Addition of Thermotolerant Nitrifying Bacteria During Pig Manure Composting Enhanced Nitrogen Retention and Modified Microbial Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Preparation and Composting Materials
2.2. Analysis of Physical and Chemical Properties
treatment)/(Germination rate of seeds × Root length of distilled water control)
2.3. High-Throughput 16S rDNA and ITS Sequencing
2.4. Quantitative Analysis of Nitrogen Conversion-Related Genes
2.5. Data Analysis
3. Results and Discussion
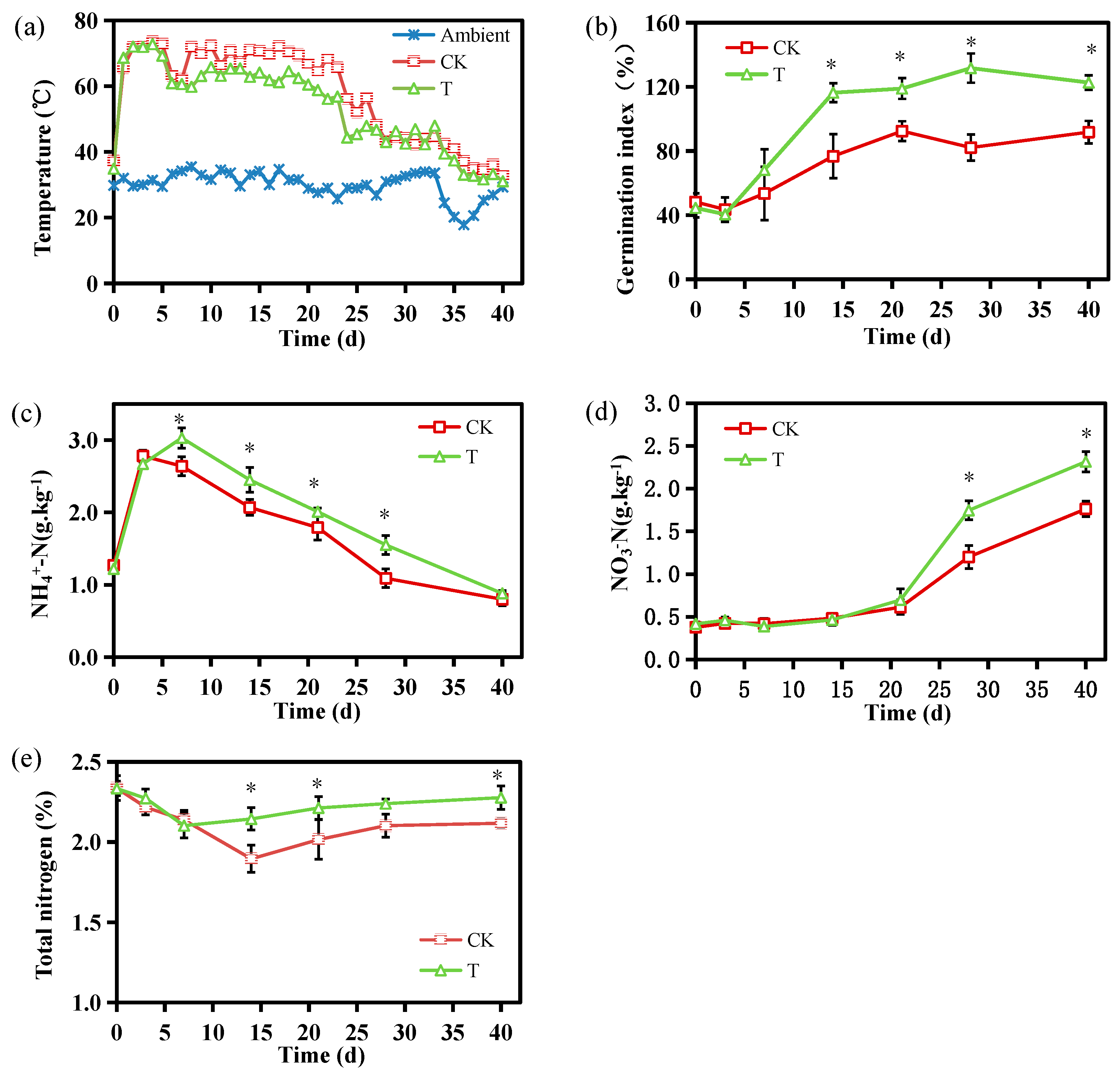
3.1. Composting-Related Temperature, GI, NH4+-N, NO3–-N, and TN Changes
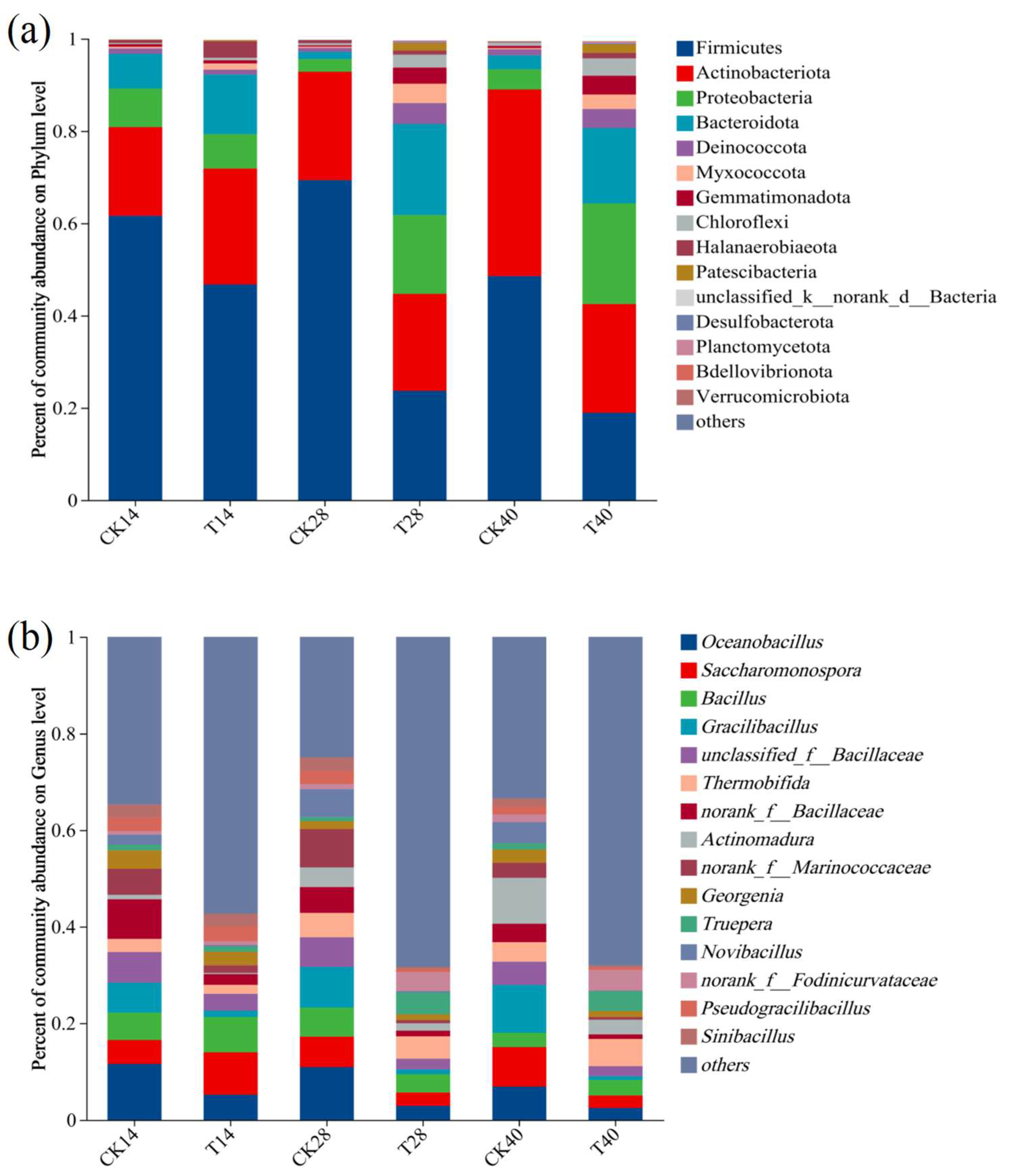
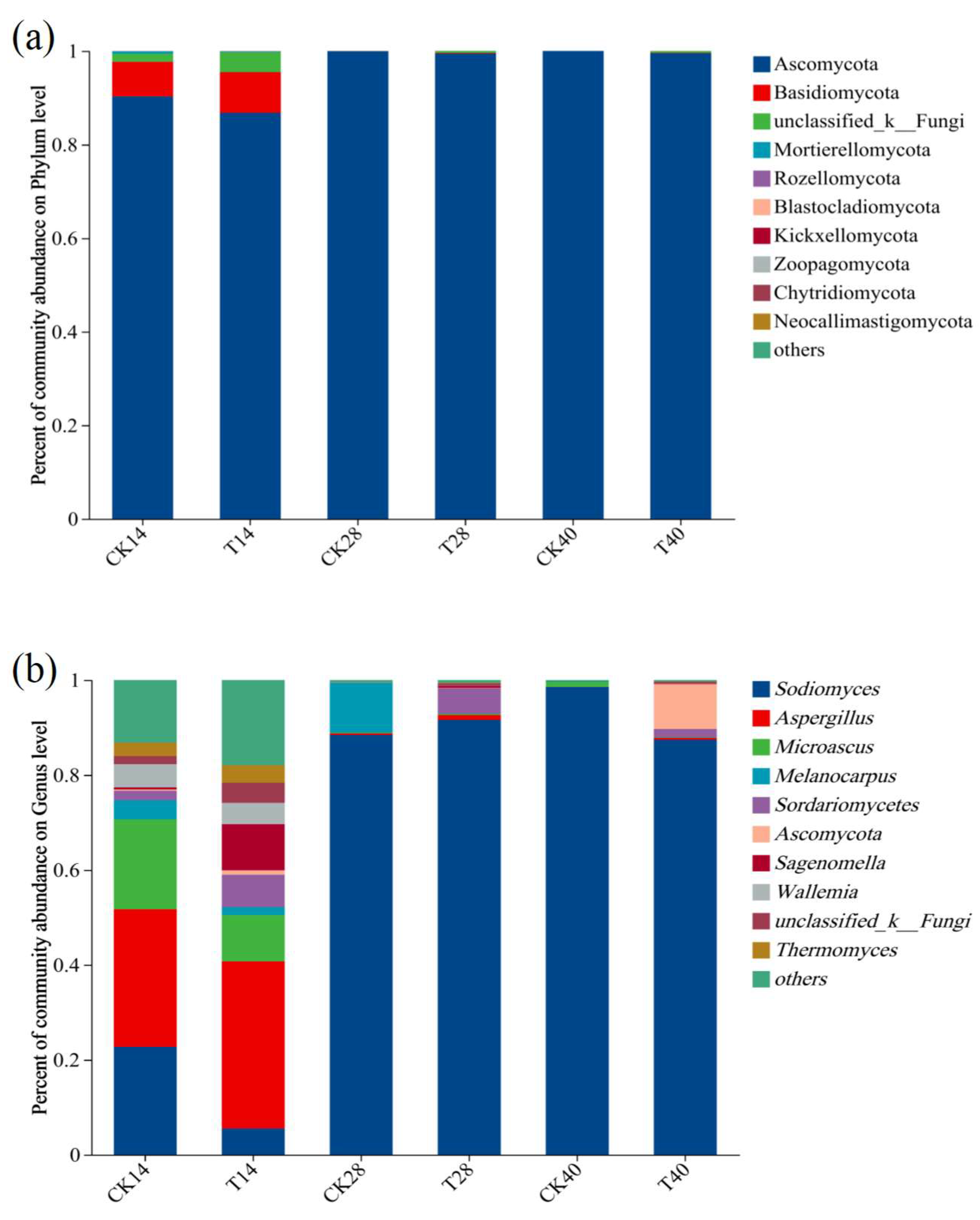
3.2. Microbial Community Dynamics over the Course of Composting
3.2.1. Bacterial Community and Functional Dynamics
3.2.2. Fungal Community Succession
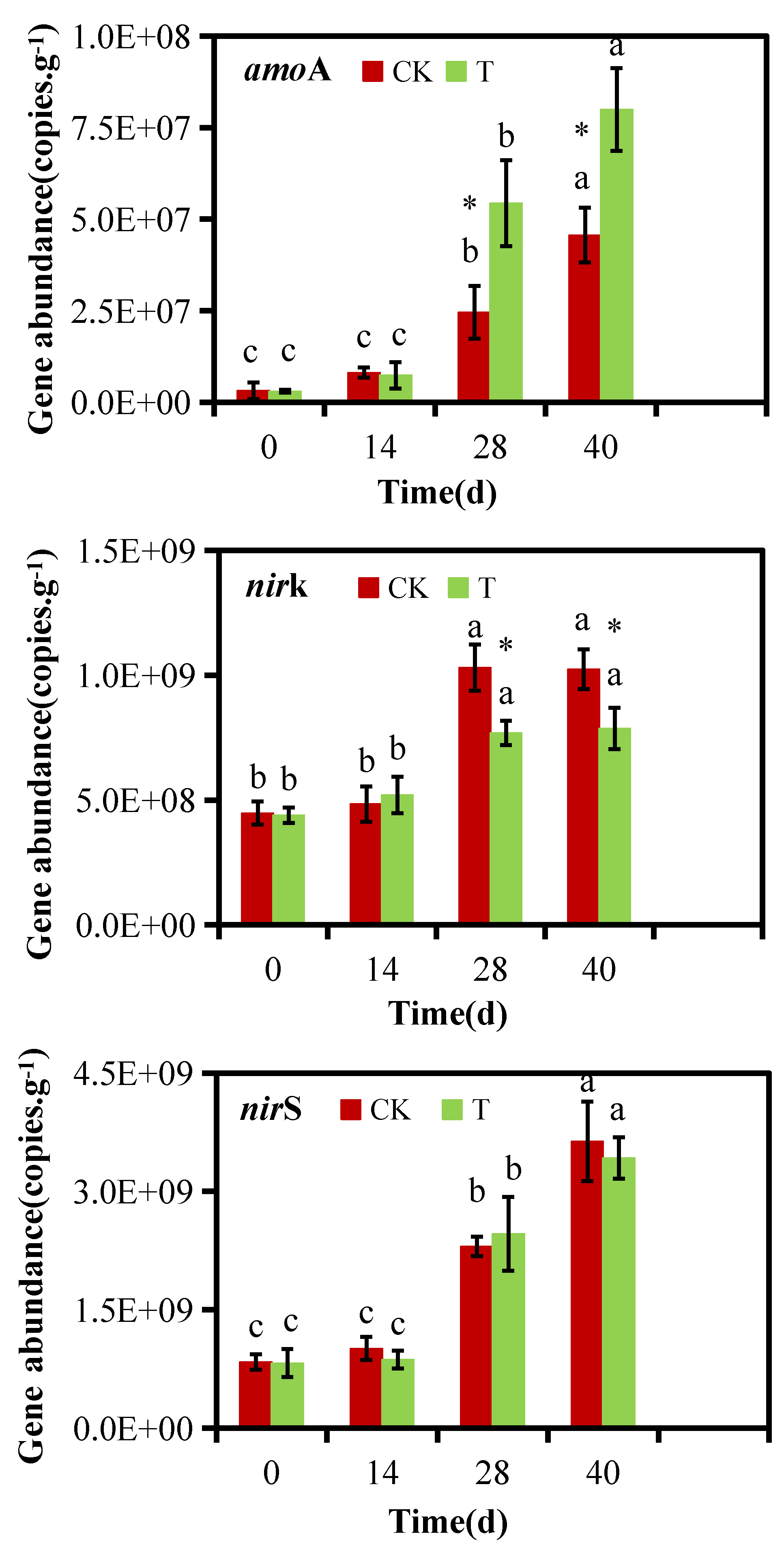
3.3. Effects of Bacterial Addition on the Levels of Nitrogen-Converting Genes
