Abstract
Anthracnose is a widespread plant disease affecting vegetables, flowers, crops, and fruit trees, causing significant economic losses. It occurs at various stages of pepper growth, leading to rotting and shedding in later stages. The aim of this study was to explore the relationship with anthracnose occurrence by analyzing the physicochemical properties and microbiota changes in the inter-root soil of pepper under different susceptibility levels to reveal the key microecological factors and dominant microbial populations and to provide reference for ecological control. Illumina Miseq sequencing was first used to evaluate the physicochemical properties and microbial taxa in pepper inter-root soil across different health statuses and identify key parameters associated with anthracnose. Subsequently, PICRUSt2 (systematic genetic Investigation of communities by Reconstruction of observed States 2) and FUNGuild (Fungi Functional Guild) V1.0 online platform were used to predict the activities of inter-root bacteria and fungi. The findings indicated that healthy peppers had significantly higher inter-root soil nutrient levels and enzyme activity compared to sensitive peppers. There were significant differences between their community structures. In alpha-diversity analysis, inter-root soil microbial richness and diversity were significantly higher in healthy peppers than in susceptible peppers. At the bacterial taxonomic level, the comparative prevalence of Acidobacteria in highly resistant plants, resistant plants, and susceptible plants decreased sequentially. At the genus level, the relative abundance of Vicinamibacteraceae and RB41 was markedly elevated in disease-resistant inter-root soils than in disease-susceptible soils. At the fungal level, the comparative prevalence of Ascomycetes in highly resistant plants, resistant plants, and susceptible plants increased sequentially. Differences in function are mainly manifested in apoptosis and mycelial development.
1. Introduction
Capsicum annuum (Capsicum annuum L.) is commonly known as the chili pepper or sweet pepper. Capsicum is rich in beneficial substances such as capsaicin, lutein, and β-carotene and is a cash crop widely grown worldwide [1]. At the same time, pepper is also one of the most widely planted vegetables in China, planted all over the country, and can be produced annually [2]. Anthracnose is a prevalent plant disease caused by Colletotrichum spp., which can seriously harm a variety of crops, vegetables, flowers, and fruit trees, causing significant economic losses [3]. When the disease occurs, yields are usually reduced by 30–50 percent, and severely affected fields can even result in crop failure [4]. The occurrence of pepper anthracnose has the characteristics of wide distribution, heavy damage, and high spreading speed [5]. It occurs both at planting and post-harvest, mainly affecting pepper fruits but sometimes also on stalks and leaves, with the disease appearing as brown or dark-brown rounded sunken spots. The latter stages of the disease usually cause rotting and shedding of the entire pepper [6]. The main pathogens that cause anthrax in peppers are Bacillus glabrata, Bacillus niger, Bacillus nigra, and Bacillus nigricans [7]. These pathogens are able to endure in the soil for extended periods and have the capacity to withstand and acclimate to adverse situations [8]. The disease has become an important constraint on modern agricultural development. Although chemical control is a fast and efficient means of pest control, it has many problems. Excessive use of chemical pesticides can seriously interfere with the inter-root microbial community, making the ecological environment more fragile. This not only leads to the loss of agrobiodiversity but also causes environmental pollution, which in turn jeopardizes human and animal health. In addition, the long-term use of chemical pesticides may also lead to increased pathogen resistance, excessive pesticide residues, and the re-emergence of pests. Consequently, the quest for innovative and efficient biopesticides to supplant chemical pesticides has emerged as a prominent focus of contemporary research.
The inter-root environment is the primary zone for information and material exchange among microorganisms, plants, and soil [9]. Inter-root microorganisms significantly impact biochemical cycle processes in the soil and are essential for root health and development [10]. Plants can recruit beneficial and pathogen-antagonistic microorganisms through root secretions or the autoimmune system, thereby remodeling the inter-root microbial community, increasing their resistance to pathogens and protecting themselves from infection [11,12]. Inter-root microorganisms affect plants most closely [13]. The inhibitory effects of these inter-root microorganisms on pathogens have been reported several times, and the patterns include hyperparasitism [14], the release of antibacterial substances [15], and competitive competition for resources, including nutrients or space, to suppress harmful microbes [16]. In the past few years, researchers have identified several bacteria from plant tissues or soil that can inhibit different plant pathogens. For example, Actinomycetes can significantly inhibit the growth of hyphae, thereby suppressing bacterial wilt [16]. A highly significant positive correlation has been shown between the occurrence of bitter melon wilt and the number of Fusarium acanthamoeba in the soil [17]. In addition, compounding different strains to form a microflora can better inhibit pathogenic microorganisms. The multi-strain synthetic flora was more effective in the control of blight, the disease index of wilt was significantly reduced, and plant growth and development were promoted [18]. An endophytic bacterial community of Dendrobium ferrugineum improves resistance to soft rot disease in ginger [19]. Based on previous studies, it was found that healthy samples had more diverse microbiological communities than diseased samples [20]. This is because inter-root microbial interactions form a complex microbial ecological network structure that inhibits pathogenic bacteria and creates a healthier soil microbiological environment [21]. Therefore, studying the composition and structure of inter-root microbial communities is important to assess their ability to resist pathogen invasion. Nonetheless, it remains ambiguous if disparities exist in the inter-root bacterial communities between disease-resistant and disease-susceptible species of Capsicum and whether these differences correlate with the extent of Capsicum anthracnose incidence.
It is unclear how specific microorganisms in the inter-root soil of pepper affect plant health. Accordingly, the focus of this experiment was to detect anthracnose-resistant soil in pepper and to investigate the relationship between their disease-suppressive properties and inter-root soil microbes. The aims of the present study were as follows: (i) to analyze the changes of environmental factors and microbiota in the inter-root soil of pepper in three different health states; (ii) to predict the interactions among anthracnose, soil factors, and inter-root microbial communities. Our research hypothesis was to assess the potential of microecological precision modulation as a basis for preventing anthracnose in the future.
