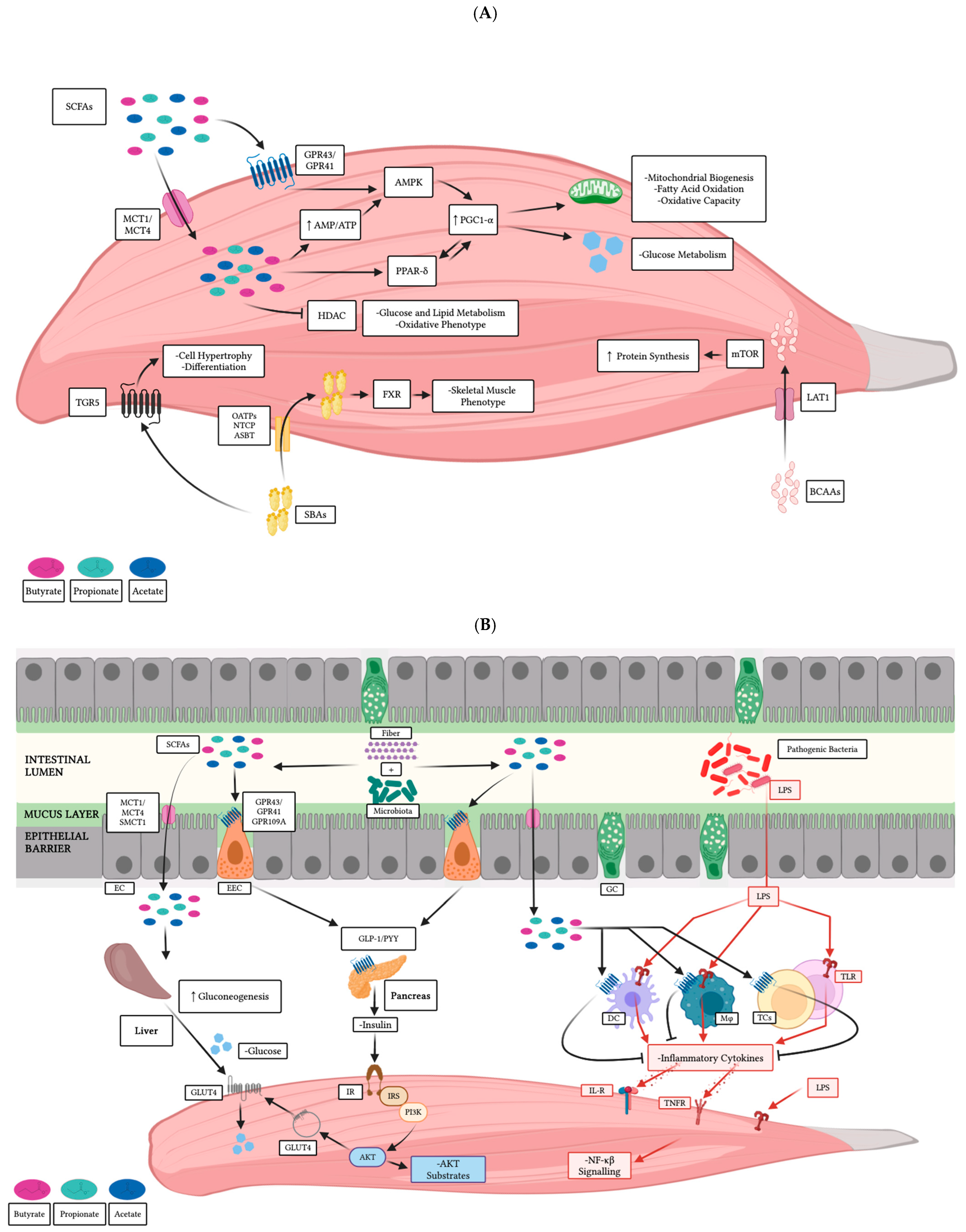
4. Gut-Derived Secondary Metabolites and Muscle Cells
4.1. Direct Effects on Skeletal Muscle
4.2. Indirect Effects on Skeletal Muscle
5. Probiotics, Growth Performance, Muscle Metabolism, and Meat Quality Parameters in Broiler Chickens
5.1. In Ovo Administration
| Broiler Breed | Probiotic Treatment | Administration | Sample | Main Results | Reference |
|---|---|---|---|---|---|
| Ross 308 | L. rhamnosus NRRL-B-442 L. paracasei DUP-13076 | In ovo spray (∼4 log CFU/egg for 18 days) | Pectoralis muscle | -↑ EW by L. rhamnosus; ↑ breast and leg weights by both probiotics -↑ myofiber and nuclei density and ↓ myofiber CSA by both probiotics -↑ expression of MYF5, MYOD, MYOG, and MRF4 by L. rhamnosus; -↑ expression of IGF1, IGF1R, FGF2 and FGF4 by both probiotics | [187] |
| Cobb-500 | E. faecium AL41 (CCM 8558) | Orally (1.0 × 109 CFU/0.2 mL of PBS for 7 days) | Pectoralis muscle | -↑ pectoralis major weight and ↑ BWG -↑ total RNA content; no differences in total DNA and total protein content; -↑ transcription levels of IGF1 and PAX7; ↓ transcription levels of MYF5 -↑ myonuclei number and fiber CSA; ↑ capillary area and capillary density | [188] |
| Cobb-500 | E. faecium AL41 (CCM 8558) | Orally (1.0 × 109 CFU/0.2 mL of PBS for 7 days; alone or in combination with S. Enteritidis PT4 1.0 × 108 CFU/0.2 mL PBS on day 4) | Pectoralis muscle | -↓ fiber CSA along with ↓ nuclei number and ↓ capillary area caused by S. Enteritidis colonization; effects alleviated by the probiotic -↑ CK activity in the presence of S. Enteritidis; effects lessened by the probiotic | [189] |
| Arbor Acres | L. plantarum L. salivarius Ligilactobacillus ingluviei (unspecified strains) | Gavage (108 CFU/mL each alone or in combination (1:1:1) at 1.0 mL/day from 22 to 42 d of age) | Pectoralis muscle | -↑ BW by L. ingluviei -↑ myofiber diameter and myofiber density by L. plantarum and L. ingluviei -↑ relative expression of MyHC SM, MyHC FRM, PFK, PDH, IDH, SDH, and Tfam genes by all probiotics; ↑ relative expression of CoxVa, PK, and myoglobin genes by L. plantarum and L. ingluviei | [190] |
| Ross 308 | L. acidophilus ATCC 20552 | Dietary (3 × 108 CFU/mL and 0.1 g/kg feed from 15 to 37 days of age) | Breast muscle | -↑ BW and breast muscle weight; ↓ FCR -↑ mRNA expression of IGF1, IGF1R and GHSR | [191] |
| Cobb-500 | L. plantarum P8 | Dietary (1 × 108 CFU/g alone or in combination with 200 μL/day DEX injection 3 mg/kg BW from d 16 to d 21) | Breast muscle | -oxidative stress negatively affected levels of MDA, SOD, GPx, Keap1, Nrf2, mtDNA copy numbers, PGC-1α, SIRT1, SIRT3, MFF, Mfn1, and OPA1; basal levels restored by the probiotic; oxidative stress caused ↓ expression of ATG5, Becline-1, Parkin, PINK1, LC3II/I, LC3B and ↑ levels of NLRP3, IL-18, Caspase-1; basal levels restored by the probiotic | [192] |
| Arbor Acres | B. subtilis DSM32324-32325 | Dietary (3.2 × 109 CFU/g at low-dose 300 mg/kg and high-dose 500 mg/kg for 35 d) | Breast and Thigh muscle | -↑ pH45min and pH24h post-mortem in breast and thigh muscle; ↑ redness and -↓ luminance, yellowness, drip loss, cooking loss, and meat shear force -↑ percentage of type I fibers and ↓ percentage of type II fibers in thigh muscle -↑ mRNA levels of MyHC I in breast and thigh muscle and ↓ mRNA levels of MyHC IIb in breast muscle; no differences in MyHC IIa; ↑ slow MyHC protein expression and -↓ fast MyHC protein expression in thigh muscle; ↑ relative mRNA and protein levels of AMPK, SIRT1, PGC-1α, CAT, SOD, GPx, Nrf2 and HO-1 in breast and thigh muscle; -↑ activity of CAT and GPx; ↑ T-AOC and ↓ MDA concentration | [193] |
| Three-yellow chickens | 1 EM | Dietary (0.5% EM of 2 × 108 CFU/kg + 3 × 107 CFU/kg alone or in combination with 0.2 mg/kg sodium selenite (S-Se) or 0.2 mg/kg selenium yeast (Y-Se) for 70 d) | Breast and Thigh muscle | -no differences in BW and FCR -↑ pH and muscle color at higher concentrations -negative correlation between shear force and ↓ muscle fiber perimeter, diameter, and CSA; negative correlation between ↑ muscle fiber density and drip loss and meat lightness -↑ mRNA expression of MYF5, MYOG and MRF4 in breast and thigh muscle; ↑ mRNA expression of MEF2A and MEF2D | [194] |
5.2. Orally and Dietary Administration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, K.F.; Gephart, J.A.; Emery, K.A.; Leach, A.M.; Galloway, J.N.; D’Odorico, P. Meeting Future Food Demand with Current Agricultural Resources. Glob. Environ. Change 2016, 39, 125–132. [Google Scholar] [CrossRef]
- Kumar, P.; Abubakar, A.A.; Verma, A.K.; Umaraw, P.; Adewale Ahmed, M.; Mehta, N.; Sazili, A.Q. New Insights in Improving Sustainability in Meat Production: Opportunities and Challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 11830–11858. [Google Scholar] [PubMed]
- Warner, R.D. The Eating Quality of Meat: IV—Water Holding Capacity and Juiciness. In Lawrie’s Meat Science, 9th ed.; Woodhead Publishing: Cambridge, UK, 2023; pp. 457–508. [Google Scholar]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 182746. [Google Scholar]
- Choi, Y.M.; Kim, B.C. Muscle Fiber Characteristics, Myofibrillar Protein Isoforms, and Meat Quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar]
- Aalhus, J.L.; Robertson, W.M.; Ye, J. Muscle Fiber Characteristics and Their Relation to Meat Quality. Appl. Muscle Biol. Meat Sci. 2009, 97, 97–114. [Google Scholar]
- Lefaucheur, L. A Second Look into Fibre Typing–Relation to Meat Quality. Meat Sci. 2010, 84, 257–270. [Google Scholar]
- Kim, Y.H.B.; Warner, R.D.; Rosenvold, K. Influence of High Pre-Rigor Temperature and Fast pH Fall on Muscle Proteins and Meat Quality: A Review. Anim. Prod. Sci. 2014, 54, 375–395. [Google Scholar]
- Chriki, S.; Renand, G.; Picard, B.; Micol, D.; Journaux, L.; Hocquette, J.-F. Meta-Analysis of the Relationships between Beef Tenderness and Muscle Characteristics. Livest. Sci. 2013, 155, 424–434. [Google Scholar]
- Berti, F.; Nogueira, J.M.; Wöhrle, S.; Sobreira, D.R.; Hawrot, K.; Dietrich, S. Time Course and Side-by-Side Analysis of Mesodermal, Pre-Myogenic, Myogenic and Differentiated Cell Markers in the Chicken Model for Skeletal Muscle Formation. J. Anat. 2015, 227, 361–382. [Google Scholar] [CrossRef]
- Liu, J.; Fu, R.; Liu, R.; Zhao, G.; Zheng, M.; Cui, H.; Li, Q.; Song, J.; Wang, J.; Wen, J. Protein profiles for muscle development and intramuscular fat accumulation at different post-hatching ages in chickens. PLoS ONE 2016, 11, e0159722. [Google Scholar]
- Velleman, S.G.; McFarland, D.C. Skeletal Muscle. In Sturkie’s Avian Physiology, 6th ed.; Academic Press: San Diego, CA, USA, 2015; pp. 379–402. [Google Scholar]
