Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol Design and Registration
2.2. Search Strategies
3. Eligibility for Study Selection
3.1. Inclusion Criteria
3.2. Populations/Subjects
3.3. Exposure
3.4. Outcomes of Interest
3.5. Exclusion Criteria
3.6. Data Documentation
3.7. Quality Assessment and Reducing the Risk of Bias
3.8. Data Analysis
4. Results
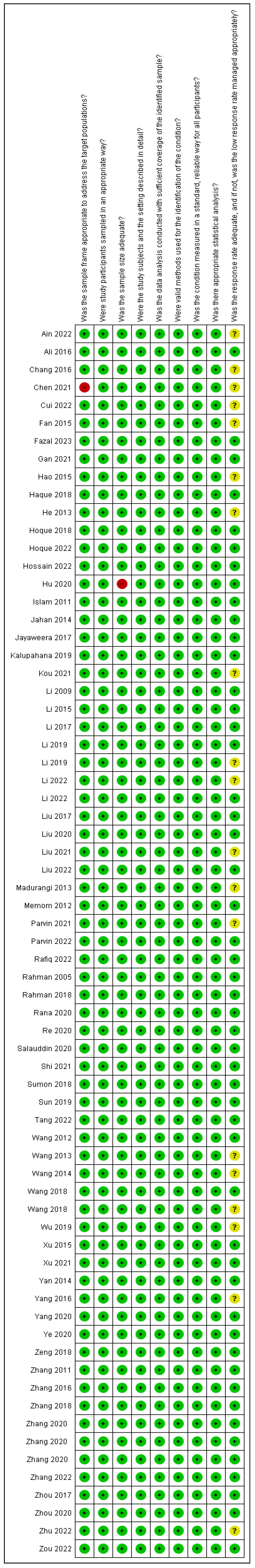
4.1. Characteristics of Studies and Quality Evaluation
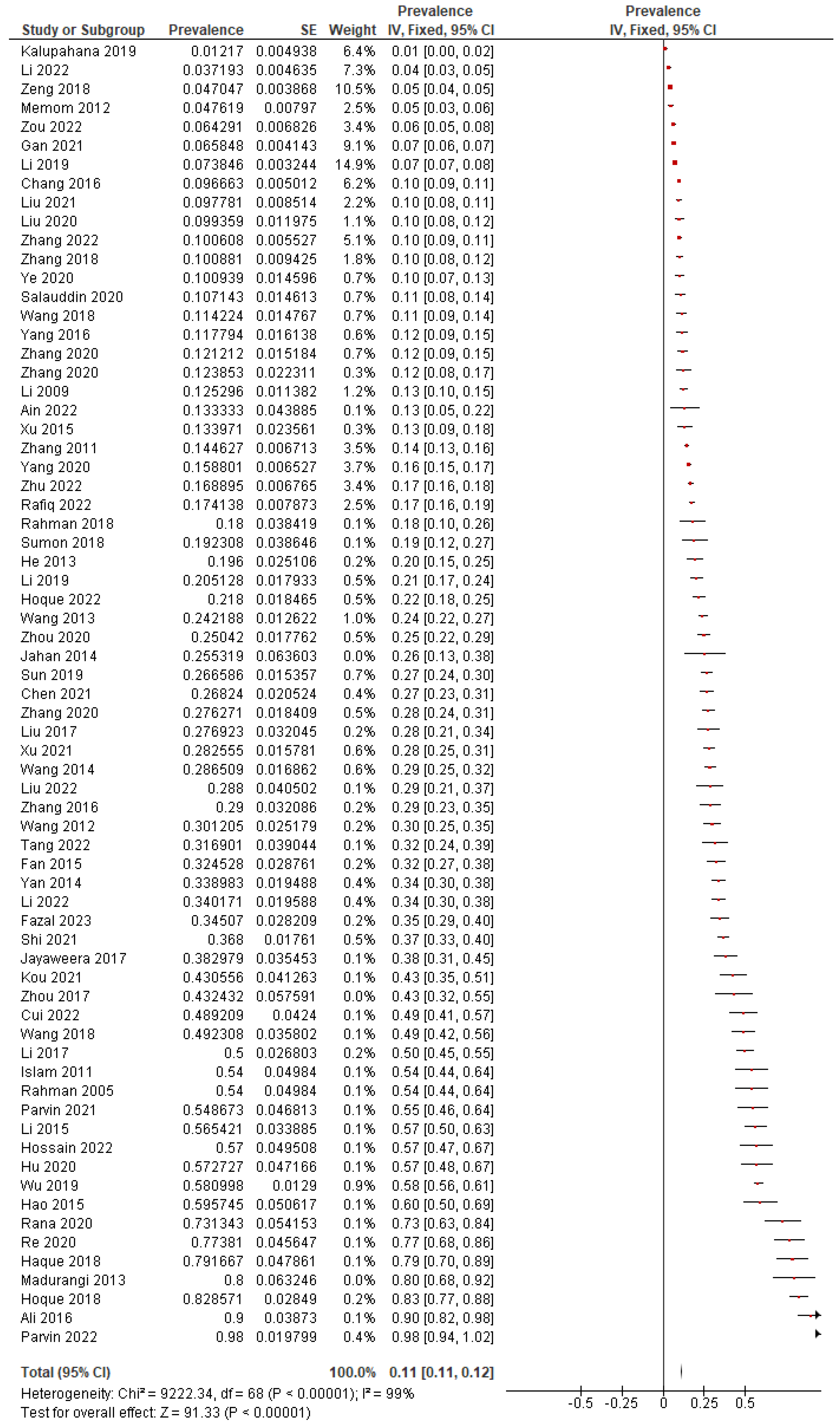
4.2. Meta-Analysis Results
4.2.1. Overall Pooled Prevalence
4.2.2. Subgroup Analyses
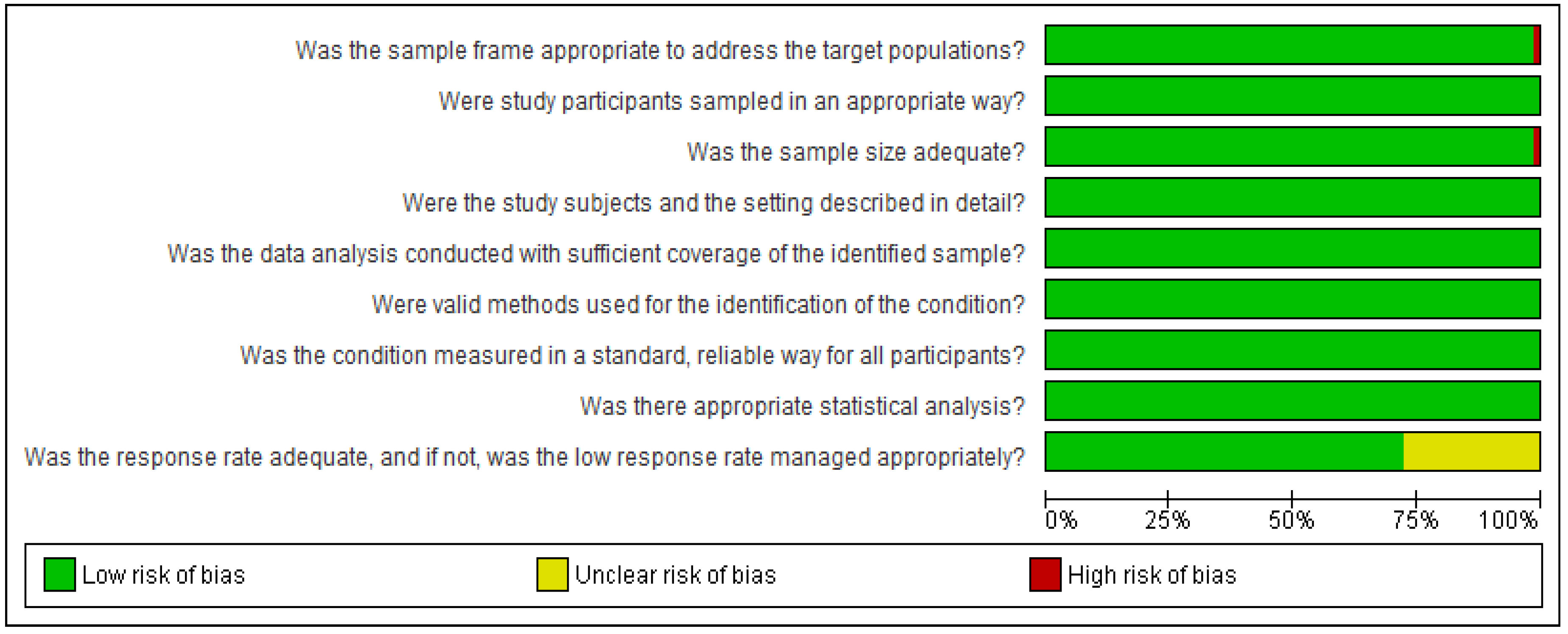
4.2.3. Risk of Bias Assessment
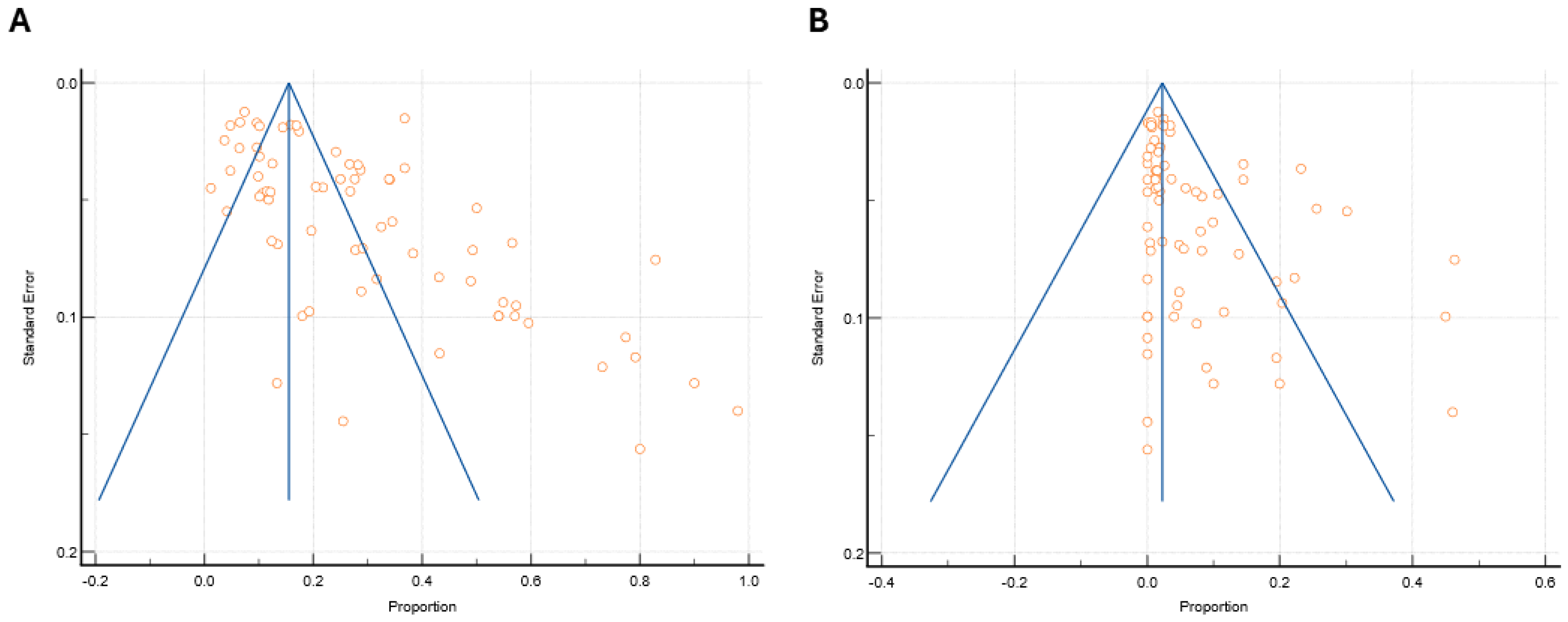
4.2.4. Publication Bias
4.2.5. Quality of Evidence According to GRADEpro Analysis
4.3. Antibiotic Resistance Characterization
4.4. Molecular Genetic Characterization
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, A.; Rai, S.; Guddattu, V.; Mukhopadhyay, C.; Saravu, K. Is Methicillin-Resistant Staphylococcus aureus Infection Associated with Higher Mortality and Morbidity in Hospitalized Patients? A Cohort Study of 551 Patients from South Western India. Risk Manag. Healthc. Policy 2018, 11, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.C.; Markey, B.K. Meticillin-Resistant Staphylococcus aureus in Animals: A Review. Vet. J. 2008, 175, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M.; the SENTRY Participants Group. Survey of Infections Due to Staphylococcus Species: Frequency of Occurrence and Antimicrobial Susceptibility of Isolates Collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 15, S114–S132. [Google Scholar] [CrossRef]
- Grema, H.A. Methicillin Resistant Staphylococcus aureus (MRSA): A Review. Adv. Anim. Vet. Sci. 2015, 3, 79–98. [Google Scholar] [CrossRef]
- Pantosti, A. Methicillin-Resistant Staphylococcus aureus Associated with Animals and Its Relevance to Human Health. Front. Microbiol. 2012, 3, 127. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M. Methicillin-Resistant Staphylococcus aureus among Animals: Current Overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef]
- Silva, V.; Monteiro, A.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Poeta, P. MRSA in Humans, Pets and Livestock in Portugal: Where We Came from and Where We Are Going. Pathogens 2022, 11, 1110. [Google Scholar] [CrossRef]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-Resistant Staphylococcus aureus in Pig Farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef]