2. Materials and Methods
2.1. Sample Collection
The pepper variety used in this experiment was “Sanying-9 pepper”, which was planted in the experimental base of the Henan Academy of Agricultural Sciences for producing and growing peppers. To investigate how anthracnose affects the peppers’ inter-root microbial community, we sampled soils with different levels of incidence. The designated groups were as follows: HR (highly resistant plants), R (resistant plants), and S (susceptible plants). Areas with 0% incidence are highly resistant, 0–5% incidence is resistant, and more than 50% incidence is susceptible. The inter-root soil was collected using the S-type five-point random sampling method [22]. That is, five soil samples were collected and combined into a composite sample using an S-shaped sampling track. For the purpose of extracting DNA and characterizing the soil, all samples of soil were rapidly frozen in liquid nitrogen for an hour before being kept in a refrigerator set at −80 °C.
2.2. Determining the Chemical Characteristics and Enzyme Activity of Soil
A total of 3 technical replicates were conducted to evaluate the 16 physicochemical characteristics of HR, R, and S inter-root soils in order to evaluate the soil conditions in the study region. These qualities included quick-acting nitrogen (QN), quick-acting phosphorus (QP), quick-acting potassium (QK), calcium/magnesium content (Ca/Mg), zinc content (Zn), iron content (Fe), copper content (Cu), soil pH, organic matter (OM), soil electrical conductivity (EC), microbial carbon biomass (MBC), soil sucrase (S-SC), soil alkaline phosphatase (S-ALP), soil urease (S-UE), and soil dehydrogenase (S-DHA) activity. The alkaline hydrolysis diffusion method was used to determine QN [23]. QP was quantified using molybdenum-antimony spectrophotometry [24]. Cu, Fe, and Ca were determined using atomic absorption spectrophotometry [25]. A conductivity monitor was used to identity the EC [26]. OM was determined by oxidation method [27]. Chloroform fumigation extraction was used to assess MBC [28]. S-ALP was determined by colorimetric method [29]. S-SC was determined by colorimetric method using 3,5-dinitrosalicylic acid [30]. S-UE was determined by colorimetric method using indanol blue [31]. S-DHA was assessed by colorimetric method [32]. In every soil plot, measurements of the soil’s characteristics were made three times. The above experimental technology service was provided by Norminkoda Biotechnology Co., Ltd. (Wuhan, China).
2.3. DNA Extraction and PCR Amplification
Microbial community genomic DNA was extracted from individual pepper assemblage samples using the CretMagTM-Powered Soil DNA Kit ((CretBiotech, Suzhou, China). DNA extracts were assayed on 1% agarose gels for DNA extracts, and a NanoDrop 2000 UV-visible Spectrophotometer (Thermal Sciences, Inc., Wilmington, NC, USA) was used to determine the concentration of DNA and the purity. The bacterial 16S was amplified by an A200 PCR thermocycler (A200, LongGene, Hangzhou, China) using primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). rRNA gene with highly variable region V3-V4 [33]. The hypervariable regions of the ITS were amplified with primer pairs ITS1F(5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′) by an A200 PCR thermocycler (A200, LongGene, China) [34]. The PCR amplification procedure for the genes was as follows: initial denaturation at 94 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension and annealing at 72 °C for 45 s, and a single extension at 72 °C for 10 min ending at 4 °C.
2.4. Illumina Novaseq Sequencing
Purified amplicons were combined in equimolar ratios and subjected to paired-end DNA sequencing on the Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) using protocols established by Baiaoweifan Biotechnology Co., Ltd. (Wuhan, China). The original sequencing reads were submitted to the NCBI Sequence Read Archive (SRA) database (Accession Number: PRJNA1211196).
2.5. Sequencing Data Processing
The original 16S rRNA gene sequencing readings underwent demultiplexing, quality filtering using fastp version 0.20.0 [35], and merging via FLASH version 1.2.7 [36] according to the following specified criteria: (i) the 300 bp reads were trimmed whenever the mean quality score dropped below 20 within a 50 bp sliding window. Reads less than 50 bp after trimming were removed, and reads with unclear characters were excluded; (ii) only overlapping sequences exceeding 10 bp were constructed based on their overlapping regions. The highest permissible mismatch ratio in the overlap region is 0.2. Unassembled reads were eliminated; and (iii) samples were categorized based on barcodes and primers, sequence orientation was corrected, precise barcode matching was ensured, and a two-nucleotide mismatch was permitted in primer matching.
Operational taxonomic units (OTUs) were clustered at 97% similarity threshold using UPARSE version 7.1 [37]; chimeric sequences were uncovered and eliminated. The taxonomy for every OTU-representative sequence was assessed using RDP Classifier version 2.2 [38] against the 16S rRNA database with a confidence level of 0.7.
2.6. Statistical Analyses
Statistical analyses were performed using IBM SPSS 20.0 (IBM Corporation, New York, NY, USA) and R software (version 3.5.2). One-way analysis of variance (ANOVA) and post hoc multiple comparison tests (Tukey HSD) were used to analyze the effect of anthracnose on soil properties and enzyme activities. Wilcoxon rank sum test was used as a test of statistical significance for comparison of the three groups. All statistical tests conducted in this study were considered significant at p < 0.05 and extremely significant at p < 0.01.
3. Results
3.1. Comparative Analysis of the Physical and Chemical Properties of HR, R, and S
Sixteen features of the inter-root soils of each group were evaluated—QN, QP, QK, Ca, Mg, Zn, Fe, Cu, soil pH, OM, EC, MBC, S-SC, S-ALP, S-UE, and S-DHA activity.
These were used to ascertain the correlation among chemical characteristics, enzyme activities, and the prevalence of anthracnose in peppers. The results in the graph (Figure 1) demonstrated that substantial alterations occurred in some of the soil physicochemical features and enzyme activities in HR, R, and S. Calcium, magnesium, zinc, and iron contents in HR, R, and S were reduced in that order, and the differences were significant. In the measurement of soil enzyme activities, no substantial differences were observed, except that the S-ALP concentration in group R was markedly higher than that in groups HR and S. Additionally, the difference in inter-root soil pH of pepper across three distinct health situations was not significant (p > 0.05), suggesting that the presence of anthracnose in pepper did not influence the inter-root soil pH.