- Gu, S.; Gao, J.; Li, Z.; Zhang, S.; Wen, C.; Sun, C.; Yan, W.; Hou, Z.; Yang, N.; Li, J. Comparative Analysis of Myofiber Characteristics, Shear Force, and Amino Acid Contents in Slow- and Fast-Growing Broilers. Foods 2024, 13, 3997. [Google Scholar] [CrossRef]
- Clark, D.; Harding, R. Myogenesis, Muscle Growth and Structure. In Poultry Quality Evaluation, 1st ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 29–49. [Google Scholar]
- Day, K.; Paterson, B.; Yablonka-Reuveni, Z. A Distinct Profile of Myogenic Regulatory Factor Detection within Pax7+ Cells at S Phase Supports a Unique Role of Myf5 during Posthatch Chicken Myogenesis. Dev. Dyn. 2009, 238, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, S.; Gilbert, E.R.; Siegel, P.B.; Zhu, Q.; Wong, E.A. Expression profiles of muscle genes in postnatal skeletal muscle in lines of chickens divergently selected for high and low body weight. Poult. Sci. 2014, 93, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Beermann, M.L.; Ardelt, M.; Girgenrath, M.; Miller, J.B. Prdm1 (Blimp-1) and the expression of fast and slow myosin heavy chain isoforms during avian myogenesis in vitro. PLoS ONE 2010, 5, e9951. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Zhang, Z.; Wang, X.; Shen, W.; Zhang, L.; Lin, S. Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry. Front. Vet. Sci. 2023, 10, 1284551. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Lee, B.; Kim, D.-H.; Lee, J.; Cressman, M.D.; Choi, Y.M.; Lee, K. Greater numbers and sizes of muscle bundles in the breast and leg muscles of broilers compared to layer chickens. Front. Physiol. 2023, 14, 1285938. [Google Scholar] [CrossRef]
- Rudar, M.; Fiorotto, M.L.; Davis, T.A. Regulation of muscle growth in early postnatal life in a swine model. Annu. Rev. Anim. Biosci. 2019, 7, 309–335. [Google Scholar] [CrossRef]
- Wu, C.; Lyu, W.; Hong, Q.; Zhang, X.; Yang, H.; Xiao, Y. Gut microbiota influence lipid metabolism of skeletal muscle in pigs. Front. Nutr. 2021, 8, 675445. [Google Scholar] [CrossRef]
- Han, Q.; Huang, X.; Yan, F.; Yin, J.; Xiao, Y. The role of gut microbiota in the skeletal muscle development and fat deposition in pigs. Antibiotics 2022, 11, 793. [Google Scholar] [CrossRef]
- Wen, C.; Gou, Q.; Gu, S.; Huang, Q.; Sun, C.; Zheng, J.; Yang, N. The cecal ecosystem is a great contributor to intramuscular fat deposition in broilers. Poult. Sci. 2023, 102, 102568. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Wang, Q.; Gu, S.; Jin, J.; Yang, N. Emerging perspectives in the gut–muscle axis: The gut microbiota and its metabolites as important modulators of meat quality. Microb. Biotechnol. 2024, 17, e14361. [Google Scholar]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, M.; Zhou, S.; Zhu, X.; Zhang, X.; Xu, Q. Trans-species fecal transplant revealed the role of the gut microbiome as a contributor to energy metabolism and development of skeletal muscle. Metabolites 2022, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cui, Y.; Ali, Q.; Zhu, X.; Li, D.; Ma, S.; Shi, Y. Gut Microbiota Modulate Rabbit Meat Quality in Response to Dietary Fiber. Front. Nutr. 2022, 9, 849429. [Google Scholar]
- Lan, R.X.; Lee, S.I.; Kim, I.H. Effects of Enterococcus faecium SLB 120 on growth performance, blood parameters, relative organ weight, breast muscle meat quality, excreta microbiota shedding, and noxious gas emission in broilers. Poult. Sci. 2017, 96, 3246–3253. [Google Scholar]
- Racines, M.P.; Solis, M.N.; Šefcová, M.A.; Herich, R.; Larrea-Álvarez, M.; Revajová, V. An overview of the use and applications of Limosilactobacillus fermentum in broiler chickens. Microorganisms 2023, 11, 1944. [Google Scholar]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 6, 14. [Google Scholar]
- Valeriano, V.D.V.; Balolong, M.P.; Kang, D.-K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar]
- Du, W.; Wang, X.; Hu, M.; Hou, J.; Du, Y.; Si, W.; Yang, L.; Xu, L.; Xu, Q. Modulating gastrointestinal microbiota to alleviate diarrhea in calves. Front. Microbiol. 2023, 14, 1181545. [Google Scholar] [PubMed]
- Wang, L.; Sun, H.; Gao, H.; Xia, Y.; Zan, L.; Zhao, C. A meta-analysis on the effects of probiotics on the performance of pre-weaning dairy calves. J. Anim. Sci. Biotechnol. 2023, 14, 3. [Google Scholar]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut Microbes and Muscle Function: Can Probiotics Make Our Muscles Stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar]
- Nakajima, A.; Nakatani, A.; Hasegawa, S.; Irie, J.; Ozawa, K.; Tsujimoto, G.; Suganami, T.; Itoh, H.; Kimura, I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE 2017, 12, e0179696. [Google Scholar]
- Tan, J.K.; McKenzie, C.; Mariño, E.; Macia, L.; Mackay, C.R. Metabolite-sensing G protein–coupled receptors—Facilitators of diet-related immune regulation. Annu. Rev. Immunol. 2017, 35, 371–402. [Google Scholar]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut–Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar]
- Pardella, E.; Ippolito, L.; Giannoni, E.; Chiarugi, P. Nutritional and metabolic signalling through GPCRs. FEBS Lett. 2022, 596, 2364–2381. [Google Scholar]
- Puhl III, H.L.; Won, Y.-J.; Lu, V.B.; Ikeda, S.R. Human GPR42 is a transcribed multisite variant that exhibits copy number polymorphism and is functional when heterologously expressed. Sci. Rep. 2015, 5, 12880. [Google Scholar]
- Maßberg, D.; Hatt, H. Human olfactory receptors: Novel cellular functions outside of the nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [PubMed]
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate histone deacetylase inhibitors. BioRes. Open Access 2012, 1, 192–198. [Google Scholar] [PubMed]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front. Immunol. 2020, 11, 546173. [Google Scholar]
- Thomas, S.P.; Denu, J.M. Short-chain fatty acids activate acetyltransferase p300. Elife 2021, 10, e72171. [Google Scholar]
- Perry, R.J.; Borders, C.B.; Cline, G.W.; Zhang, X.-M.; Alves, T.C.; Petersen, K.F.; Rothman, D.L.; Kibbey, R.G.; Shulman, G.I. Propionate increases hepatic pyruvate cycling and anaplerosis and alters mitochondrial metabolism. J. Biol. Chem. 2016, 291, 12161–12170. [Google Scholar]
- Geng, T.; Li, P.; Okutsu, M.; Yin, X.; Kwek, J.; Zhang, M.; Yan, Z. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am. J. Physiol.-Cell Physiol. 2010, 298, C572–C579. [Google Scholar]
- Chai, W.; Dong, Z.; Wang, N.; Wang, W.; Tao, L.; Cao, W.; Liu, Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide–dependent mechanism. Diabetes 2012, 61, 888–896. [Google Scholar]
- Gérard, P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef]