- Van Rijen, M.M.L.; Van Keulen, P.H.; Kluytmans, J.A. Increase in a Dutch Hospital of Methicillin-Resistant Staphylococcus aureus Related to Animal Farming. Clin. Infect. Dis. 2008, 46, 261–263. [Google Scholar] [CrossRef]
- Ayliffe, G.A.J. The Progressive Intercontinental Spread of Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 1997, 24 (Suppl. S1), S74–S79. [Google Scholar] [CrossRef]
- Wong, J.W.H.; Ip, M.; Tang, A.; Wei, V.W.I.; Wong, S.Y.S.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and Risk Factors of Community-Associated Methicillin-Resistant Staphylococcus aureus Carriage in Asia-Pacific Region from 2000 to 2016: A Systematic Review and Meta-Analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Belhout, C.; Elgroud, R.; Butaye, P. Methicillin-Resistant Staphylococcus aureus (MRSA) and Other Methicillin-Resistant Staphylococci and Mammaliicoccus (MRNaS) Associated with Animals and Food Products in Arab Countries: A Review. Vet. Sci. 2022, 9, 317. [Google Scholar] [CrossRef]
- Silva, V.; Araújo, S.; Monteiro, A.; Eira, J.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Lemsaddek, T.S.; Poeta, P. Staphylococcus aureus and MRSA in Livestock: Antimicrobial Resistance and Genetic Lineages. Microorganisms 2023, 11, 124. [Google Scholar] [CrossRef]
- Sapugahawatte, D.N.; Li, C.; Yeoh, Y.K.; Dharmaratne, P.; Zhu, C.; Ip, M. Swine Methicillin-Resistant Staphylococcus aureus Carrying Toxic-Shock Syndrome Toxin Gene in Hong Kong, China. Emerg. Microbes Infect. 2020, 9, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- Ferradas, C.; Cotter, C.; Shahbazian, J.H.; Iverson, S.A.; Baron, P.; Misic, A.M.; Brazil, A.M.; Rankin, S.C.; Nachamkin, I.; Ferguson, J.M.; et al. Risk Factors for Antimicrobial Resistance among Staphylococcus Isolated from Pets Living with a Patient Diagnosed with Methicillin-Resistant Staphylococcus aureus Infection. Zoonoses Public Health 2022, 69, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.; Conceição, T.; Poirel, L.; de Lencastre, H.; Aires-De-Sousa, M. Epidemiology and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus aureus Isolates Colonizing Pigs with Different Exposure to Antibiotics. PLoS ONE 2019, 14, e0225497. [Google Scholar] [CrossRef]
- Al Amin, M.; Hoque, M.N.; Siddiki, A.Z.; Saha, S.; Kamal, M.M. Antimicrobial Resistance Situation in Animal Health of Bangladesh. Vet. World 2020, 13, 2713–2727. [Google Scholar] [CrossRef]