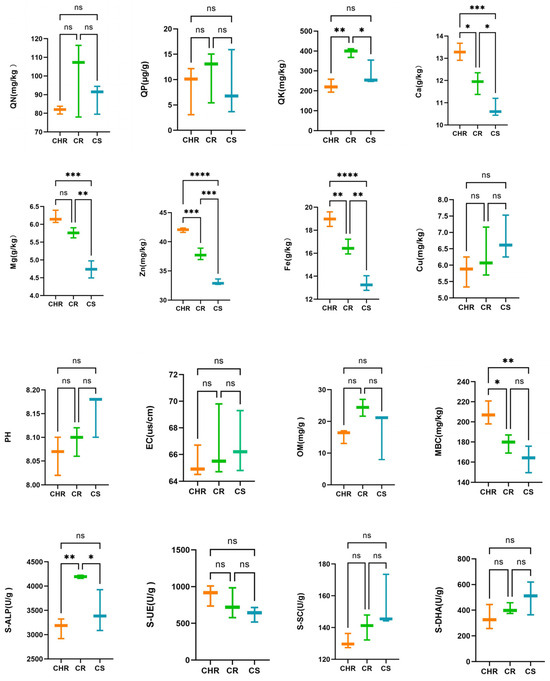
Figure 1.
Effect of resistance and susceptibility to pepper anthracnose on chemical characteristics of inter-root soil and enzymatic activities. The symbol ‘*’ denotes significant differences, the symbol ‘ns’ indicates no significant difference. (p < 0.05) across the three groups as determined by one-way ANOVA and HSD test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.2. Analysis of Microbial Diversity and High-Throughput Sequencing Data in HR, R, and S
The species accumulation curves for the bacterial and fungal community sequencing results were plotted (Figure 2A,E) to assess the adequacy of the sample size in this study and to estimate community richness. The findings demonstrated that the number of newly discovered species essentially hit a plateau when the number of samples was above ten. Nine samples were adequate in this investigation to represent the species composition of the community. We drew Venn diagrams to examine common and unique species in the three samples. The bacterial community generated 3711 OTUs (Figure 2B), and the fungal community generated 2283 OTUs (Figure 2F). We calculated alpha-diversity indices using QIIME2 (qiime version v.1.8.0) and plotted alpha-diversity index box plots to reflect changes in the richness and variety of microbial communities within the samples. The Chao1 and ACE indices represent the abundance of microbial communities, and the Shannon and Simpson indices represent the variety of microbial communities. It can be seen from the figure that in the bacterial community, the Chao1 index and ACE index of HR were markedly elevated compared to S and R (Figure 2C). The Shannon diversity index of HR was markedly greater than that of S and R. However, in comparison of the Simpson indices, R had the lowest index, i.e., the highest diversity, but it was not statistically significant due to p-value greater than 0.05 (Figure 2D). Among the fungal communities, HR had higher abundance and diversity than H and R, but none of the results were statistically significant.
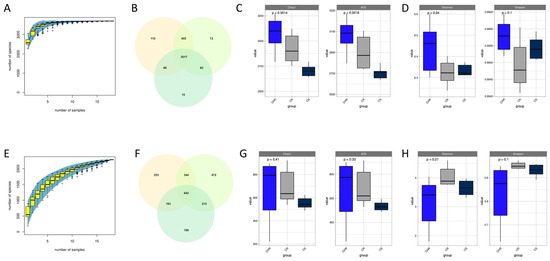
Figure 2.
α-diversity of inter-root microbial communities. Species accumulation curves for bacteria (A) and fungi (E). Venn diagrams were constructed to illustrate the shared and unique bacterial (B) and fungal (F) taxa identified across the three groups. Box plots illustrate the variation in the Chao1 index, ACE index, and Shannon and Simpson indices for bacteria (panels (C,D)) and fungi (panels (G,H)) across the three groups.
3.3. Cluster Analysis of Microbial Communities and Similarities and Differences in Their Structures in HR, R, and S
The multidimensional soil microbial variables were downscaled into two variables by principal coordinate analysis (PCoA) (Figure 3) of microbiological consortia from three different plots. For the bacterial community, the contribution of the first principal coordinate (PCoA1) was 30.67%, the contribution of the second principal coordinate (PCoA2) was 12.33%, and the cumulative contribution of the two was 43.00%. From Figure 2A, it is evident that the soil samples from HR and R, S areas were farther apart from each other, indicating that the disparity in bacterial community makeup between the highly resistant sample plots and the resistant and susceptible sample plots was more pronounced. Regarding the fungal community, the contribution of PCoA1 was 37.2%, that of PCoA2 was 12.7%, and the cumulative contribution of the two was 49.9%. As can be seen in Figure 3B, the soil samples from the HR area and the R, S area were farther apart from each other, indicating that the distinction in fungal community composition between the two sample sites was more pronounced. This is consistent with the bacterial results.

Figure 3.
Plot of PCoA analysis of bacteria (A) and fungi (B). The horizontal and vertical coordinates represent the first and second principal components and their contribution to the sample difference, respectively. The branch lengths of the Bray–Curtis multi-sample clustering tree (C) for bacteria indicate the distance between samples; greater similarity across samples increases the likelihood of their grouping together.