3.4. Associations Between Microbes, Available Nitrogen, and Nitrogen Conversion-Associated Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Zhou, H.; Meng, H.; Ding, J.; Shen, Y.; Cheng, H.; Zhang, X.; Li, R.; Fan, S. Amino acid profile characterization during the co-composting of a livestock manure and maize straw mixture. J. Clean. Prod. 2021, 278, 123494. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, L.; Liu, Y.; Li, X.; Li, Z. Evaluation of composting parameters, technologies and maturity indexes for aerobic manure composting: A meta-analysis. Sci. Total Environ. 2023, 886, 163929. [Google Scholar] [CrossRef] [PubMed]
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.O.; Iroh, I.N.; Ezeogu, L.I. Composting technology in waste stabilization: On the methods, challenges and future prospects. J. Environ. Manag. 2017, 190, 140–157. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Li, R.; Chang, C.C.; Pan, J.; Zhang, Z. Effect of clay on greenhouse gas emissions and humification during pig manure composting as supported by spectroscopic evidence. Sci. Total Environ. 2020, 737, 139712. [Google Scholar] [CrossRef]
- Beck-Friis, B.; Smårs, S.; Jonsson, H.; Kirchmann, H. SE—Structures and environment: Gaseous emissions of carbon dioxide, ammonia and nitrous oxide from organic household waste in a compost reactor under different temperature regimes. J. Agric. Eng. Res. 2001, 78, 423–430. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Gao, Y.; Tan, W.; Xi, B. Additives for reducing nitrogen loss during composting: A review. J. Clean. Prod. 2021, 307, 127308. [Google Scholar] [CrossRef]
- Yang, Y.; Awasthi, M.K.; Ren, X.; Guo, H.; Lv, J. Effect of bean dregs on nitrogen transformation and bacterial dynamics during pig manure composting. Bioresour. Technol. 2019, 288, 121430. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Qiao, Y.; Li, S.; Chen, Y.; Hu, C. Mitigation of carbon and nitrogen losses during pig manure composting: A meta-analysis. Sci. Total Environ. 2021, 783, 147103. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Li, J.; Liu, Z.; Xin, Y.; Wang, L.; Chen, J.; Gun, S. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123167. [Google Scholar] [CrossRef]