- Chan, O.S.K.; Wu, P.; Cowling, B.; Lee, E.; Yeung, M.; St-Hilaire, S.; Tun, H.; Wernli, D.; Lam, W. Prescribing Antibiotics Prudently—A Survey of Policy Implementation Drivers among Physicians and Veterinarians. One Health 2024, 18, 100752. [Google Scholar] [CrossRef]
- Wu, Z. Antibiotic Use and Antibiotic Resistance in Food-Producing Animals in China; OECD Publishing: Paris, France, 2019; Volume 134, pp. 1–25. [Google Scholar] [CrossRef]
- Xiao, Y. Antimicrobial Stewardship in China: Systems, Actions and Future Strategies. Clin. Infect. Dis. 2018, 67, S135–S141. [Google Scholar] [CrossRef]
- Piso, R.J.; Käch, R.; Pop, R.; Zillig, D.; Schibli, U.; Bassetti, S.; Meinel, D.; Egli, A. A Cross-Sectional Study of Colonization Rates with Methicillin-Resistant Staphylococcus aureus (MRSA) and Extended-Spectrum Beta-Lactamase (ESBL) and Carbapenemase-Producing Enterobacteriaceae in Four Swiss Refugee Centres. PLoS ONE 2017, 12, e0170251. [Google Scholar] [CrossRef]
- Moxnes, J.F.; de Blasio, B.F.; Leegaard, T.M.; Moen, A.E.F. Methicillin-Resistant Staphylococcus aureus (MRSA) Is Increasing in Norway: A Time Series Analysis of Reported MRSA and Methicillin-Sensitive S. aureus Cases, 1997–2010. PLoS ONE 2013, 8, e70499. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The Development of a Critical Appraisal Tool for Use in Systematic Reviews Addressing Questions of Prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Purrello, S.M.; Daum, R.S.; Edwards, G.F.S.; Lina, G.; Lindsay, J.; Peters, G.; Stefani, S. Meticillin-Resistant Staphylococcus aureus (MRSA) Update: New Insights into Bacterial Adaptation and Therapeutic Targets. J. Glob. Antimicrob. Resist. 2014, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Zhou, J.; Lin, D.; Bai, C.; Zhang, T.; Lin, J.; Zheng, H.; Wang, X.; Ye, J.; Ye, X.; et al. A Large Meta-Analysis of the Global Prevalence Rates of S. aureus and MRSA Contamination of Milk. Crit. Rev. Food Sci. Nutr. 2018, 58, 2213–2228. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Rev. Esp. Nutr. Humana Diet. 2016, 20, 148–160. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Barua, N.; Rahman, N.; Ip, M.; Luo, M.; Liyanapathirana, V.; Prevalence, F.A. PROSPERO International Prospective Register of Systematic Reviews Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry in Mainland China, Hong Kong SAR, Sri Lanka, and Bangladesh: Systematic Review and Meta-Analysis. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023420188 (accessed on 15 January 2024).