3.4. Community Composition and Abundance of Dominant Species in HR, R, and S
To examine the community structure of microorganisms in the soil across the three sample groups, we analyzed the relative abundance of soil bacteria and fungus classified by genus and phylum (Figure 4). For the bacterial community, there are 30 bacterial phyla with proportional abundance greater than 1% at the phylum standard. The predominant phyla were Acidobacteriota and Proteobacteria. As seen in the heatmap, Acidobacteriota showed a gradual decrease in relative abundance in HR, R, and S (Figure 4A). At the genus level, there are 30 genera of bacteria with relative abundance above 1%. The genera with higher relative abundance were Vicinamibacteraceae, RB41, and Vicinamibacterales. As seen in the heatmap, the proportional abundance of Subgroup-2, AD3 in HR and R was greater than that in group S, but the proportional abundance of MND1 in group S was higher than that in groups HR and R (Figure 4B). For the fungal community, there are 18 fungal phyla with relative abundance above 1% at the phylum-level standard. Among them, the predominant phyla were Ascomycota and Basidiomycota. As seen in the heat map, the relative abundance of Ascomycota in HR, R, and S gradually increased, and the relative abundance of Basidiomycota in HR, R, and S gradually decreased (Figure 4C). At the genus level, there are 30 genera of fungi with relative abundance exceeding 1%. The genus with high relative abundance were the fungi, uncultured Botryotrichum_atrogriseum. As can be seen from the heatmap, the relative abundance of uncultured Agaricales and uncultured Trechisporates was higher in HR than in R and S.
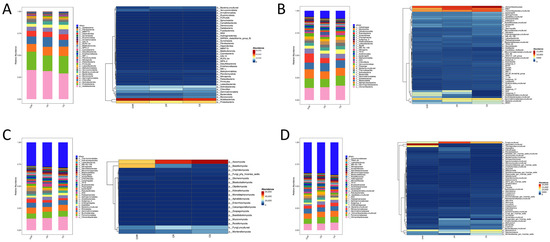
Figure 4.
Composition of microbial communities at the phylum and genus level (top 30 by relative abundance). Bacteria at the phylum (A) and genus (B) classifications. Fungi at the phylum (C) and genus (D) level.
3.5. Functional Analysis of the HR, R, and S Microbial Communities
We compared the functional abundance of bacterial and fungal communities based on sequence analysis of the ITS markers of the 16S rRNA gene. PICRUSt 2 (systematic genetic Investigation of communities by Reconstruction of observed States 2) and FUNGuild (Fungi Functional Guild) V1.0 [30] online platform were utilized to forecast microbial function, thereby establishing a foundation for comprehending the microbial community and its potential relationships with host peppers. For the bacterial communities, we plotted histograms of differential function (Figure 5A). The graphs show functional genes with significantly different abundances in different groups, and the length of the bars represents the relative abundance of significantly different functional genes. As seen from the figure, the functions of bacterial chemotaxis and flagellar assembly in HR were significantly higher than those of R and S. Geraniol degradation and synthesis, the degradation of ketone bodies, and the citrate cycle were found to be in high functional abundance in S. Based on ANOVA analysis of functional gene abundance (Figure 5B), the functional abundance of aminoacyl-tRNA biosynthesis, alanine, aspartate, and glutamate metabolism were all higher in functional abundance, but none were significantly different.
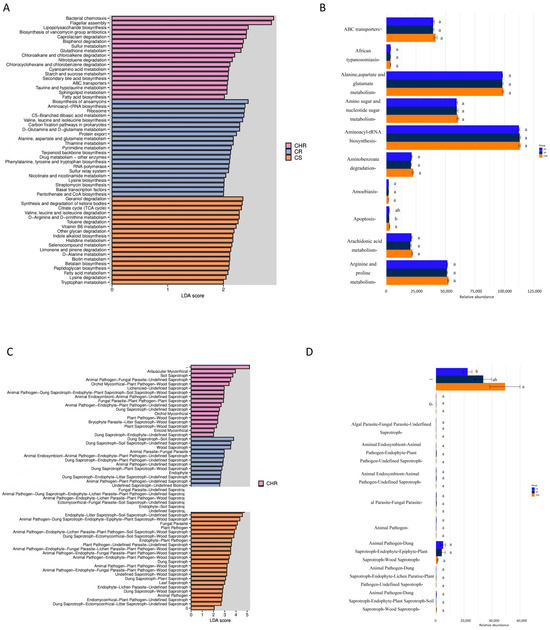
Figure 5.
Prediction and analysis of metabolic function based on Picrust II and FUNGuild for bacterial communities (A,B) and fungal communities (C,D) of HR, R, and S. The effect values of linear discriminant analysis (LEfSe) were greater than 2.0. The linear discriminant analysis effect values (LEfSe) for bacterial (A) and fungal (C) communities exceeded 2.0 when the p-value was below 0.05. The histograms illustrate the linear discriminant analysis scores of the microbial communities. Communities that met the LDA significance threshold greater than 2.0 are shown. ANOVA analysis derived from the prevalence of functional genes in bacteria (B) and fungi (D). The horizontal coordinates indicate the relative abundance of a species in different subgroups, the vertical coordinates indicate the differential species, and different colors indicate different groups. Different letters indicate the significance of differences.
FUNGuild is a database providing functional annotations for fungi, encompassing information for approximately 12,000 species. It predicts fungal functionality by delineating the structural makeup of fungal communities for annotation, and we analyzed the functional prediction results of three sample plots and performed ANOVA analysis (Figure 5C,D). The findings indicated that the functional abundance of arbuscular mycorrhizal was markedly greater in HR compared to R and S. The animal pathogen-dung saprotroph-endophyte-epiphyte-plant saprotroph-wood saprotroph had higher but not significantly different functional abundance.
4. Discussion
Pepper (Capsicum spp.) is popular for its unique flavor, and demand is increasing [39]. With the expansion of the scale of pepper production, pepper anthracnose is also gradually spreading, becoming a major problem hindering the healthy development of the pepper industry [40]. Pepper varieties, continuous cropping, sticky soil, heavy treatment and light prevention, and failure to clean up leaves with Anthracnose germs and residual leaves in time can cause anthracnose to occur [41,42]. Chemical control serves as the primary method for disease management. Excessive use of chemical agents not only damages the ecological environment but also causes harm to humans [43]. Biological control is frequently regarded as a more economical option relative to chemical and physical methods [44]. Microorganisms significantly contribute to plant disease resistance [45]; for example, the induction of disease resistance in goldenseal by an antagonistic bacterium of stem rot of the nematode tree [46]. Therefore, it is essential to investigate the physicochemical properties and microbial community composition of the inter-root soil of pepper to facilitate the biological control of pepper anthracnose.