- Mancin, L.; Wu, G.D.; Paoli, A. Gut microbiota–bile acid–skeletal muscle axis. Trends Microbiol. 2023, 31, 254–269. [Google Scholar]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched chain amino acids: Beyond nutrition metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, X. Dietary amino acids and the gut–microbiome–immune axis: Physiological metabolism and therapeutic prospects. Compreh. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar]
- Gorissen, S.H.M.; Phillips, S.M. Branched-chain amino acids (leucine, isoleucine, and valine) and skeletal muscle. In Nutrition and Skeletal Muscle, 1st ed.; Academic Press: London, UK, 2019; pp. 283–298. [Google Scholar]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [PubMed]
- Kaur, H.; Ali, S.A. Probiotics and gut microbiota: Mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar]
- Sharma, B.; Dabur, R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: A systematic review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE 2012, 7, e43928. [Google Scholar]
- Sgibnev, A.; Kremleva, E. Influence of Hydrogen Peroxide, Lactic Acid, and Surfactants from Vaginal Lactobacilli on the Antibiotic Sensitivity of Opportunistic Bacteria. Probiotics Antimicrob. Proteins. 2017, 9, 131–141. [Google Scholar]
- Woo, J.; Ahn, J. Probiotic-Mediated Competition, Exclusion and Displacement in Biofilm Formation by Food-borne Pathogens. Lett. Appl. Microbiol. 2013, 56, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Gueimonde, M.; Salminen, S. Probiotics in Adhesion of Pathogens: Mechanisms of Action. In Bioactive Foods in Promoting Health, 1st ed.; Academic Press: San Diego, CA, USA, 2010; pp. 353–370. [Google Scholar]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging Molecular Insights into the Interaction between Probiotics and the Host Intestinal Mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef]
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Magalhaes, J.G.; Tattoli, I.; Girardin, S.E. The Intestinal Epithelial Barrier: How to Distinguish Between the Microbial Flora and Pathogens. In Seminars in Immunology; Academic Press: London, UK, 2007; Volume 19, pp. 106–115. [Google Scholar]
- Fesseha, H.; Demlie, T.; Mathewos, M.; Eshetu, E. Effect of Lactobacillus Species Probiotics on Growth Performance of Dual-Purpose Chicken. Vet. Med. Res. Rep. 2021, 75, 75–83. [Google Scholar] [CrossRef]
- Cengiz, Ö.; Köksal, B.H.; Tatlı, O.; Sevim, Ö.; Ahsan, U.; Üner, A.G.; Ulutaş, P.A.; Beyaz, D.; Büyükyörük, S.; Yakan, A.; et al. Effect of Dietary Probiotic and High Stocking Density on the Performance, Carcass Yield, Gut Microflora, and Stress Indicators of Broilers. Poult. Sci. 2015, 94, 2395–2403. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, M.; Tu, Y.; Zhang, N.; Deng, K.; Ma, T.; Diao, Q. Effect of Oral Administration of Probiotics on Growth Performance, Apparent Nutrient Digestibility and Stress-Related Indicators in Holstein Calves. J. Anim. Physiol. Anim. Nutr. 2016, 100, 33–38. [Google Scholar] [CrossRef]
- Kelsey, A.J.; Colpoys, J.D. Effects of Dietary Probiotics on Beef Cattle Performance and Stress. J. Vet. Behav. 2018, 27, 8–14. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, N.; Zhou, M.; Tu, Y.; Deng, K.; Diao, Q. Effects of Dietary Probiotics on Growth Performance, Faecal Microbiota and Serum Profiles in Weaned Piglets. Anim. Prod. Sci. 2014, 54, 616–621. [Google Scholar] [CrossRef]
- Liu, T.; Bai, Y.; Wang, C.; Zhang, T.; Su, R.; Wang, B.; Duan, Y.; Sun, L.; Jin, Y.; Su, L. Effects of Probiotics Supplementation on the Intestinal Metabolites, Muscle Fiber Properties, and Meat Quality of Sunit Lamb. Animals 2023, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, Y.; Chen, Y.; Yang, N.; Wang, X.-J.; Zhu, D. Systematic Identification of Genes Involved in Divergent Skeletal Muscle Growth Rates of Broiler and Layer Chickens. BMC Genom. 2009, 10, 87. [Google Scholar]
- Petracci, M.; Cavani, C. Muscle Growth and Poultry Meat Quality Issues. Nutrients 2011, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of Broiler Chicken Meat Quality and Factors Affecting Them: A Review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar]
- Geiger, A.E.; Daughtry, M.R.; Gow, C.M.; Siegel, P.B.; Shi, H.; Gerrard, D.E. Long-Term Selection of Chickens for Body Weight Alters Muscle Satellite Cell Behaviors. Poult. Sci. 2018, 97, 2557–2567. [Google Scholar]
- Jankowski, M.; Mozdziak, P.; Petitte, J.; Kulus, M.; Kempisty, B. Avian Satellite Cell Plasticity. Animals 2020, 10, 1322. [Google Scholar] [CrossRef]
- Zanou, N.; Gailly, P. Skeletal Muscle Hypertrophy and Regeneration: Interplay between the Myogenic Regulatory Factors (MRFs) and Insulin-Like Growth Factors (IGFs) Pathways. Cell. Mol. Life Sci. 2013, 70, 4117–4130. [Google Scholar] [CrossRef]
- Buckingham, M. Gene Regulatory Networks and Cell Lineages That Underlie the Formation of Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2017, 114, 5830–5837. [Google Scholar] [CrossRef]
- Sakakibara, I.; Wurmser, M.; Dos Santos, M.; Santolini, M.; Ducommun, S.; Davaze, R.; Guernec, A.; Sakamoto, K.; Maire, P. Six1 Homeoprotein Drives Myofiber Type IIA Specialization in Soleus Muscle. Skelet. Muscle 2016, 6, 30. [Google Scholar]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Teixeira, A.; Rodrigues, S. Consumer Perceptions towards Healthier Meat Products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar]
- Maltin, C.; Balcerzak, D.; Tilley, R.; Delday, M. Determinants of Meat Quality: Tenderness. Proc. Nutr. Soc. 2003, 62, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.; Huo, W.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Fiber Characteristics and Meat Quality of Different Muscular Tissues from Slow- and Fast-Growing Broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [PubMed]
- Kim, G.D.; Jeong, J.Y.; Jung, E.Y.; Yang, H.S.; Lim, H.T.; Joo, S.T. The Influence of Fiber Size Distribution of Type IIB on Carcass Traits and Meat Quality in Pigs. Meat Sci. 2013, 94, 267–273. [Google Scholar]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.-H.; Amjad, Z. Ruminant Meat Flavor Influenced by Different Factors with Special Reference to Fatty Acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of Fresh Meat Quality through Manipulation of Muscle Fiber Characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar]
- Yan, H.; Diao, H.; Xiao, Y.; Li, W.; Yu, B.; He, J.; Chen, D. Gut Microbiota Can Transfer Fiber Characteristics and Lipid Metabolic Profiles of Skeletal Muscle from Pigs to Germ-Free Mice. Sci. Rep. 2016, 6, 31786. [Google Scholar]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar]
- Chen, X.; Guo, Y.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Arginine Promotes Skeletal Muscle Fiber Type Transformation from Fast-Twitch to Slow-Twitch via Sirt1/AMPK Pathway. J. Nutr. Biochem. 2018, 61, 155–162. [Google Scholar]