- Pega, F.; Momen, N.C.; Bero, L.; Whaley, P. Towards a Framework for Systematic Reviews of the Prevalence of Exposure to Environmental and Occupational Risk Factors. Environ. Health 2022, 21, 64. [Google Scholar] [CrossRef]
- Munn, Z.; MClinSc, S.M.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Whitehead, A.; Simmonds, M. Sequential Methods for Random-Effects Meta-Analysis. Stat. Med. 2011, 30, 903–921. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Rahman, N.; Leung, A.; Ip, M. Is There a Role of Faecal Microbiota Transplantation in Reducing Antibiotic Resistance Burden in Gut? A Systematic Review and Meta-Analysis. Ann. Med. 2021, 53, 662–681. [Google Scholar] [CrossRef]
- Van Enst, W.A.; Ochodo, E.; Scholten, R.J.; Hooft, L.; Leeflang, M.M. Investigation of Publication Bias in Meta-Analyses of Diagnostic Test Accuracy: A Meta-Epidemiological Study. BMC Med. Res. Methodol. 2014, 14, 70. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H.; Murad, M.H.; Hong, C.; Qu, Z.; Cole, S.R.; Chen, Y. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.T.; Arif, M.; Ema, F.A.; Shathi, M.S.A.; Islam, M.A.; Khatun, M.M. Multidrug-Resistant Staphylococcus aureus Isolated from Chicken Nuggets Sold at Superstores in Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2022, 9, 601–609. [Google Scholar] [CrossRef]
- Ali, Y.; Islam, M.A.; Muzahid, N.H.; Sikder, M.O.F.; Hossain, M.A.; Marzan, L.W. Characterization, Prevalence and Antibiogram Study of Staphylococcus aureus in Poultry. Asian Pac. J. Trop. Biomed. 2017, 7, 253–256. [Google Scholar] [CrossRef]
- Chang, Y.; Gao, H.; Zhu, Z.; Ye, S.; Yang, Y.; Shen, X.; Zhang, D.; Song, Q. High Prevalence and Properties of Enterotoxin-Producing Staphylococcus aureus ST5 Strains of Food Sources in China. Foodborne Pathog. Dis. 2016, 13, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ali, T.; Li, J.; Song, L.; Shen, S.; Li, T.; Zhang, C.; Cheng, M.; Zhao, Q.; Wang, H. New Clues about the Global MRSA ST398: Emergence of MRSA ST398 from Pigs in Qinghai, China. Int. J. Food Microbiol. 2022, 378, 109820. [Google Scholar] [CrossRef]
- Haque, Z.F.; Sabuj, A.A.M.; Mahmud, M.M.; Pondit, A.; Islam, M.A.; Saha, S. Characterization of Staphylococcus aureus from Milk and Dairy Products Sold in Some Local Markets of Mymensingh District of Bangladesh. J. Nutr. Food Sci. 2018, 8, 1000743. [Google Scholar] [CrossRef]
- Hossain, M.J.; Sohidullah, M.; Alam, M.A.; Al Mamun, M.S.; Badr, Y.; Altaib, H.; Rahman, M.M. Molecular Detection of Methicillin Resistant Staphylococcus aureus (MRSA) in Poultry in Bangladesh: Having Public Health Significance. Eur. J. Vet. Med. 2022, 2, 17–21. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, M.M.; Uddin, M.S.; Kobayashi, N.; Ahmed, M.U. Detection of Methicillin-resistant Staphylococcus aureus (MRSA) from Animal and Human Origin in Bangladesh by Polymerase Chain Reaction. Bangladesh J. Vet. Med. 2011, 9, 161–166. [Google Scholar] [CrossRef]
- Jahan, M.; Rahman, M.; Parvej, M.S.; Chowdhury, S.M.Z.H.; Haque, M.E.; Talukder, M.A.K.; Ahmed, S. Isolation and Characterization of Staphylococcus aureus from Raw Cow Milk in Bangladesh. J. Adv. Vet. Anim. Res. 2015, 2, 49–55. [Google Scholar] [CrossRef]
- Kou, X.; Cai, H.; Huang, S.; Ni, Y.; Luo, B.; Qian, H.; Ji, H.; Wang, X. Prevalence and Characteristics of Staphylococcus aureus Isolated From Retail Raw Milk in Northern Xinjiang, China. Front. Microbiol. 2021, 12, 705947. [Google Scholar] [CrossRef]
- Li, S.M.; Fang, L.X.; Li, L.; Zhao, M.; Lu, X.; Gu, W.Q.; Liao, X.P.; Sun, J.; Xiong, Y.Q.; Liu, Y.H. Investigation on the Antibiotic Resistance of Staphylococcus Methicillin-Resistant MLSB from Food Animals in Six Provinces of China. Sci. Agric. Sin. 2019, 52, 1646–1656. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Pan, Y.; Liu, C.; Liang, S.; Zeng, Z. The Emergence and Molecular Study of Methicillin-Resistant Staphylococcus aureus ST239, ST59, ST9, and ST630 in Food Animals, Chongqing, China. Vet. Microbiol. 2022, 265, 109329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, X.; Xu, L.; Tong, P.; Zhu, M.; Peng, B.; Yao, G. Prevalence, Antimicrobial Resistance, and Molecular Characterization of Staphylococcus aureus Isolated from Animals, Meats, and Market Environments in Xinjiang, China. Foodborne Pathog. Dis. 2021, 18, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Madurangi, G.H.P. Prevalence of Escherichia coli, Salmonella serovars and Staphylococcus aureus in Retail Chicken from Badulla District, Sri Lanka. UWU Erepository. 2013. Available online: http://ir.kdu.ac.lk/handle/345/1568 (accessed on 23 January 2024).