4.1. Relationship Between Physical and Chemical Properties of Inter-Root Soil and Plant Health
Microbial activity and plant growth are significantly impacted by the physicochemical characteristics of the soil and the activities of its enzymes [34]. Quick-acting nutrients in soil mainly include soil alkaline exchangeable calcium/magnesium, effective iron, quick-acting potassium, alkaline dissolved nitrogen, etc. [47]. Lack of nutrients tends to result in the creation of plant diseases. Yang Fan et al. in their investigation into the impact of soil fast-acting nutrient content on the yield of peppers found that the number of diseased fruits of peppers in the experimental area lacking phosphorus fertilizer ratios was relatively high [48]. One study showed that calcium deficiencies can lead to blossom rot in tomatoes [49]. This aligns with the findings of the current investigation, in which calcium, magnesium, zinc, and iron contents in HR, R, and S sequentially decreased with significant differences. However, the differences in N, P, and K were not significant, which may pertain to the composition of beneficial nutrients in the soil or the nutrient absorption mechanism of the pepper plant, and the relationship between these three factors and anthracnose needs to be further studied. The activity of various soil enzymes is a crucial factor for plant growth and development [50]. Additionally, they contribute to the promotion of benign soil ecological cycles and the prevention of pest diseases [51]. S-ALP is considered to be an important driver of the microbial activation process of organophosphorus and can increase the effective phosphorus content of soil [52]. In this experiment, the content of phosphatase in the inter-root soil of disease-resistant peppers was markedly elevated compared to that in disease-susceptible areas, which aligns with findings from prior research, and we hypothesized that it was because the high content of the enzyme suppressed the development of anthracnose. There was no significant correlation between the three treatment groups of S-SC, S-UE, and S-DHA with resistant and susceptible soils, indicating that the activities of these three enzymes were not influenced by variations in vegetation susceptibility to disease. Studies have shown that alkaline phosphatase activity is negatively correlated with soil pH [53], which is in general agreement with the results of this experiment. Lacey et al. found that disease severity decreased with decreasing acidic pH [54]. Senechkin et al. found that wilt incidence was lower in high-pH soils [55]. It has also been suggested that there is no correlation between the severity of plant diseases and pH [56], and the results of the present experiments are in agreement with that result. We also determined the electrical conductivity (EC) and the organic matter content of the soil. High EC values create a reverse osmotic pressure, which dries out and browns the root system, preventing it from absorbing water and nutrients [57]. N increase in soil organic matter content improves plant growth and yield [58]. However, there was no significant difference between the two results of this experiment. The activities of most enzymes in soil are affected by soil microbiomass carbon [51], which increased with the increase of plant disease resistance in this experiment. Therefore, we hypothesized that microbial amount carbon MBC plays an important role in anthracnose suppression.
4.2. Diversity and Community Composition of Inter-Root Soil Microorganisms in Relation to Plant Health
Inter-root microorganisms are regarded as one of the most intricate ecosystems on Earth, encompassing a multitude of biological species and serving as a conduit between the soil and the plant [59]. The variety and makeup of soil microorganisms influence the multifunctionality of soil ecosystems and suppress plant diseases [60]. Diverse microbial community composition has been demonstrated to more effectively inhibit pathogens [59]. Zhou xin et al. found that bacterial and fungal diversity were higher in the inter-root soil of tomato with lower incidence [61]. Chen shu juan et al. discovered that the lowest microbial diversity was found in the inter-root soil of poorly growing blueberries [62]. In this experiment, disease-resistant soil had more bacteria in terms of both abundance and diversity than disease-susceptible soil; however, the diversity difference was not statistically significant. The results of abundance and diversity of fungi were the same as those of bacteria, both of which were higher in disease-resistant than in susceptible soils, but the differences were not significant. The results indicate that disease-resistant varieties have a greater ability to recruit beneficial microorganisms under susceptible soil conditions. This is consistent with previous results, but the difference was not significant. We hypothesize that it is due to the fact that these factors may not be consistent indicators [63].
Further species analysis of soil bacteria in the different treatments at the phylum taxonomic level revealed that Acidobacteriota and Proteobacteria were the dominant bacteria in the three treatments, which is consistent with the findings of earlier research [64,65,66]. Acidobacteriota and Proteobacteria can be used as indicators of soil nutrient status, with Acidobacteriota as nutrient-poor bacteria that can expand across a broad range of amounts of carbon sources [67]. As eutrophic bacteria, the relative abundance of Proteobacteria usually increases at high nitrogen levels [68]. With a variety of metabolic and genetic roles, Acidobacteriota are a significant component of the soil bacterial community [69], usually associated with disease suppression [70,71]. In this experiment, the proportional quantity of Acidobacteriota in the soil increased as the degree of disease resistance increased. We hypothesized that this was due to the inhibition of pepper anthracnose by Acidobacteriota. At the genus level, Vicinamibacteraceae, RB41 were the predominant bacterium found in healthy plants’ inter-root soil. Vicinamibacteraceae belongs to Acidobacteriota, which was found by Yang Fajun to be closely related to plant uptake of nitrogen, phosphorus, and potassium [72]. Wang Jing et al. found that this bacterium may better promote inter-plant root uptake of nutrients, which is closely linked to the utilization of nitrogen and phosphorus nutrients [73]. Wang Honglan et al. found in the study of inter-root soil of Qiangwu that its function was related to promoting soil nutrient decomposition, conversion utilization and uptake, and improving plant resistance to stresses [74]. Synthesizing previous studies, we hypothesized that the high enrichment of Vicinamibacteraceae in disease-resistant soils promotes the uptake and utilization of soil nutrients, which consequently promotes plant growth and enhances plant resistance. RB41 is a common and abundant soil bacterium that is able to utilize carbon sources and nutrients in the soil and participates in the soil carbon cycle, accounting for more than half of the carbon utilization in the soil, along with Bradyrhizobium and Streptomyces [75]. As stated by Liu Wei et al., RB41 abundance was positively correlated with urease, which may indirectly increase soil alkaline dissolved nitrogen by affecting soil carbon cycling [76]. In this study, RB41, urease, and quick-acting nitrogen levels were higher in disease-resistant inter-root soils than in disease-susceptible soils, consistent with their results. It has been shown that the genus MND1’s abundance enhances the quality of the soil. They are crucial for the solubilization of inorganic phosphorus, as well as the mineralization of organic phosphorus [77]. Furthermore, they are engaged in nitrogen cycling activities, such as ammonia oxidation and nitrogen fixing [78]. In this study, however, the amount of MND1 in sensitive soil was higher than that in healthy soil. We hypothesize that this is due to the influence of the complex microbial ecological network structure, about which the specific mechanism of influence remains to be studied.