- Guo, H.; Gu, J.; Wang, X.; Nasir, M.; Yu, J.; Lei, L.; Wang, J.; Zhao, W.; Dai, X. Beneficial effects of bacterial agent/bentonite on nitrogen transformation and microbial community dynamics during aerobic composting of pig manure. Bioresour. Technol. 2020, 298, 122384. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Guo, F.; Zhang, X.; Dong, W.; Zhang, X.; Zhang, X.; Zhang, C.; Cheng, K.; Li, Y.; et al. Composting pig manure and sawdust with urease inhibitor: Succession of nitrogen functional genes and bacterial community. Environ. Sci. Pollut. R. 2020, 27, 36160–36171. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.B.; Mao, H.L.; Chu, C.; Tian, Y. Changes in structure and function of fungal community in cow manure composting. Bioresour. Technol. 2018, 255, 123–130. [Google Scholar] [CrossRef]
- Duan, Y.M.; Awasthi, S.K.; Chen, H.Y.; Liu, T.; Zhang, Z.Q.; Zhang, L.S.; Awasthi, M.K.; Taherzadeh, M.J. Evaluating the impact of bamboo biochar on the fungal community succession during chicken manure composting. Bioresour. Technol. 2019, 272, 308–314. [Google Scholar] [CrossRef]
- Li, J.; Bao, H.Y.; Xing, W.J.; Yang, J.; Liu, R.F.; Wang, X.; Lv, L.H.; Tong, X.G.; Wu, F.Y. Succession of fungal dynamics and their influence on physicochemical parameters during pig manure composting employing with pine leaf biochar. Bioresour. Technol. 2020, 297, 122377. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Cledon, M.; Brar, S.; Ramirez, A. Nitrification of vegetable wastes using nitrifying bacteria. Ecol. Eng. 2018, 121, 83–88. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.G.; Chen, L.; Meng, L.Q.; Zhang, S.M. Impacts of adding thermotolerant bacteria on nitrogenous gas emissions and bacterial community structure during sewage sludge composting. Bioresour. Technol. 2023, 368, 128359. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Dong, S.; Lan, S.; Zhou, H.; Tan, Z.; Li, X. Mixed nitrifying bacteria culture under different temperature dropping strategies: Nitrification performance, activity, and community. Chemosphere 2018, 195, 800–809. [Google Scholar] [CrossRef]
- NY/T 1116-2014; Fertilizers—Determination of Nitrate Nitrogen, Ammonium Nitrogen, Amide Nitrogen Contents. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2014.