- Lamon, S.; Russell, A.P. The Role and Regulation of PGC-1α and PGC-1β in Skeletal Muscle Adaptation. In The Plasticity of Skeletal Muscle; Sakuma, K., Ed.; Springer: Singapore, 2017; pp. 175–202. [Google Scholar]
- Kong, S.; Cai, B.; Nie, Q. PGC-1α Affects Skeletal Muscle and Adipose Tissue Development by Regulating Mitochondrial Biogenesis. Mol. Genet. Genom. 2022, 297, 621–633. [Google Scholar]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1α-Mediated Adaptations in Skeletal Muscle. Pflügers Arch.-Eur. J. Physiol. 2010, 460, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, J.; He, J.; Zheng, P.; Yu, J. Leucine Promotes Differentiation of Porcine Myoblasts through the Protein Kinase B (Akt)/Forkhead Box O1 Signalling Pathway. Br. J. Nutr. 2018, 119, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Men, X.-M.; Deng, B.; Tao, X.; Qi, K.-K.; Xu, Z.-W. Wnt Gene Expression in Adult Porcine Longissimus Dorsi and Its Association with Muscle Fiber Type, Energy Metabolism, and Meat Quality. J. Integr. Agric. 2017, 16, 144–150. [Google Scholar] [CrossRef]
- Elia, D.; Madhala, D.; Ardon, E.; Reshef, R.; Halevy, O. Sonic Hedgehog Promotes Proliferation and Differentiation of Adult Muscle Cells: Involvement of MAPK/ERK and PI3K/Akt Pathways. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1438–1446. [Google Scholar] [CrossRef]
- Qiu, K.; Xu, D.; Wang, L.; Zhang, X.; Jiao, N.; Gong, L.; Yin, J. Association Analysis of Single-Cell RNA Sequencing and Proteomics Reveals a Vital Role of Ca2+ Signaling in the Determination of Skeletal Muscle Development Potential. Cells 2020, 9, 1045. [Google Scholar] [CrossRef]
- Mallinson, J.; Meissner, J.; Chang, K.-C. Calcineurin Signaling and the Slow Oxidative Skeletal Muscle Fiber Type. Int. Rev. Cell Mol. Biol. 2009, 277, 67–101. [Google Scholar]
- Witwicka, H.; Nogami, J.; Syed, S.A.; Maehara, K.; Padilla-Benavides, T.; Ohkawa, Y.; Imbalzano, A.N. Calcineurin Broadly Regulates the Initiation of Skeletal Muscle-Specific Gene Expression by Binding Target Promoters and Facilitating the Interaction of the SWI/SNF Chromatin Remodeling Enzyme. Mol. Cell. Biol. 2019, 39, e00063-19. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Toda, K.; Yamauchi, Y.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Yoshimoto, S.; Xiao, J.-z. Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis. Nutrients 2020, 12, 219. [Google Scholar] [CrossRef]
- Chen, L.-H.; Huang, S.-Y.; Huang, K.-C.; Hsu, C.-C.; Yang, K.-C.; Li, L.-A.; Chan, C.-H.; Huang, H.-Y. Lactobacillus paracasei PS23 Decelerated Age-Related Muscle Loss by Ensuring Mitochondrial Function in SAMP8 Mice. Aging 2019, 11, 756. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, 1900603. [Google Scholar]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial Bacteria Inhibit Cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [PubMed]
- An, J.M.; Kang, E.A.; Han, Y.M.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Kim, D.H.; Hahm, K.B. Dietary Intake of Probiotic Kimchi Ameliorated IL-6-Driven Cancer Cachexia. J. Clin. Biochem. Nutr. 2019, 65, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 91530. [Google Scholar]
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lü, X. Current Status and Potentiality of Class II Bacteriocins from Lactic Acid Bacteria: Structure, Mode of Action and Applications in the Food Industry. Trends Food Sci. Technol. 2022, 120, 387–401. [Google Scholar]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of Global Trends in Classification, Methods of Preparation and Application of Bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef]
- Petrova, M.I.; Lievens, E.; Verhoeven, T.L.A.; Macklaim, J.M.; Gloor, G.; Schols, D.; Vanderleyden, J.; Reid, G.; Lebeer, S. The Lectin-Like Protein 1 in Lactobacillus rhamnosus GR-1 Mediates Tissue-Specific Adherence to Vaginal Epithelium and Inhibits Urogenital Pathogens. Sci. Rep. 2016, 6, 37437. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion Mechanisms Mediated by Probiotics and Prebiotics and Their Potential Impact on Human Health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar]
- MacKenzie, D.A.; Jeffers, F.; Parker, M.L.; Vibert-Vallet, A.; Bongaerts, R.J.; Roos, S.; Walter, J.; Juge, N. Strain-Specific Diversity of Mucus-Binding Proteins in the Adhesion and Aggregation Properties of Lactobacillus reuteri. Microbiology 2010, 156, 3368–3378. [Google Scholar]
- Holst, B.; Glenting, J.; Holmstrøm, K.; Israelsen, H.; Vrang, A.; Antonsson, M.; Ahrné, S.; Madsen, S.M. Molecular Switch Controlling Expression of the Mannose-Specific Adhesin, Msa, in Lactobacillus plantarum. Appl. Environ. Microbiol. 2019, 85, e02954-18. [Google Scholar]
- Lu, Y.; Han, S.; Zhang, S.; Wang, K.; Lv, L.; McClements, D.J.; Xiao, H.; Berglund, B.; Yao, M.; Li, L. The Role of Probiotic Exopolysaccharides in Adhesion to Mucin in Different Gastrointestinal Conditions. Curr. Res. Food Sci. 2022, 5, 581–589. [Google Scholar] [PubMed]
- Bönisch, E.; Oh, Y.J.; Anzengruber, J.; Hager, F.F.; López-Guzmán, A.; Zayni, S.; Hinterdorfer, P.; Kosma, P.; Messner, P.; Duda, K.A.; et al. Lipoteichoic Acid Mediates Binding of a Lactobacillus S-Layer Protein. Glycobiology 2018, 28, 148–158. [Google Scholar] [PubMed]
- Alp, D.; Kuleaşan, H. Determination of competition and adhesion abilities of lactic acid bacteria against gut pathogens in a whole-tissue model. Biosci. Microbiota Food Health 2020, 39, 250–258. [Google Scholar] [PubMed]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics Importance and Their Immunomodulatory Properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar]
- Peters, V.B.M.; Van De Steeg, E.; Van Bilsen, J.; Meijerink, M. Mechanisms and Immunomodulatory Properties of Pre- and Probiotics. Benef. Microbes 2019, 10, 225–236. [Google Scholar]
- Khomayezi, R.; Adewole, D. Probiotics, Prebiotics, and Synbiotics: An Overview of Their Delivery Routes and Effects on Growth and Health of Broiler Chickens. World Poult. Sci. J. 2022, 78, 57–81. [Google Scholar]
- Shokryazdan, P.; Faseleh Jahromi, M.; Boo Liang, J.; Ramasamy, K.; Sieo, C.C.; Wan Ho, Y. Effects of a Lactobacillus salivarius Mixture on Performance, Intestinal Health and Serum Lipids of Broiler Chickens. PLoS ONE 2017, 12, e0175959. [Google Scholar]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on the Growth Performance and Intestinal Health of Broilers Challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018, 9, 25. [Google Scholar]
- Šefcová, M.A.; Ortega-Paredes, D.; Larrea-Álvarez, C.M.; Mina, I.; Guapás, V.; Ayala-Velasteguí, D.; Leoro-Garzón, P.; Molina-Cuasapaz, G.; Vinueza-Burgos, C.; Revajová, V.; et al. Effects of Lactobacillus fermentum Administration on Intestinal Morphometry and Antibody Serum Levels in Salmonella –Infantis–Challenged Chickens. Microorganisms 2023, 11, 256. [Google Scholar]