The makeup of fungal communities is simpler than that of bacterial communities. Ascomycota and Basidiomycota appeared as the main endophytic fungal dominant groups, which agrees with the findings of Yao Suhang et al. [79]. Ascomycota has high species diversity and evolutionary rate [80] and dominates soil fungal communities globally [81]. It is not only highly adaptable to arid environments but also plays an important function in decomposing soil organic matter [82]. Ascomycota are essential for the degradation of plant apoplasts with high lignin content [83]. Ectomycorrhizal fungi (ECMF), which are also included in Ascomycota and Basidiomycota, form a mutually beneficial symbiotic relationship with plant roots and have a significant part to play in the nitrogen and phosphorus cycle [84]. It is therefore a key fungal taxon for pepper resistance to anthracnose. Compared to disease-susceptible soils, disease-resistant inter-root soils had a substantially higher relative abundance of uncultured Basidiomycota and Trechisporates. Basidiomycota play an important role in the breakdown of cellulose and lignin and are an integral part of forest ecosystems [85]. Uncultured Trechisporates is an unclassified order under the Basidiomycota whose functional studies have not been clarified and need to be further explored.
4.3. Relationship Between Inter-Root Soil Microbial Function and Plant Health
Much research has been carried out to show that the functional composition of microorganisms is intimately related to the environment [86,87]. We examined the functional abundance of bacterial and fungal communities using sequencing analysis of ITS markers of 16S rRNA genes. PICRUST 2 and FUNGuild predictions showed that soil bacterial and fungal communities were rich in functional diversity. Ansamycins are a class of macrocyclic lactam antibiotics mainly produced by microorganisms with complex structures, almost all of which have significant physiological activities [88,89]. Many of its derivatives have been used in clinical treatments [90,91]. The biosynthesis function of biosynthesis of ansamycins is in high abundance in both disease-resistant and disease-sensitive soil areas. We hypothesize that the inter-root soil of pepper may produce certain metabolites or enrich a certain microflora closely associated with the synthesis of such compounds. Furthermore, the functional abundance of biosynthesis of vancomycin group antibiotics, valine, leucine, and isoleucine biosynthesis were also high. Vancomycin is a glycopeptide antibiotic, which can prevent the bacterial cell wall from synthesizing and from producing polypeptides and phospholipids so as to kill bacteria and avoid bacterial infection [92]. Valine, leucine, and isoleucine as branched-chain amino acids (BCAAs) are biosynthetically important in plant disease resistance defense. Naoki et al. found that exogenous proteogenic amino acids can confer plant resistance, such as isoleucine and leucine, that trigger resistance to rice blast. All of these functions enhance plant disease resistance.
Functional prediction of FUNGuild revealed that the inter-root soil of susceptible plants with animal pathogen-dung saprotroph-endophyte-epiphyte-plant saprotroph-wood saprotroph had maximum functional abundance. These plant diseases may have a direct bearing on this outcome. In healthy plant inter-root soil, arbuscular mycorrhizal had a much higher functional abundance than in R and S. Arbuscular mycorrhizal are the most widely distributed soil symbiotic fungi on earth; they can form symbiotic relationships with more than 80% of terrestrial plants, absorb carbohydrates produced by plant photosynthesis through the symbiotic relationship, and transport the photosynthesized products downward to convert them into difficult-to-biodegrade organic matter, and their clumped structures near the root system are considered to be an important place for plant nutrient exchange [93]. In addition, arbuscular mycorrhizal accelerate soil organic matter decomposition, and the associated soil proteins secreted after apoptosis can be released into the soil as a carbon source [94]. It can also alleviate the growth inhibition of plants under stressful environments to varying degrees and improve plant resistance and adaptability [95]. Weili et al. found that arbuscular mycorrhizal reduced anthracnose in tea seedlings [96]. Yang Yalin et al. found that there was a decreasing trend in the abundance and diversity index of AMF spores in disease-susceptible oil tea soil [97]. Based on previous studies, we hypothesized that it might be due to the fact that the arbuscular mycorrhizal competed with the pathogen for ecological niches at the inter-root level, reducing the chances of infestation by the pathogen.
Of course, interpreting these variations as merely soil–microbe interactions is insufficient; the direct effects of these microbial and chemical characteristics on plant health require more study. We combined correlation analyses to develop a correlation mechanism map (Figure 6) that reveals possible pathways between environmental factors, inter-root bacteria and fungi, and pepper resistance and susceptibility to anthracnose. Whether specific microbial communities and soil factors in healthy pepper inter-root soils have the ability to suppress disease and the optimal microbial ecological network structure remain to be further investigated.