- NY/T 525-2021; Organic Fertilizer. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- Liu, B.; Chen, W.; Wang, Z.; Guo, Z.; Li, Y.; Xu, L.; Wu, M.; Yin, H. The impact of Bacillus coagulans X3 on available nitrogen content, bacterial community composition, and nitrogen functional gene levels when composting cattle manure. Agronomy 2024, 14, 587. [Google Scholar] [CrossRef]
- Manu, M.K.; Kumar, R.; Garg, A. Performance assessment of improved composting system for food waste with varying aeration and use of microbial inoculum. Bioresour. Technol. 2017, 234, 167–177. [Google Scholar]
- GB/T 36195-2018; Technical Specification for Sanitation Treatment of Livestock and Poultry Manure. State Administration for Market Regulation of the People’s Republic of China: Beijing, China, 2018.
- Zhao, Y.; Li, W.; Chen, L.; Meng, L.; Zheng, Z. Effect of enriched thermotolerant nitrifying bacteria inoculation on reducing nitrogen loss during sewage sludge composting. Bioresour. Technol. 2020, 311, 123461. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Huang, D.; Huang, G.; Hu, T.; Jiang, X.; Feng, C.; Chen, Y.; Tang, L.; Liu, H. Composting of lead-contaminated solid waste with inocula of white-rot fungus. Bioresour. Technol. 2007, 98, 320–326. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Liu, B.; Wang, K.; Su, C.; Wu, C. Ammonia emission and biodegradation of organic carbon during sewage sludge composting with different extra carbon source. Int. Biodeterior. Biodegrad. 2013, 85, 624–630. [Google Scholar] [CrossRef]
- Tian, X.; Gao, R.; Li, Y.; Liu, Y.; Zhang, X.; Pan, J.; Tang, K.; Scriber, K.E., II; Amoah, I.D.; Zhang, Z.; et al. Enhancing nitrogen conversion and microbial dynamics in swine manure composting process through inoculation with a microbial consortium. J. Clean. Prod. 2023, 423, 138819. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Huang, Y.; Huang, H. Inoculation with nitrogen turnover bacterial agent appropriately increasing nitrogen and promoting maturity in pig manure composting. Waste Manag. 2015, 39, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T. Effect of biochar and bacteria inoculum addition on cow dung composting. Bioresour. Technol. 2020, 297, 122407. [Google Scholar] [CrossRef]
- Xue, S.D.; Zhou, L.N.; Zhong, M.Z.; Awasthi, A.K.; Mao, H. Bacterial agents affected bacterial community structure to mitigate greenhouse gas emissions during sewage sludge composting. Bioresour. Technol. 2021, 337, 125397. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, C.H.; Li, W.; Wu, W.X.; Tang, Z.R.; Tian, Y.; Xi, B.D. Ammonia assimilation is key for the preservation of nitrogen during industrial-scale composting of chicken manure. Waste Manag. 2023, 170, 50–61. [Google Scholar] [CrossRef]
- Martínez-Ruiz, B.E.; Cooper, M.; Fastner, J.; Szewzyk, U. Manganese-oxidizing bacteria isolated from natural and technical systems remove cylindrospermopsin. Chemosphere 2020, 238, 124625. [Google Scholar] [CrossRef]
- Wu, X.Y.; Amanze, C.; Yu, Z.J.; Li, J.K.; Liu, Y.D.; Shen, L.; Yu, R.L.; Wu, X.L.; Xu, X.W.; Tan, S.Y.; et al. Evaluation of fungal community assembly and function during food waste composting with Aneurinibacillus sp. LD3 inoculant. Bioresour. Technol. 2022, 363, 127923. [Google Scholar] [CrossRef]