- Rahman, M.M.; Amin, K.B.; Rahman, S.M.M.; Khair, A.; Rahman, M.; Hossain, A.; Rahman, A.K.M.A.; Parvez, M.S.; Miura, N.; Alam, M.M. Investigation of Methicillin-Resistant Staphylococcus aureus among Clinical Isolates from Humans and Animals by Culture Methods and Multiplex PCR. BMC Vet. Res. 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tao, X.; Xia, X.; Yang, B.; Xi, M.; Meng, J.; Zhang, J.; Xu, B. Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus in Retail Raw Chicken in China. Food Control 2013, 29, 103–106. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Jiang, T.; Peng, Z.; Xu, J.; Yi, L.; Li, F.; Fanning, S.; Baloch, Z. Prevalence and Characterization of Staphylococcus aureus Cultured from Raw Milk Taken from Dairy Cows with Mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, L.; Xu, X.; Zhou, G. Prevalence, Genetic Characterization and Biofilm Formation in Vitro of Staphylococcus aureus Isolated from Raw Chicken Meat at Retail Level in Nanjing, China. Food Control 2018, 86, 11–18. [Google Scholar] [CrossRef]
- Yan, X.; Yu, X.; Tao, X.; Zhang, J.; Zhang, B.; Dong, R.; Xue, C.; Grundmann, H.; Zhang, J. Staphylococcus aureus ST398 from Slaughter Pigs in Northeast China. Int. J. Med. Microbiol. 2014, 304, 379–383. [Google Scholar] [CrossRef]
- Liu, K.; Tao, L.; Li, J.; Fang, L.; Cui, L.; Li, J.; Meng, X.; Zhu, G.; Bi, C.; Wang, H. Characterization of Staphylococcus aureus isolates from cases of clinical bovine mastitis on large-scale Chinese dairy farms. Front. Vet. Sci. 2020, 7, 580129. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, X.; Wang, X.; Li, H. Detection of Antibiotic Resistance, Virulence Gene, and Drug Resistance Gene of Staphylococcus aureus Isolates from Bovine Mastitis. Microbiol. Spectr. 2022, 10, e0047122. [Google Scholar] [CrossRef]
- Parvin, M.S.; Ali, M.Y.; Mandal, A.K.; Talukder, S.; Islam, M.T. Sink Survey to Investigate Multidrug Resistance Pattern of Common Foodborne Bacteria from Wholesale Chicken Markets in Dhaka City of Bangladesh. Sci. Rep. 2022, 12, 10818. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Islam, M.R.; Siddiky, N.A.; Samad, M.A.; Chowdhury, S.; Hossain, K.M.M.; Rume, F.I.; Hossain, M.K.; Mahbub-E-Elahi, A.T.M.; Ali, M.Z.; et al. Antimicrobial Resistance Profile of Common Foodborne Pathogens Recovered from Livestock and Poultry in Bangladesh. Antibiotics 2022, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.-H.; Zhang, X.-F.; Wang, J.; Ma, Z.-B.; Chen, L.; Zeng, Z.-L. Emergence of Methicillin-Resistant Staphylococcus aureus ST398 in Pigs in China. Int. J. Antimicrob. Agents 2018, 51, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, L.; Li, G.; Pan, Y.; Lu, Y.; Chen, J.; Xiong, W.; Zeng, Z. Prevalence and Genetic Characteristics of FosB-Positive Staphylococcus aureus in Duck Farms in Guangdong, China in 2020. J. Antimicrob. Chemother. 2023, 78, 802–809. [Google Scholar] [CrossRef]
- Hoque, M.N.; Das, Z.C.; Rahman, A.N.M.A.; Haider, M.G.; Islam, M.A. Molecular Characterization of Staphylococcus aureus Strains in Bovine Mastitis Milk in Bangladesh. Int. J. Vet. Sci. Med. 2018, 6, 53–60. [Google Scholar] [CrossRef]
- Fazal, M.A.; Rana, E.A.; Akter, S.; Alim, M.A.; Barua, H.; Ahad, A. Molecular Identification, Antimicrobial Resistance and Virulence Gene Profiling of Staphylococcus spp. Associated with Bovine Sub-Clinical Mastitis in Bangladesh. Vet. Anim. Sci. 2023, 21, 100297. [Google Scholar] [CrossRef]
- Rahman, M.T.; Kobayashi, N.; Mahbub Alam, M.; Ishino, M. Genetic Analysis of cecA Homologues in Staphylococcus sciuri Strains Derived from Mastitis in Dairy Cattle. Microb. Drug Resist. 2005, 11, 205–214. [Google Scholar] [CrossRef]
- Rana, E.A.; Das, T.; Dutta, A.; Rahman, M.; Bostami, M.B.; Akter, N.; Barua, H. Coagulase-Positive Methicillin-Resistant Staphylococcus aureus Circulating in Clinical Mastitic Goats in Bangladesh. Vet. World 2020, 13, 1303–1310. [Google Scholar] [CrossRef]
- Hao, D.; Xing, X.; Li, G.; Wang, X.; Zhang, M.; Zhang, W.; Xia, X.; Meng, J. Prevalence, Toxin Gene Profiles, and Antimicrobial Resistance of Staphylococcus aureus Isolated from Quick-Frozen Dumplings. J. Food Prot. 2015, 78, 218–223. [Google Scholar] [CrossRef]
- Hoque, M.N.; Talukder, A.K.; Saha, O.; Hasan, M.M.; Sultana, M.; Rahman, A.A.; Das, Z.C. Antibiogram and Virulence Profiling Reveals Multidrug Resistant Staphylococcus aureus as the Predominant Aetiology of Subclinical Mastitis in Riverine Buffaloes. Vet. Med. Sci. 2022, 8, 2631–2645. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Meng, L.; Dong, L.; Zhao, S.; Lan, X.; Wang, J.; Zheng, N. Prevalence, Antimicrobial Susceptibility, and Molecular Characterization of Staphylococcus aureus Isolated from Dairy Herds in Northern China. J. Dairy. Sci. 2017, 100, 8796–8803. [Google Scholar] [CrossRef]
- Li, J.P.; Zhou, H.J.; Yuan, L.; He, T.; Hu, S.H. Prevalence, Genetic Diversity, and Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolated from Bovine Mastitis in Zhejiang Province, China. J. Zhejiang Univ. Sci. B 2009, 10, 753–760. [Google Scholar] [CrossRef]
- Liu, H.; Dong, L.; Zhao, Y.; Meng, L.; Wang, J.; Wang, C.; Zheng, N. Antimicrobial Susceptibility, and Molecular Characterization of Staphylococcus aureus Isolated From Different Raw Milk Samples in China. Front. Microbiol. 2022, 13, 840670. [Google Scholar] [CrossRef]