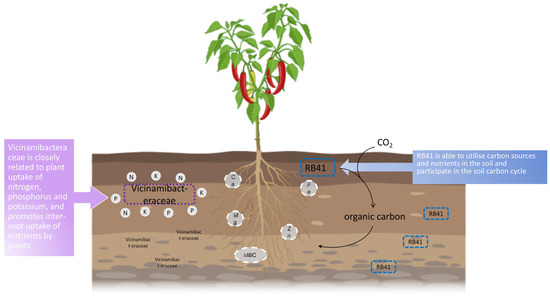
Figure 6.
Diagram of possible mechanisms of inhibition of pepper anthracnose by inter-root soil bacteria and fungi in combination with soil factors. Vicinamibacteraceae is closely related to plant uptake of nitrogen, phosphorus, and potassium and promotes inter-root uptake of nutrients by plants. RB41 is able to utilize carbon sources and nutrients in the soil and participate in the soil carbon cycle.
5. Conclusions
Despite using the same growth conditions for peppers, there were significant differences observed in soil physical and chemical properties and microbial communities under the three different health conditions. MBC increased as plant disease resistance increased, and soil MBC levels may inhibit anthracnose development. The relative abundance of Vicinamibacteraceae and RB41 is higher in healthy soils. This promotes the decomposition, translational utilization, and uptake of nutrients such as nitrogen, phosphorus, and potassium. At the fungal taxonomic level, Ascomycota and Basidiomycota, as the main dominant groups of endophytic fungi, play an important role in the decomposition of soil organic matter, the degradation of lignin, and nitrogen and phosphorus cycling. Differences in function are mainly manifested in apoptosis and arbuscular mycorrhizal fungi.
In conclusion, the correlation analysis and functional prediction of key environmental factors, microbiota, and pepper health indices in the inter-root soil of peppers deepened the understanding of the inhibitory effects of soil microorganisms on Capsicum anthracnose and provided a reference for the management of peppers infections. In addition, we predicted interactions between anthracnose, soil factors, and inter-root microbial communities, providing a basis for future prevention of anthracnose through microecological regulation.
Author Contributions
F.Y. and Y.M. planted the plants and collected the root materials. Y.M. and M.L. were conducive to the collection and analysis of sequencing data. All authors (Y.M., M.L., Y.H., Y.W., X.C., G.S., H.X., Q.Y. and F.Y.) contributed to the design of the experiments and preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Independent Innovation Project of Technology System of Bulk Vegetable Industry in Henan Province (HARS-22-07-G4), the Youth Backbone Teacher Training Program of Colleges and Universities in Henan Province (No. 2023GGJS029), Major Science and Technology Special Project of Henan Province (241100110200), Henan Academy of Agricultural Sciences (2024ZC035), the National Characteristic Vegetable Industry Technology System (CARS-24-G-15), Key R&D Projects in Henan Province (2211111110100), and the Henan Academy of Agricultural Sciences and Technology Innovation Team (2024TD38, 2024TD43).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw sequence data have been made available in the NCBI Sequence Read Archive (SRA) database under the bioproject accession number PRJNA1211196.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohd Hassan, N.; Yusof, N.A.; Yahaya, A.F.; Mohd Rozali, N.N.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yang, S.; Dai, X.; Hu, B.; Xu, H.; Zhu, F.; Pei, S.; Yuan, F. The Rapid Development of China’s Chili Pepper Industry Over the Past 40 Years. Acta Hortic. Sin. 2025, 52, 247–258. [Google Scholar]
- Alice, C.; Francesca, N.; Riccardo, B.; Elena, B. Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, B.; Cheng, C.; Fu, B.; Qi, M. Comparative Transcriptomics Analysis Reveals the Differences in Transcription between Resistant and Susceptible Pepper (Capsicum annuum L.) Varieties in Response to Anthracnose. Plants 2024, 13, 527. [Google Scholar] [CrossRef]
- Shen, H.; Yang, Q.; Xie, X.; Deng, H.; Chen, F.; Sun, D.; Jiang, S.; Lin, B. Study on Biological Characteristics of Colletotrichum acutatum in Pepper. Guangdong Agric. Sci. 2021, 48, 110–117. [Google Scholar]
- Ruangwong, O.U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol Mechanisms of Trichoderma koningiopsis PSU3-2 against Postharvest Anthracnose of Chili Pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, H.; Xiao, Z.; Mo, W.; Cheng, H.; Peng, L.; Ding, H. Identification and fungicide sensitivity of pathogen causing anthracnose of pepper. J. South China Agric. Univ. 2023, 44, 430–437. [Google Scholar]
- Freeman, S. Management, survival strategies, and host range of colletotrichum acutatum on strawberry. HortScience 2008, 43, 66–68. [Google Scholar] [CrossRef]
- Fu, L.; Xiong, W.; Dini-Andreote, F.; Wang, B.; Tao, C.; Ruan, Y.; Shen, Z.; Li, R.; Shen, Q. Changes in bulk soil affect the disease-suppressive rhizosphere microbiome against Fusarium wilt disease. Front. Agric. Sci. Eng. 2020, 7, 307–316. [Google Scholar] [CrossRef]
- Birt, H.W.; Tharp, C.L.; Custer, G.F.; Dini-Andreote, F. Root phenotypes as modulators of microbial microhabitats. Front. Plant Sci. 2022, 13, 1003868. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.E.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.; Feussner, I.; Pieterse, C.M. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed]
- Ge, A.H.; Liang, Z.H.; Xiao, J.L.; Zhang, Y.; Zeng, Q.; Xiong, C.; Han, L.L.; Wang, J.T.; Zhang, L.M. Microbial assembly and association network in watermelon rhizosphere after soil fumigation for Fusarium wilt control. Agric. Ecosyst. Environ. 2021, 312, 107336. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef]