- Šefcová, M.; Larrea-Álvarez, M.; Larrea-Álvarez, C.; Karaffová, V.; Revajová, V.; Gancarčíková, S.; Ševčíková, Z.; Herich, R. Lactobacillus fermentum Administration Modulates Cytokine Expression and Lymphocyte Subpopulation Levels in Broiler Chickens Challenged with Campylobacter coli. Foodborne Pathog. Dis. 2020, 17, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Šefcová, M.A.; Larrea-Álvarez, M.; Larrea-Álvarez, C.M.; Karaffová, V.; Ortega-Paredes, D.; Vinueza-Burgos, C.; Ševčíková, Z.; Levkut, M.; Herich, R.; Revajová, V. The Probiotic Lactobacillus fermentum Biocenol CCM 7514 Moderates Campylobacter jejuni -Induced Body Weight Impairment by Improving Gut Morphometry and Regulating Cecal Cytokine Abundance in Broiler Chickens. Animals 2021, 11, 235. [Google Scholar] [CrossRef]
- Šefcová, M.; Larrea-Álvarez, M.; Larrea-Álvarez, C.; Revajová, V.; Karaffová, V.; Koščová, J.; Nemcová, R.; Ortega-Paredes, D.; Vinueza-Burgos, C.; Levkut, M.; et al. Effects of Lactobacillus fermentum Supplementation on Body Weight and Pro-Inflammatory Cytokine Expression in Campylobacter jejuni -Challenged Chickens. Vet. Sci. 2020, 7, 121. [Google Scholar] [PubMed]
- Yang, C.; Wang, S.; Li, Q.; Zhang, R.; Xu, Y.; Feng, J. Effects of Probiotic Lactiplantibacillus plantarum HJLP-1 on Growth Performance, Selected Antioxidant Capacity, Immune Function Indices in the Serum, and Cecal Microbiota in Broiler Chicken. Animals 2024, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a Probiotic, Enterococcus faecium, on Growth Performance, Intestinal Morphology, Immune Response, and Cecal Microflora in Broiler Chickens Challenged with Escherichia coli K88. Poultry Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef]
- Qiu, K.; Li, C.-L.; Wang, J.; Qi, G.-H.; Gao, J.; Zhang, H.-J.; Wu, S.-G. Effects of Dietary Supplementation with Bacillus subtilis, as an Alternative to Antibiotics, on Growth Performance, Serum Immunity, and Intestinal Health in Broiler Chickens. Front. Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef]
- Li, Z.; Long, L.; Jin, X.; Li, Y.; Wu, Q.; Chen, X.; Geng, Z.; Zhang, C. Effects of Clostridium butyricum on Growth Performance, Meat Quality, and Intestinal Health of Broilers. Front. Vet. Sci. 2023, 10, 1107798. [Google Scholar]
- Saleh, A.A.; Hayashi, K.; Ijiri, D.; Ohtsuka, A. Beneficial Effects of Aspergillus awamori in Broiler Nutrition. World’s Poult. Sci. J. 2014, 70, 857–864. [Google Scholar] [CrossRef]
- Monirujjaman, M.D.; Ferdouse, A. Metabolic and Physiological Roles of Branched-Chain Amino Acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Molinaro, A.; Wahlström, A.; Marschall, H.-U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar]
- Calvigioni, M.; Bertolini, A.; Codini, S.; Mazzantini, D.; Panattoni, A.; Massimino, M.; Celandroni, F.; Zucchi, R.; Saba, A.; Ghelardi, E. HPLC-MS-MS Quantification of Short-Chain Fatty Acids Actively Secreted by Probiotic Strains. Front. Microbiol. 2023, 14, 1124144. [Google Scholar]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut Microbes from the Phylogenetically Diverse Genus Eubacterium and Their Various Contributions to Gut Health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [PubMed]
- Pushpass, R.-A.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating Bile Acids as a Link between the Gut Microbiota and Cardiovascular Health: Impact of Prebiotics, Probiotics and Polyphenol-Rich Foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar]
- Chu, C.; Yu, L.; Li, Y.; Guo, H.; Zhai, Q.; Chen, W.; Tian, F. Lactobacillus plantarum CCFM405 against Rotenone-Induced Parkinson’s Disease Mice via Regulating Gut Microbiota and Branched-Chain Amino Acids Biosynthesis. Nutrients 2023, 15, 1737. [Google Scholar] [CrossRef]
- Viollet, B.; Andreelli, F. AMP-Activated Protein Kinase and Metabolic Control. Diabetes Perspect. Drug Ther. 2011, 203, 303–330. [Google Scholar]
- Hardie, D.G. AMP-Activated Protein Kinase: Maintaining Energy Homeostasis at the Cellular and Whole-Body Levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 452696. [Google Scholar]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020, 21, 8056. [Google Scholar] [CrossRef]
- Crossland, H.; Constantin-Teodosiu, D.; Greenhaff, P.L. The Regulatory Roles of PPARs in Skeletal Muscle Fuel Metabolism and Inflammation: Impact of PPAR Agonism on Muscle in Chronic Disease, Contraction and Sepsis. Int. J. Mol. Sci. 2021, 22, 9775. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Engel, F.B. PPARβ/δ: Linking Metabolism to Regeneration. Int. J. Mol. Sci. 2018, 19, 2013. [Google Scholar] [CrossRef] [PubMed]
- Manickam, R.; Wahli, W. Roles of Peroxisome Proliferator-Activated Receptor β/δ in Skeletal Muscle Physiology. Biochimie 2017, 136, 42–48. [Google Scholar] [PubMed]
- Remels, A.H.V.; Langen, R.C.J.; Gosker, H.R.; Russell, A.P.; Spaapen, F.; Voncken, J.W.; Schrauwen, P.; Schols, A.M.W.J. PPARγ Inhibits NF-κB-Dependent Transcriptional Activation in Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E174–E183. [Google Scholar]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARγ as a Molecular Target of EPA Anti-Inflammatory Activity during TNF-α-Impaired Skeletal Muscle Cell Differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar]
- Amiri, P.; Hosseini, S.A.; Roshanravan, N.; Saghafi-Asl, M.; Tootoonchian, M. The Effects of Sodium Butyrate Supplementation on the Expression Levels of PGC-1α, PPARα, and UCP-1 Genes, Serum Level of GLP-1, Metabolic Parameters, and Anthropometric Indices in Obese Individuals on Weight Loss Diet: A Study Protocol for a Triple-Blind, Randomized, Placebo-Controlled Clinical Trial. Trials 2023, 24, 489. [Google Scholar]
- Bharathy, N.; Ling, B.M.T.; Taneja, R. Epigenetic Regulation of Skeletal Muscle Development and Differentiation. In Epigenetics: Development and Disease, 1st ed.; Springer: Cham, Switzerland, 2013; pp. 139–150. [Google Scholar]
- Gaur, V.; Connor, T.; Sanigorski, A.; Martin, S.D.; Bruce, C.R.; Henstridge, D.C.; Steffensen, C.; Warren, R.; Prabhakar, P.; McGee, S.L. Disruption of the Class IIa HDAC Corepressor Complex Increases Energy Expenditure and Lipid Oxidation. Cell Rep. 2016, 16, 2802–2810. [Google Scholar]
- Walsh, M.E.; Van Remmen, H. Emerging Roles for Histone Deacetylases in Age-Related Muscle Atrophy. Nutr. Healthy Aging 2016, 4, 17–30. [Google Scholar]