- Parvin, M.S.; Ali, M.Y.; Talukder, S.; Nahar, A.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Prevalence and Multidrug Resistance Pattern of Methicillin Resistant S. aureus Isolated from Frozen Chicken Meat in Bangladesh. Microorganisms 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Tang, Y.; Meng, C.; Ingmer, H.; Jiao, X. Prevalence and Characterization of Staphylococcus aureus and Staphylococcus argenteus in Chicken from Retail Markets in China. Food Control 2019, 96, 158–164. [Google Scholar] [CrossRef]
- Ren, Q.; Liao, G.; Wu, Z.; Lv, J.; Chen, W. Prevalence and Characterization of Staphylococcus aureus Isolates from Subclinical Bovine Mastitis in Southern Xinjiang, China. J. Dairy. Sci. 2020, 103, 3368–3380. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yu, Z.; Ho, H.; Wang, J.; Wu, W.; Xing, M.; Wang, Y.; Rahman, S.M.E.; Han, R. Occurrence, Antimicrobial Resistance Patterns, and Genetic Characterization of Staphylococcus aureus Isolated from Raw Milk in the Dairy Farms over Two Seasons in China. Microb. Drug Resist. 2021, 27, 99–110. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Xia, X.; Yang, B.; Xi, M.; Meng, J. Antimicrobial Susceptibility and Molecular Typing of Methicillin-Resistant Staphylococcus aureus in Retail Foods in Shaanxi, China. Foodborne Pathog. Dis. 2014, 11, 281–286. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, F.; Wu, Q.; Zhang, J.; Pang, R.; Zeng, H.; Yang, X.; Chen, M.; Wang, J.; et al. Prevalence and Characterization of Food-Related Methicillin-Resistant Staphylococcus aureus (MRSA) in China. Front. Microbiol. 2019, 10, 304. [Google Scholar] [CrossRef]
- Xu, J.; Tan, X.; Zhang, X.; Xia, X.; Sun, H. The Diversities of Staphylococcal Species, Virulence and Antibiotic Resistance Genes in the Subclinical Mastitis Milk from a Single Chinese Cow Herd. Microb. Pathog. 2015, 88, 29–38. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Yu, S.; Wu, Q.; Guo, W.; Huang, J.; Cai, S. Prevalence of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus in Retail Ready-to-Eat Foods in China. Front. Microbiol. 2016, 7, 816. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Shang, X.; Li, H.; Zhang, H.; Cui, D.; Wang, X.; Wang, L.; Yan, Z.; Sun, Y. Short Communication: Detection and Molecular Characterization of Methicillin-Resistant Staphylococcus aureus Isolated from Subclinical Bovine Mastitis Cases in China. J. Dairy. Sci. 2020, 103, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Hou, F.; Xu, D.; Huang, Q.; Chen, X.; Zeng, Z.; Peng, Y.; Fang, R. Prevalence and Characterisation of Class 1 and 2 Integrons in Multi-Drug Resistant Staphylococcus aureus Isolates from Pig Farms in Chongqing, China. J. Vet. Res. 2020, 64, 381–386. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, M.; Li, H.; Yang, H.; Li, X.; Song, X.; Wang, Z. Prevalence and Molecular Characterization of Staphylococcus aureus Isolated from Goats in Chongqing, China. BMC Vet. Res. 2017, 13, 352. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Yin, C. Antibiotic Resistance and Molecular Characteristics of Staphylococcus aureus Isolated from Pigs in Hunan, China. Pol. J. Vet. Sci. 2020, 23, 563–570. [Google Scholar] [CrossRef]
- Li, X.; Xie, L.; Huang, H.; Li, Z.; Li, G.; Liu, P.; Xiao, D.; Zhang, X.; Xiong, W.; Zeng, Z. Prevalence of Livestock-Associated MRSA ST398 in a Swine Slaughterhouse in Guangzhou, China. Front. Microbiol. 2022, 13, 914764. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, R.S.; Duim, B.; Verstappen, K.M.; Gamage, C.D.; Dissanayake, N.; Ranatunga, L.; Graveland, H.; Wagenaar, J.A. MRSA in Pigs and the Environment as a Risk for Employees in Pig-Dense Areas of Sri Lanka. Front. Sustain. Food Syst. 2019, 3, 25. [Google Scholar] [CrossRef]
- Salauddin, M.; Akter, M.R.; Hossain, M.K.; Nazir, K.H.M.N.H.; Noreddin, A.; El Zowalaty, M.E. Molecular Detection of Multidrug Resistant Staphylococcus aureus Isolated from Bovine Mastitis Milk in Bangladesh. Vet. Sci. 2020, 7, 36. [Google Scholar] [CrossRef]
- Sumon, S.M.M.R.; Haider, M.G.; Islam, M.A.; Siddiki, S.H.M.F.; Karim, M.R. Prevalence and Antibiogram Profile of Staphylococcus aureus Isolated from Milk Samples of Lactating Cows with Subclinical Mastitis in Gazipur, Bangladesh. An. Bangladesh Agric. 2018, 22, 51–60. [Google Scholar]
- Zhang, L.; Li, Y.; Bao, H.; Wei, R.; Zhou, Y.; Zhang, H.; Wang, R. Population Structure and Antimicrobial Profile of Staphylococcus aureus Strains Associated with Bovine Mastitis in China. Microb. Pathog. 2016, 97, 103–109. [Google Scholar] [CrossRef]
- Chen, C.; Sun, C.; Li, J.; Ji, X.; Wang, Y.; Song, C.; Wang, G. Characterisation of Staphylococcus aureus Isolates from Bovine Mastitis in Ningxia, Western China. J. Glob. Antimicrob. Resist. 2021, 25, 232–237. [Google Scholar] [CrossRef]
- Fan, R.; Li, D.; Wang, Y.; He, T.; Feßler, A.T.; Schwarz, S.; Wu, C. Presence of the optrA Gene in Methicillin-Resistant Staphylococcus sciuri of Porcine Origin. Antimicrob. Agents Chemother. 2016, 60, 7200–7205. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Shu, G.; Fu, H.; Yan, Q.; Zhang, W.; Tang, H.; Yin, L.; Zhao, L.; Lin, J. Antimicrobial Resistance and Genotyping of Staphylococcus aureus Obtained from Food Animals in Sichuan Province, China. BMC Vet. Res. 2021, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Hu, J.; Chen, J.; Lu, Y.; Kong, L.; Diao, L.; Zhang, F.; Xiong, W.; Zeng, Z. Relationship Between Biofilm Formation and Molecular Typing of Staphylococcus aureus from Animal Origin. Sci. Agric. Sin. 2022, 55, 602–612. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Qi, J.; Chen, H.; Zhao, C.; Zhang, F.; Li, H.; Wang, H. Food-Animal Related Staphylococcus aureus Multidrug-Resistant ST9 Strains with Toxin Genes. Foodborne Pathog. Dis. 2013, 10, 782–788. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Zhou, T.; Zhang, Y.; Yang, B.; Xi, M.; Sheng, J.; Zhi, S.; Xia, X. Antimicrobial Susceptibility Testing and Genotypic Characterization of Staphylococcus aureus from Food and Food Animals. Foodborne Pathog. Dis. 2012, 9, 95–101. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, Z.; Wang, Y.; Cao, X.; Logue, C.M.; Wang, B.; Yang, J.; Shen, J.; Wu, C. Molecular Characterization of Methicillin-Resistant Staphylococcus aureus Strains from Pet Animals and Veterinary Staff in China. Vet. J. 2011, 190, e125–e129. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Barkema, H.W.; Ali, T.; Liu, G.; Deng, Y.; Naushad, S.; Kastelic, J.P.; Han, B. Virulence Gene Profiles: Alpha-Hemolysin and Clonal Diversity in Staphylococcus aureus Isolates from Bovine Clinical Mastitis in China. BMC Vet. Res. 2018, 14, 63. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Yan, H. Genotypic Characteristics and Correlation of Epidemiology of Staphylococcus aureus in Healthy Pigs, Diseased Pigs, and Environment. Antibiotics 2020, 9, 839. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, X.; Chen, X.; Zou, G.; Huang, Q.; Meng, X.; Pei, X.; Chen, Z.; Zhou, R.; Hu, D.; et al. Prevalence and Virulence Determinants of Staphylococcus aureus in Wholesale and Retail Pork in Wuhan, Central China. Foods 2022, 11, 4114. [Google Scholar] [CrossRef]
- Zou, G.; Matuszewska, M.; Bai, F.; Wang, S.; Wang, S.; Li, H.; Ke, Y.; Tang, C.; Li, J.; Tang, J.; et al. Genomic Analyses of Staphylococcus aureus Isolated from Yaks in Ganzi Tibetan Autonomous Prefecture, China. J. Antimicrob. Chemother. 2022, 77, 910–920. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Tan, W.; Cui, H.; Zhu, Z.; Yang, C.; Huang, Q.; Meng, X.; Li, S. Prevalence and Antimicrobial Resistance of Salmonella and Staphylococcus aureus in Fattening Pigs in Hubei Province, China. Microb. Drug Resist. 2021, 27, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Zhang, J.; Fu, X.; Wan, Y.; Pan, H.; Wu, C.; Wang, X. Prevalence and Characterization of Staphylococcus Aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Retail Yak Butter in Tibet, China. J. Dairy. Sci. 2021, 104, 9596–9606. [Google Scholar] [CrossRef]
- Zhang, D.X.; Li, Y.; Yang, X.Q.; Su, H.Y.; Wang, Q.; Zhang, Z.H.; Liu, Y.C.; Tian, C.L.; Cui, C.C.; Liu, M.C. In Vitro Antibiotic Susceptibility, Virulence Genes Distribution and Biofilm Production of Staphylococcus aureus Isolates from Bovine Mastitis in the Liaoning Province of China. Infect. Drug Resist. 2020, 13, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, L.; Wang, L.; Xue, H.; Zhao, X. Characterization of Methicillin-Resistant and-Susceptible Staphylococcal Isolates from Bovine Milk in Northwestern China. PLoS ONE 2015, 10, e0116699. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, B.; Hulth, A.; Schwarz, S.; Ji, X.; Nilsson, L.E.; Ma, S.; Sun, Q.; Bi, Z.; Wang, Y.; et al. Genomic Analysis of Staphylococcus aureus along a Pork Production Chain and in the Community, Shandong Province, China. Int. J. Antimicrob. Agents 2019, 54, 8–15. [Google Scholar] [CrossRef]
- Jayaweera, J.A.A.S.; Kumbukgolla, W.W. Antibiotic Resistance Patterns of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolated from Livestock and Associated Farmers in Anuradhapura, Sri Lanka. Germs 2017, 7, 132–139. [Google Scholar] [CrossRef]
- Li, B.; Yao, J.; Wei, J.; Shao, D.; Liu, K.; Qiu, Y.; Ma, Z. Characterisation of Fluoroquinolone Resistance in Methicillin-Resistant Staphylococcus aureus of Human and Pig Origin. J. Glob. Antimicrob. Resist. 2018, 12, 77–78. [Google Scholar] [CrossRef]
- Li, J.; Jiang, N.; Ke, Y.; Feßler, A.T.; Wang, Y.; Schwarz, S.; Wu, C. Characterization of Pig-Associated Methicillin-Resistant Staphylococcus aureus. Vet. Microbiol. 2017, 201, 183–187. [Google Scholar] [CrossRef]
- Memon, J.; Kashif, J.; Yaqoob, M.; Wang, L.P.; Yang, Y.C.; Fan, H. Molecular characterization and antimicrobial sensitivity of pathogens from sub-clinical and clinical mastitis in eastern China. Pak. Vet. J. 2013, 33, 170–174. [Google Scholar]
- Ribeiro, C.M.; Stefani, L.M.; Lucheis, S.B.; Okano, W.; Cruz, J.C.M.; Souza, G.V.; Casagrande, T.A.C.; Bastos, P.A.S.; Pinheiro, R.R.; Arruda, M.M.; et al. Methicillin-Resistant Staphylococcus aureus in Poultry and Poultry Meat: A Meta-Analysis. J. Food Prot. 2018, 81, 1055–1062. [Google Scholar] [CrossRef] [PubMed]


| Number of Studies | MRSA Prevalence % (95% CI) | * I2% | p-Value (Between-Group) | |
|---|---|---|---|---|
| Country | <0.00001 | |||
| Bangladesh | 17 | 12.29 (7.09 to 18.67) | 96.00% | |
| China and HK | 49 | 4.65 (3.59 to 5.86) | 9.00% | |
| Sri Lanka | 3 | 3.83 (0.003 to 15.09) | 96.00% | |
| 69 | ||||
| Detection method for MRSA | <0.00001 | |||
| Broth microdilution or agar dilution | 15 | 1.67 (0.96 to 2.56) | 88.00% | |
| mecA and/or mecC PCR | 28 | 5.20 (3.58 to 7.11) | 97.00% | |
| Disk diffusion | 22 | 5.54 (3.18 to 8.51) | 96.00% | |
| Selective media | 2 | 5.56 (0.41 to 26.47) | 99.00% | |
| No method mentioned (excluded from subgroup analysis) | 2 | --- | --- | |
| 69 | ||||
| Pre-enrichment before S. aureus detection | <0.00001 | |||
| Yes | 53 | 5.41 (3.94 to 7.11) | 97.00% | |
| No | 7 | 2.54 (1.61 to 3.68) | 72.00% | |
| Not mentioned (excluded from subgroup analysis) | 9 | --- | --- | |
| 69 | ||||
| Study period | <0.00001 | |||
| 2000–2009 | 2 | 1.19 (0.67 to 8.74) | ** Not applicable | |
| 2010–2019 | 35 | 3.62 (2.31 to 5.20) | 97.00% | |