- Neher, D.A.; Weicht, T.R.; Bates, S.T.; Leff, J.W.; Fierer, N. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013, 8, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Grum-Grzhimaylo, A.A.; Falkoski, D.L.; Heuvel, J.V.D.; Valero-Jimenez, C.A.; Min, B.; Choi, I.G.; Lipzen, A.; Daum, C.G.; Aanen, D.K.; Tsang, A. The obligate alkalophilic soda-lake fungus Sodiomyces alkalinus has shifted to a protein diet. Mol. Ecol. 2018, 27, 14912. [Google Scholar] [CrossRef]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.C.R.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the gut (Gallus gallus) microbiota following the consumption of zinc biofortified wheat (Triticum aestivum)-based diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.P.; Ma, S.S.; He, X.Q.; Han, L.J.; Huang, G.Q. Nitrogen transformation and dynamic changes in related functional genes during functional-membrane covered aerobic composting. Bioresour. Technol. 2021, 332, 125087. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, G.; Zhang, J.; Chen, Y.; Yu, M.; Lu, L.; Li, H.; Zhu, Y.; Yuan, Y.; Huang, A. Response of denitrifying genes coding for nitrite (nirK or nirS) and nitrous oxide (nosZ) reductases to different physico-chemical parameters during agricultural waste composting. Appl. Microbiol. Biotechnol. 2015, 99, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Tom, O.D.; Christopher, Q.; Alon, S.; Özcan, C.E.; Tm, L.S.; Michael, S.R.; Sandra, L.M.; Sebastian, L.; Murat, E.A. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat. Microbiol. 2018, 3, 804–813. [Google Scholar]
- Mironov, V.; Vanteeva, A.; Sokolova, D.; Merkel, A.; Nikolaev, Y. Microbiota dynamics of mechanically separated organic fraction of municipal solid waste during composting. Microorganisms 2021, 9, 1877. [Google Scholar] [CrossRef]


| Moisture (%) | pH | Total Carbon (%) a | Total Nitrogen (%) a | C/N Ratio a | |
|---|---|---|---|---|---|
| Pig manure | 58.82 ± 1.32 | 8.13 ± 0.04 | 35.43 ± 0.52 | 2.05 ± 0.10 | 17.33 ± 0.61 |
| Sawdust | 8.28 ± 0.34 | 6.66 ± 0.05 | 52.12 ± 0.03 | 0.78 ± 0.02 | 67.11 ± 0.32 |
| Gene | Primer | Primer Sequence (5′–3′) |
|---|---|---|
| amoA | bamoA1F | GGGGTTTCTACTGGTGGT |
| bamoA2R | CCCCTCKGSAAAGCCTTCTTC | |
| nirS | Cd3aF | GTSAACGTSAAGGARACSGG |
| R3cdR | GASTTCGGRTGSGTCTTGA | |
| nirK | nirK1aCuF | ATCATGGTSCTGCCGCG |
| nirKR3CuR | GCCTCGATCAGRTTGTGGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Guo, Z.; Chen, W.; Wang, Z.; Xu, L.; Gao, S.; Wu, Y.; Zeng, Y.; Tang, B.; Wu, M.; et al. Addition of Thermotolerant Nitrifying Bacteria During Pig Manure Composting Enhanced Nitrogen Retention and Modified Microbial Composition. Microorganisms 2025, 13, 719. https://doi.org/10.3390/microorganisms13040719
Liu B, Guo Z, Chen W, Wang Z, Xu L, Gao S, Wu Y, Zeng Y, Tang B, Wu M, et al. Addition of Thermotolerant Nitrifying Bacteria During Pig Manure Composting Enhanced Nitrogen Retention and Modified Microbial Composition. Microorganisms. 2025; 13(4):719. https://doi.org/10.3390/microorganisms13040719
Chicago/Turabian StyleLiu, Biao, Zhaohui Guo, Wei Chen, Zhen Wang, Lijuan Xu, Shuaishuai Gao, Yingben Wu, Yan Zeng, Bingxuan Tang, Minxi Wu, and et al. 2025. "Addition of Thermotolerant Nitrifying Bacteria During Pig Manure Composting Enhanced Nitrogen Retention and Modified Microbial Composition" Microorganisms 13, no. 4: 719. https://doi.org/10.3390/microorganisms13040719
APA StyleLiu, B., Guo, Z., Chen, W., Wang, Z., Xu, L., Gao, S., Wu, Y., Zeng, Y., Tang, B., Wu, M., & Yin, H. (2025). Addition of Thermotolerant Nitrifying Bacteria During Pig Manure Composting Enhanced Nitrogen Retention and Modified Microbial Composition. Microorganisms, 13(4), 719. https://doi.org/10.3390/microorganisms13040719