- He, R.; Liu, B.; Geng, B.; Li, N.; Geng, Q. The Role of HDAC3 and Its Inhibitors in Regulation of Oxidative Stress and Chronic Diseases. Cell Death Discov. 2023, 9, 131. [Google Scholar]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Zhao, R. Butyrate Alleviates High Fat Diet-Induced Obesity through Activation of Adiponectin-Mediated Pathway and Stimulation of Mitochondrial Function in the Skeletal Muscle of Mice. Oncotarget 2016, 7, 56071. [Google Scholar]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile Salt Hydrolases: Gatekeepers of Bile Acid Metabolism and Host-Microbiome Crosstalk in the Gastrointestinal Tract. PLoS Pathog. 2019, 15, e1007581. [Google Scholar] [PubMed]
- Tamai, Y.; Eguchi, A.; Shigefuku, R.; Kitamura, H.; Tempaku, M.; Sugimoto, R.; Kobayashi, Y.; Iwasa, M.; Takei, Y.; Nakagawa, H. Association of Lithocholic Acid with Skeletal Muscle Hypertrophy through TGR5-IGF-1 and Skeletal Muscle Mass in Cultured Mouse Myotubes, Chronic Liver Disease Rats and Humans. eLife 2022, 11, e80638. [Google Scholar] [PubMed]
- Sun, L.; Li, F.; Tan, W.; Zhao, W.; Li, Y.; Zhu, X.; Gao, P.; Shu, G.; Wang, S.; Jiang, Q.; et al. Lithocholic Acid Promotes Skeletal Muscle Regeneration through the TGR5 Receptor: Lithocholic Acid Promotes Skeletal Muscle Regeneration. Acta Biochim. Biophys. Sin. 2023, 55, 51. [Google Scholar]
- Mazuy, C.; Helleboid, A.; Staels, B.; Lefebvre, P. Nuclear Bile Acid Signaling through the Farnesoid X Receptor. Cell. Mol. Life Sci. 2015, 72, 1631–1650. [Google Scholar]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The Impact of Short-Chain Fatty Acids on GLP-1 and PYY Secretion from the Isolated Perfused Rat Colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Howard, J.K.; Cani, P.D.; Bewick, G.A. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018, 8, 74. [Google Scholar]
- Gheller, B.J.; Blum, J.E.; Merritt, E.K.; Cummings, B.P.; Thalacker-Mercer, A.E. Peptide YY (PYY) Is Expressed in Human Skeletal Muscle Tissue and Expanding Human Muscle Progenitor Cells. Front. Physiol. 2019, 10, 437309. [Google Scholar]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar]
- Matheus, V.A.; Oliveira, R.B.; Maschio, D.A.; Tada, S.F.; Soares, G.M.; Mousovich-Neto, F.; Costa, R.G.; Mori, M.A.; Barbosa, H.C.; Collares-Buzato, C.B. Butyrate restores the fat/lean mass ratio balance and energy metabolism and reinforces the tight junction-mediated intestinal epithelial barrier in prediabetic mice independently of its anti-inflammatory and epigenetic actions. J. Nutr. Biochem. 2023, 120, 109409. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, J.; Zhang, W.; Nie, C. Impacts of sodium butyrate on intestinal mucosal barrier and intestinal microbial community in a weaned piglet model. Front. Microbiol. 2023, 13, 1041885. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, Z.; Yang, Z.; Qiao, X. Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides. Biomed. Pharmacother. 2023, 162, 114586. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.H.; Park, J.H.; Jeon, W.M.; Han, K.S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Kumari, A.; Bhawal, S.; Kapila, S.; Kapila, R. Probiotic lactobacilli mediate their immunoregulatory functions in intestinal cells via modulation of H3 histone acetylation. J. Appl. Microbiol. 2023, 134, lxac045. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; Ledue, F.; Blottière, H.M.; Lapaque, N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Min, J.; Wang, J.; Wu, H.; Zeng, Y.; Chen, S.; Chu, Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell. Immunol. 2012, 277, 66–73. [Google Scholar] [CrossRef]
- Du, H.-X.; Yue, S.-Y.; Niu, D.; Liu, C.; Zhang, L.-G.; Chen, J.; Chen, Y.; Guan, Y.; Hua, X.L.; Li, C.; et al. Gut microflora modulates Th17/Treg cell differentiation in experimental autoimmune prostatitis via the short-chain fatty acid propionate. Front. Immunol. 2022, 13, 915218. [Google Scholar] [CrossRef]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Zheng, Y.; Zhang, M.; Fei, W.; Sun, D.; Zhao, M.; Ye, Y.; Zheng, C. Short-chain fatty acids regulate B cell differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br. J. Pharmacol. 2022, 179, 4315–4329. [Google Scholar] [PubMed]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36, 66. [Google Scholar]
- Cobb500 Broiler Performance and Nutrition Supplement. Available online: https://www.cobb-vantress.com/resource/featured?q=nutrition (accessed on 14 September 2024).
- Aviagen Ross Broiler Guide. Available online: https://en.aviagen.com (accessed on 14 September 2024).
- Arbor Acres Broiler Management Handbook. Available online: https://aviagen.com/eu/brands/arbor-acres/ (accessed on 14 September 2024).
- Alcocer, H.M.; Xu, X.; Gravely, M.E.; Gonzalez, J.M. In Ovo Feeding of Commercial Broiler Eggs: An Accurate and Reproducible Method to Affect Muscle Development and Growth. J. Vis. Exp. 2021, 175, e63006. [Google Scholar]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken Embryo Development: Metabolic and Morphological Basis for In Ovo Feeding Technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar]
- Xu, X.; Jackson, A.R.; Gonzalez, J.M. The Effects of In Ovo Nicotinamide Riboside Dose on Broiler Myogenesis. Poult. Sci. 2021, 100, 100926. [Google Scholar]
- Muyyarikkandy, M.S.; Schlesinger, M.; Ren, Y.; Gao, M.; Liefeld, A.; Reed, S.; Amalaradjou, M.A. In Ovo Probiotic Supplementation Promotes Muscle Growth and Development in Broiler Embryos. Poult. Sci. 2023, 102, 102744. [Google Scholar]
- Albrecht, E.; Zitnan, R.; Karaffova, V.; Revajova, V.; Čechová, M.; Levkut Jr., M.; Röntgen, M. Effects of the Probiotic Enterococcus faecium on Muscle Characteristics of Chickens. Life 2022, 12, 1695. [Google Scholar]
- Zitnan, R.; Albrecht, E.; Kalbe, C.; Miersch, C.; Revajova, V.; Levkut Jr., M.; Röntgen, M. Muscle Characteristics in Chicks Challenged with Salmonella Enteritidis and the Effect of Preventive Application of the Probiotic Enterococcus faecium. Poult. Sci. 2019, 98, 2014–2025. [Google Scholar]
- Cai, L.; Wang, X.; Zhu, X.; Xu, Y.; Qin, W.; Ren, J.; Yan, X. Lactobacillus -Derived Protoporphyrin IX and SCFAs Regulate the Fiber Size via Glucose Metabolism in the Skeletal Muscle of Chickens. mSystems 2024, 9, e00214-24. [Google Scholar]
- Saleh, A.A.; Amber, K.; Mohammed, A.A. Dietary Supplementation with Avilamycin and Lactobacillus acidophilus Effects Growth Performance and the Expression of Growth-Related Genes in Broilers. Anim. Prod. Sci. 2020, 60, 1704–1710. [Google Scholar]
- Yuan, J.; Zhao, F.; Liu, Y.; Liu, H.; Zhang, K.; Tian, X.; Wang, Y. Effects of Lactiplantibacillus plantarum on Oxidative Stress, Mitophagy, and NLRP3 Inflammasome Activation in Broiler Breast Meat. Poult. Sci. 2023, 102, 103128. [Google Scholar] [PubMed]
- Wang, H.; Xiao, C.; Li, J.; Liang, R.; Liu, Y.; Song, Z.; Zhu, L. Dietary Bacillus subtilis Benefits Meat Quality by Regulating the Muscle Fiber Type and Antioxidant Capacity of Broilers. Poult. Sci. 2024, 103, 104267. [Google Scholar] [PubMed]