| 2020–now | 32 | 6.25 (4.45 to 8.32) | 97.00% | |
| 69 | ||||
| Study location | <0.00001 | |||
| Urban | 40 | 4.15 (2.88 to 5.63) | 99.00% | |
| Rural | 11 | 9.49 (4.49 to 16.10) | 98.00% | |
| Not mentioned (excluded from subgroup analysis) | 18 | -- | -- | |
| 69 | ||||
| Study Population | MRSA Prevalence % (95% CI) | * I2% | p-Value (Between-Group) | |
|---|---|---|---|---|
| <0.00001 | ||||
| Buffalos (Excluded from subgroup analysis) | 1 | -- | -- | |
| Cats | 2 | 0.11 (0.005 to 0.36) | ** Not applicable | |
| Chickens | 10 | 3.05 (1.12 to 5.88) | 95.00% | |
| Cows | 11 | 1.91 (0.56 to 4.05) | 75.00% | |
| Dogs | 2 | 4.98 (0.0004 to 19.07) | 70.00% | |
| Ducks | 3 | 1.26 (0.45 to 2.48) | 53.00% | |
| Goats | 4 | 2.60 (0.16 to 7.87) | 9.00% | |
| Pigs | 19 | 5.96 (3.59 to 8.87) | 96.00% | |
| Raw Milk | 26 | 4.23 (2.30 to 6.73) | 99.00% | |
| Sheep (Excluded from subgroup analysis) | 1 | -- | -- | |
| Yaks | 2 | 1.25 (0.10 to 3.65) | 65.00% | |
| Beef | 4 | 2.20 (0.20 to 6.28) | 96.00% | |
| Chicken meat | 8 | 8.42 (3.15 to 15.92) | 93.00% | |
| Duck meat (Excluded from subgroup analysis) | 1 | -- | -- | |
| Pork | 8 | 3.94 (1.26 to 8.02) | 89.00% | |
| Certainty Assessment | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | No. of Events | Total Samples | Relative Ratio (95% * CI) | ||
| Prevalence of MRSA among livestock, including poultry, cattle, pigs, and companion animals (follow-up: range 6 weeks to 8 weeks; assessed with prevalence, scale from 0 to 1) | |||||||||||
| 68 | Non-randomized studies | Not serious | Serious | Not serious | Serious | Strong association, all plausible residual confounding evidence would reduce the demonstrated effect | 1766 | 55,417 | 0.03 (0.01 to 0.02) | ⨁⨁⨁⨁ High | Critical |
| Prevalence of MRSA among livestock according to detection method; broth microdilution or agar dilution (follow-up: range 4 weeks to 6 weeks; assessed with prevalence; scale from 0 to 1) | |||||||||||
| 15 | Non-randomized studies | Not serious | Serious | Not serious | Not serious | None | 209 | 19,442 | 0.01 (001 to 0.01) | ⨁⨁⨁◯ Moderate | Important |
| Prevalence of MRSA among livestock according to detection method; mecA or mecC (follow-up: range 6 weeks to 8 weeks; assessed with prevalence; scale from 0 to 1) | |||||||||||
| 28 | Non-randomized studies | Not serious | Serious | Not serious | Serious | None | 775 | 25,151 | 0.03 (0.01 to 0.01) | ⨁⨁◯◯ Low | Important |
| Prevalence of MRSA among livestock according to detection method; disk diffusion (follow-up: range 6 weeks to 8 weeks; assessed with prevalence, scale from 0 to 1) | |||||||||||
| 22 | Non-randomized studies | Not serious | Serious | Not serious | Not serious | None | 617 | 12,824 | 0.05 (0.02 to 0.05) | ⨁⨁⨁◯ Moderate | Important |
| Prevalence of MRSA among livestock in the pre-enrichment samples (follow-up: range 6 weeks to 8 weeks; assessed with prevalence, scale from 0 to 1) | |||||||||||
| 53 | Non-randomized studies | Not serious | Serious | Not serious | Serious | Publication bias suspected, strong association, all plausible residual confounding evidence would reduce the demonstrated effect | 1283 | 39,599 | 0.03 (0.01 to 0.02) | ⨁⨁⨁◯ Moderate | Important |
| Prevalence of MRSA among livestock in the samples without pre-enrichment (follow-up: range 6 weeks to 8 weeks; assessed with prevalence, scale from 0 to 1) | |||||||||||
| 7 | Non-randomized studies | Not serious | Serious | Not serious | Not serious | Strong association, all plausible residual confounding evidence would reduce the demonstrated effect | 123 | 5327 | 0.02 (0.02 to 0.02) | ⨁⨁⨁⨁ High | Important |
| Prevalence of MRSA among livestock based on farm location; urban farm (follow-up: range 4 weeks to 6 weeks; assessed with prevalence, scale from 0 to 1) | |||||||||||
| 40 | Non-randomized studies | Not serious | Serious | Not serious | Serious | None | 834 | 27,540 | 0.03 (0.02 to 0.025) | ⨁⨁◯◯ Low | Important |
| Prevalence of MRSA among livestock based on farm location; rural farm (follow-up: range 4 weeks to 8 weeks; assessed with prevalence, scale from 0 to 1) | |||||||||||
| 10 | Non-randomized studies | Not serious | Not serious | Not serious | Not serious | None | 405 | 10,722 | 0.037 (0.01 to 0.02) | ⨁⨁⨁⨁ High | Important |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, N.; Rahman, N.; Tin, M.C.F.; Yang, L.; Alim, A.; Akther, F.; Handapangoda, N.; Manathunga, T.A.; Jinadasa, R.N.; Liyanapathirana, V.; et al. Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis. Microorganisms 2025, 13, 704. https://doi.org/10.3390/microorganisms13040704
Barua N, Rahman N, Tin MCF, Yang L, Alim A, Akther F, Handapangoda N, Manathunga TA, Jinadasa RN, Liyanapathirana V, et al. Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis. Microorganisms. 2025; 13(4):704. https://doi.org/10.3390/microorganisms13040704
Chicago/Turabian StyleBarua, Nilakshi, Nannur Rahman, Martha C. F. Tin, Liuyue Yang, Abdul Alim, Farhana Akther, Nelum Handapangoda, Thamali Ayeshcharya Manathunga, Rasika N. Jinadasa, Veranja Liyanapathirana, and et al. 2025. "Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis" Microorganisms 13, no. 4: 704. https://doi.org/10.3390/microorganisms13040704
APA StyleBarua, N., Rahman, N., Tin, M. C. F., Yang, L., Alim, A., Akther, F., Handapangoda, N., Manathunga, T. A., Jinadasa, R. N., Liyanapathirana, V., Luo, M., & Ip, M. (2025). Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis. Microorganisms, 13(4), 704. https://doi.org/10.3390/microorganisms13040704