- Xue, J.; Fang, C.; Mu, R.; Zhuo, R.; Xiao, Y.; Qing, Y.; Fang, R. Potential Mechanism and Effects of Different Selenium Sources and Different Effective Microorganism Supplementation Levels on Growth Performance, Meat Quality, and Muscle Fiber Characteristics of Three-Yellow Chickens. Front. Nutr. 2022, 9, 869540. [Google Scholar]
- Dankowiakowska, A.; Bogucka, J.; Sobolewska, A.; Tavaniello, S.; Maiorano, G.; Bednarczyk, M. Effects of In Ovo Injection of Prebiotics and Synbiotics on the Productive Performance and Microstructural Features of the Superficial Pectoral Muscle in Broiler Chickens. Poult. Sci. 2019, 98, 5157–5165. [Google Scholar]
- Dunislawska, A.; Siwek, M.; Slawinska, A.; Lepczynski, A.; Herosimczyk, A.; Kolodziejski, P.A.; Bednarczyk, M. Metabolic Gene Expression in the Muscle and Blood Parameters of Broiler Chickens Stimulated In Ovo with Synbiotics. Animals 2020, 10, 687. [Google Scholar]
- Powell, D.J.; McFarland, D.C.; Cowieson, A.J.; Muir, W.I.; Velleman, S.G. The Effect of Nutritional Status on Myogenic Satellite Cell Proliferation and Differentiation. Poultry Sci. 2013, 92, 2163–2173. [Google Scholar]
- Chen, W.; Lv, Y.T.; Zhang, H.X.; Ruan, D.; Wang, S.; Lin, Y.C. Developmental Specificity in Skeletal Muscle of Late-Term Avian Embryos and Its Potential Manipulation. Poultry Sci. 2013, 92, 2754–2764. [Google Scholar]
- Clark, D.L.; Walter, K.G.; Velleman, S.G. Incubation Temperature and Time of Hatch Impact Broiler Muscle Growth and Morphology. Poultry Sci. 2017, 96, 4085–4095. [Google Scholar]
- Luo, Y.; De Souza, C.; Ramachandran, M.; Wang, S.; Yi, H.; Ma, Z.; Zhang, L.; Lin, K. Precise Oral Delivery Systems for Probiotics: A Review. J. Control. Release 2022, 352, 371–384. [Google Scholar]
- Torp, A.M.; Bahl, M.I.; Boisen, A.; Licht, T.R. Optimizing Oral Delivery of Next Generation Probiotics. Trends Food Sci. Technol. 2022, 119, 101–109. [Google Scholar]
- Herich, R.; Kokinčáková, T.; Lauková, A.; Levkutová, M. Effect of Preventive Application of Enterococcus faecium EF55 on Intestinal Mucosa during Salmonellosis in Chickens. Czech J. Anim. Sci. 2010, 55, 42–47. [Google Scholar]
- Levkut, M.; Revajová, V.; Lauková, A.; Ševčíková, Z.; Spišáková, V.; Faixová, Z.; Levkut, J.; Levkut, M. Leukocytic Responses and Intestinal Mucin Dynamics of Broilers Protected with Enterococcus faecium EF55 and Challenged with Salmonella enteritidis. Res. Vet. Sci. 2012, 93, 195–201. [Google Scholar] [PubMed]
- Li, S.; He, Y.; Mann, D.A.; Deng, X. Global Spread of Salmonella Enteritidis via Centralized Sourcing and International Trade of Poultry Breeding Stocks. Nat. Commun. 2021, 12, 5109. [Google Scholar] [PubMed]
- Rychlik, I.; Elsheimer-Matulova, M.; Kyrova, K. Gene Expression in the Chicken Caecum in Response to Infections with Non-Typhoid Salmonella. Vet. Res. 2014, 45, 119. [Google Scholar]
- Shibat El-hamd, D.; Mohamed, H. Effect of Probiotics on Salmonella Enteritidis Infection in Broiler Chickens. Egypt. J. Chem. Environ. Health 2016, 2, 298–314. [Google Scholar]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and Prebiotics in Animal Feeding for Safe Food Production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar]
- Chaucheyras-Durand, F.; Durand, H. Probiotics in Animal Nutrition and Health. Benef. Microbes 2009, 1, 3–9. [Google Scholar]
- Hudec, E.; Mudroňová, D.; Marcinčák, S.; Bartkovský, M.; Makiš, A.; Faldyna, M.; Ratvaj, M.; Karaffová, V. The Effect of Limosilactobacillus fermentum 2i3 and 0.6% Addition of Humic Substances on Production Parameters and the Immune System of Broilers. Poult. Sci. 2024, 103, 103884. [Google Scholar]
- Zhang, K.; Li, X.; Zhao, J.; Wang, Y.; Hao, X.; Liu, K.; Liu, H. Protective Effects of Chlorogenic Acid on the Meat Quality of Oxidatively Stressed Broilers Revealed by Integrated Metabolomics and Antioxidant Analysis. Food Funct. 2022, 13, 2238–2252. [Google Scholar]
- Liu, H.; Zhao, F.; Zhang, K.; Zhao, J.; Wang, Y. Investigating the Growth Performance, Meat Quality, Immune Function and Proteomic Profiles of Plasmal Exosomes in Lactobacillus plantarum -Treated Broilers with Immunological Stress. Food Funct. 2021, 12, 11790–11807. [Google Scholar]
- Zhao, J.; Zhao, F.; Li, X.; Yuan, J.; Zhang, K.; Liu, H.; Wang, Y. Multi-Omics Reveals the Mechanisms Underlying Lactiplantibacillus plantarum P8-Mediated Attenuation of Oxidative Stress in Broilers Challenged with Dexamethasone. Anim. Nutr. 2023, 14, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, H.; Shi, L.; Zhang, X.; Sheng, R.; Yin, F.; Gooneratne, R. Study of Bacillus subtilis on Growth Performance, Nutrition Metabolism, and Intestinal Microflora of 1 to 42 d Broiler Chickens. Anim. Nutr. 2017, 3, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.K.; Podmaniczky, B.; Kürti, P.; Tenk, I.; Glávits, R.; Virág, G.Y.; Szabó, Z.S. Effect of Different Concentrations of Bacillus subtilis on Growth Performance, Carcase Quality, Gut Microflora, and Immune Response of Broiler Chickens. Br. Poult. Sci. 2011, 52, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Mahmoud, M.A.; Zaki, R.S.; Cheng, H.W. Effect of a Probiotic Supplement (Bacillus subtilis) on Struggling Behavior, Immune Response, and Meat Quality of Shackled Broiler Chickens Exposed to Preslaughter Stress. Poult. Sci. 2024, 103, 104051. [Google Scholar] [CrossRef]
- Saleh, A.A.; Shukry, M.; Farrag, F.; Soliman, M.M.; Abdel-Moneim, A.M.E. Effect of Feeding Wet Feed or Wet Feed Fermented by Bacillus licheniformis on Growth Performance, Histopathology, and Growth and Lipid Metabolism Marker Genes in Broiler Chickens. Animals 2021, 11, 83. [Google Scholar] [CrossRef]
- Akinola, O.S.; Onakomaiya, A.O.; Agunbiade, J.A.; Oso, A.O. Growth Performance, Apparent Nutrient Digestibility, Intestinal Morphology, and Carcass Traits of Broiler Chickens Fed Dry, Wet, and Fermented-Wet Feed. Livest. Sci. 2015, 177, 103–109. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Guo, S.; Tan, J. Effects of Clostridium butyricum and Enterococcus faecium on Growth Performance, Lipid Metabolism, and Cecal Microbiota of Broiler Chickens. Appl. Microbiol. Biotechnol. 2013, 97, 6477–6488. [Google Scholar] [CrossRef]
- Saneyasu, T.; Kimura, S.; Kitashiro, A.; Tsuchii, N.; Tsuchihashi, T.; Inui, M.; Honda, K.; Kamisoyama, H. Differential Regulation of the Expression of Lipid Metabolism-Related Genes with Skeletal Muscle Type in Growing Chickens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 189, 1–5. [Google Scholar] [CrossRef]
- Zheng, A.; Luo, J.; Meng, K.; Li, J.; Zhang, S.; Li, K.; Liu, G.; Cai, H.; Bryden, W.L.; Yao, B. Proteome Changes Underpin Improved Meat Quality and Yield of Chickens (Gallus gallus) Fed the Probiotic Enterococcus faecium. BMC Genom. 2014, 15, 1167. [Google Scholar] [CrossRef]
- Hassan, N.; Mostafa, I.; Elhady, M.A.; Ibrahim, M.A.; Amer, H. Effects of Probiotic Feed Additives (Biosol and Zemos) on Growth and Related Genes in Broiler Chickens. Ital. J. Anim. Sci. 2022, 21, 62–73. [Google Scholar] [CrossRef]
- Singh, N.; Gaur, S. GRAS Fungi: A New Horizon in Safer Food Product. In Fungi in Sustainable Food Production, 2nd ed.; Springer: Cham, Switzerland, 2021; pp. 27–37. [Google Scholar]
- Saleh, A.A.; Eid, Y.Z.; Ebeid, T.A.; Ohtsuka, A.; Yamamoto, M.; Hayashi, K. Feeding Aspergillus awamori reduces skeletal muscle protein breakdown and stimulates growth in broilers. Anim. Sci. J. 2012, 83, 594–598. [Google Scholar] [PubMed]
- Saleh, A.A.; Amber, K.; El-Magd, M.A.; Atta, M.S.; Mohammed, A.A.; Ragab, M.M.; Abd El-Kader, H. Integrative effects of feeding Aspergillus awamori and fructooligosaccharide on growth performance and digestibility in broilers: Promotion muscle protein metabolism. Biomed. Res. Int. 2014, 2014, 946859. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics, and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [PubMed]
- Sarangi, N.R.; Babu, L.K.; Kumar, A.; Pradhan, C.R.; Pati, P.K.; Mishra, J.P. Effect of dietary supplementation of prebiotic, probiotic, and synbiotic on growth performance and carcass characteristics of broiler chickens. Vet. World 2016, 9, 313. [Google Scholar]
- Śliżewska, K.; Markowiak-Kopeć, P.; Żbikowski, A.; Szeleszczuk, P. The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 2020, 10, 4281. [Google Scholar]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar]
- Froebel, L.K.; Jalukar, S.; Lavergne, T.A.; Lee, J.T.; Duong, T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult. Sci. 2019, 98, 6668–6676. [Google Scholar]
- Stasiak, K.; Slawinska, A.; Bogucka, J. Effects of probiotics, prebiotics and synbiotics injected in ovo on the microstructure of the breast muscle in different chicken genotypes. Animals 2021, 11, 2944. [Google Scholar] [CrossRef]
- Lan, R.; Wang, Y.; Wang, H.; Zhang, J. Dietary Chitosan Oligosaccharide Supplementation Improves Meat Quality by Improving Antioxidant Capacity and Fiber Characteristics in the Thigh Muscle of Broilers. Antioxidants 2024, 13, 366. [Google Scholar] [CrossRef]
- Zhao, Y.; Balasubramanian, B.; Guo, Y.; Qiu, S.-J.; Jha, R.; Liu, W.-C. Dietary Enteromorpha Polysaccharides Supplementation Improves Breast Muscle Yield and Is Associated with Modification of mRNA Transcriptome in Broiler Chickens. Front. Vet. Sci. 2021, 8, 663988. [Google Scholar]
- Urban, J.; Kareem, K.Y.; Matuszewski, A.; Bień, D.; Ciborowska, P.; Lutostański, K.; Michalczuk, M. Enhancing Broiler Chicken Health and Performance: The Impact of Phytobiotics on Growth, Gut Microbiota, Antioxidants, and Immunity. Phytochem. Rev. 2024, 1–15. [Google Scholar] [CrossRef]
- Šefcová, M.A.; Santacruz, F.; Larrea-Álvarez, C.M.; Vinueza-Burgos, C.; Ortega-Paredes, D.; Molina-Cuasapaz, G.; Rodríguez, J.; Calero-Cáceres, W.; Revajová, V.; Fernández-Moreira, E.; et al. Administration of Dietary Microalgae Ameliorates Intestinal Parameters, Improves Body Weight, and Reduces Thawing Loss of Fillets in Broiler Chickens: A Pilot Study. Animals 2021, 11, 3601. [Google Scholar]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition–A Review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar]
- Selionova, M.I.; Trukhachev, V.I.; Zagarin, A.Y.; Kulikov, E.I.; Dmitrenko, D.M.; Martynova, V.N.; Kravchenko, A.K.; Vertiprakhov, V.G. Expression of Genes Related to Meat Productivity, Metabolic and Morphological Significance of Broiler Chickens with the Use of Nutritional Phytochemicals. Animals 2024, 14, 2958. [Google Scholar] [CrossRef] [PubMed]
- Chodkowska, K.A.; Abramowicz-Pindor, P.A.; Tuśnio, A.; Gawin, K.; Taciak, M.; Barszcz, M. Effect of Phytobiotic Composition on Production Parameters, Oxidative Stress Markers and Myokine Levels in Blood and Pectoral Muscle of Broiler Chickens. Animals 2022, 12, 2625. [Google Scholar] [CrossRef]
- de Freitas Dionizio, A.; de Souza Khatlab, A.; Alcalde, C.R.; Gasparino, E.; Feihrmann, A.C. Supplementation with Free Methionine or Methionine Dipeptide Improves Meat Quality in Broilers Exposed to Heat Stress. J. Food Sci. Technol. 2021, 58, 205–215. [Google Scholar]
- Subramaniyan, S.A.; Kang, D.R.; Park, J.R.; Siddiqui, S.H.; Ravichandiran, P.; Yoo, D.J.; Na, C.S.; Shim, K.S. Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins. Animals 2019, 9, 357. [Google Scholar] [CrossRef]
- Lu, P.; Morawong, T.; Molee, A.; Molee, W. L-Arginine Alters Myogenic Genes Expression but Does Not Affect Breast Muscle Characteristics by In Ovo Feeding Technique in Slow-Growing Chickens. Front. Vet. Sci. 2022, 9, 1030873. [Google Scholar]
- Shehata, A.A.; Basiouni, S.; Tellez-Isaias, G.; Eisenreich, W.; Hafez, H.M. Probiotics as Alternative to Antibiotics in Poultry: Challenges and Prospects. Altern. Antibiot. Pathog. Poult. 2024, 59, 59–78. [Google Scholar]
- Zommiti, M.; Chikindas, M.L.; Ferchichi, M. Probiotics—Live Biotherapeutics: A Story of Success, Limitations, and Future Prospects—Not Only for Humans. Probiotics Antimicrob. Proteins 2020, 12, 1266–1289. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herich, R.; Szabóová, R.; Karaffová, V.; Racines, M.P.; Šefcová, M.A.; Larrea-Álvarez, M. A Narrative Review on the Impact of Probiotic Supplementation on Muscle Development, Metabolic Regulation, and Fiber Traits Related to Meat Quality in Broiler Chickens. Microorganisms 2025, 13, 784. https://doi.org/10.3390/microorganisms13040784
Herich R, Szabóová R, Karaffová V, Racines MP, Šefcová MA, Larrea-Álvarez M. A Narrative Review on the Impact of Probiotic Supplementation on Muscle Development, Metabolic Regulation, and Fiber Traits Related to Meat Quality in Broiler Chickens. Microorganisms. 2025; 13(4):784. https://doi.org/10.3390/microorganisms13040784
Chicago/Turabian StyleHerich, Robert, Renáta Szabóová, Viera Karaffová, Maria Paula Racines, Miroslava Anna Šefcová, and Marco Larrea-Álvarez. 2025. "A Narrative Review on the Impact of Probiotic Supplementation on Muscle Development, Metabolic Regulation, and Fiber Traits Related to Meat Quality in Broiler Chickens" Microorganisms 13, no. 4: 784. https://doi.org/10.3390/microorganisms13040784
APA StyleHerich, R., Szabóová, R., Karaffová, V., Racines, M. P., Šefcová, M. A., & Larrea-Álvarez, M. (2025). A Narrative Review on the Impact of Probiotic Supplementation on Muscle Development, Metabolic Regulation, and Fiber Traits Related to Meat Quality in Broiler Chickens. Microorganisms, 13(4), 784. https://doi.org/10.3390/microorganisms13040784