- Parratt, S.R.; Laine, A.L. Pathogen dynamics under both bottom-up host resistance and top-down hyperparasite attack. J. Appl. Ecol. 2018, 55, 2976–2985. [Google Scholar] [CrossRef]
- Aleksej, Z.; Sergej, A.; Olga, P.; Daniel, R.M.; Peer, B. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. USA 2015, 112, 6449–6454. [Google Scholar]
- Shi, B.; Xie, Y.; Guan, F.; Yang, X.; Zhang, J.; Wang, K.; Wan, X. Analysis on Population Fluctuation of Rhizosphere Microorganism of Resistant and Susceptible Bitter Gourd. Chin. Agric. Sci. Bull. 2024, 40, 118–124. [Google Scholar]
- Chengjian, W. Analysis of Tobacco Rhizosphere Microbiota and Construction of Biocontrol Microbial Community Against Bacterial Wilt Disease; Guangxi University: Nanning, China, 2024. [Google Scholar] [CrossRef]
- Sabu, R.; Aswani, R.; Prabhakaran, P.; Krishnakumar, B.; Radhakrishnan, E.K. Differential Modulation of Endophytic Microbiome of Ginger in the Presence of Beneficial Organisms, Pathogens and Both as Identified by DGGE Analysis. Curr. Microbiol. 2018, 75, 1033–1037. [Google Scholar] [CrossRef]
- Yin, J. Study on the Prevention and Control of Tomato Bacterial Wilt Using Microbiome; Huazhong Agricultural University: Wuhan, China, 2023. [Google Scholar]
- Zhang, Y.; Chen, Z.; Chen, F.; Yan, J.; Wu, J.; Xi, Q.; Ge, S. Investigation of Microbial Community Characteristics in Pepper Rhizosphere at Seedling Stage Under Varied Soil Conditions. Guangdong Agric. Sci. 2024, 51, 129–141. [Google Scholar]
- Warner, L.S. Randomized Response: A Survey Technique for Eliminating Evasive Answer Bias. J. Am. Stat. Assoc. 2012, 60, 63–69. [Google Scholar] [CrossRef]
- Song, X.; Pan, Y.; Li, L.; Wu, X.; Wang, Y. Composition and diversity of rhizosphere fungal community in Coptis chinensis Franch. continuous cropping fields. PLoS ONE 2018, 13, e0193811. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kreller, C.R.; Greenberg, M.M. Preparation and analysis of oligonucleotides containing the c40 -oxidized abasic site and related mechanistic probes. J. Org. Chem. 2005, 70, 8122–8129. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Du, A.P.; Wang, Z.C.; Zhu, W.K.; Li, C.; Wu, L.C. Effects of different rotation periods of Eucalyptus plantations on soil physiochemical properties, enzyme activities, microbial biomass and microbial community structure and diversity. For. Ecol. Manag. 2020, 456, 117683. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Xiang, X.; Sun, R.; Yang, T.; He, D.; Zhang, K.; Ni, Y.; Zhu, Y.G.; Adams, J.M.; et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Xu, J.D.; Huang, T.X.; Zhong, J.; Yu, H.; Qiu, J.P.; Guo, J.H. Combination of beneficial bacteria improves blueberry production and soil quality. Food Sci. Nutr. 2020, 8, 5776–5784. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Alves, K.J.; da Silva, M.C.P.; Cotta, S.R.; Ottoni, J.R.; van Elsas, J.D.; de Oliveira, V.M.; Andreote, F.D. Mangrove soil as a source for novel xylanase and amylase as determined by cultivation-dependent and cultivation-independent methods. Braz. J. Microbiol. 2020, 51, 217–228. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Klein, D.A.; Loh, T.C.; Goulding, R.L. A rapid procedure to evaluate dehydrogenase activity of soils low in organic matter. Soil Biol. Biochem. 1971, 3, 385–387. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Eissa, N.; Bernstein, C.N.; Khafipour, E.; Ghia, J.E. Antepartum Antibiotic Treatment Increases Offspring Susceptibility to Experimental Colitis: A Role of the Gut Microbiota. PLoS ONE 2015, 10, e0142536. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, H.; Liang, S.; Chang, G.; Ma, K.; Niu, L.; Mi, G.; Tang, Y.; Tian, B.; Shi, X. High-Throughput Sequencing Reveals the Effect of the South Root-Knot Nematode on Cucumber Rhizosphere Soil Microbial Community. Agronomy 2023, 13, 1726. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Shan, Q.; Wan, Y.; Liang, J.; He, W.; Zeng, J. HS–SPME combined with GC–MS and GC–O for characterization of key aroma-active compounds in fruity and grassy peppers (Capsicum chinense Jacq.). Food Chem. X 2024, 24, 101944. [Google Scholar] [CrossRef]
- Ma, S.; Dong, R.; Wang, S.; Zhang, Z.; Wang, J. Study on the current situation of anthracnose occurrence and control measures of pepper. World Trop. Agric. Inf. 2024, 11, 54–56. [Google Scholar]
- Yerken, G. Occurrence and control measures of anthracnose in chili peppers. Farmer’s Friend 2017, 2, 75. [Google Scholar]
- Youshi, Y.; Chunzhong, T. Causes of pepper anthracnose in Jinhu County, Jiangsu Province and comprehensive control measures. Agric. Eng. Technol. 2021, 41. [Google Scholar] [CrossRef]
- Ren, L.; Qin, N.; Ning, J.; Yin, H.; Lü, H. Capsicum Endophytic Bacterial Strain LY7 and Prochloraz Synergistically Control Chilli Anthracnose. J. Fungi 2024, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Western Farm Press. How Soil Microbes Help Plants Resist Disease; Western Farm Press: St. Charles, IL, USA, 2020. [Google Scholar]
- Wanyu, L. Screening of Antagonistic Bacteria Against Stem Rot of Anoectochilus roxburghii and Study on Induction of Plant Resistance Mechanisms; Tongfang Knowledge Network (Beijing) Technology Co., Ltd.: Beijing, China, 2023. [Google Scholar] [CrossRef]
- Mishra, A.; Pattnaik, T.M.; Das, D.; Das, M. Vertical Distribution of Available Plant Nutrients in Soils of Mid Central Valley at Odisha Zone, India. Am. J. Exp. Agric. 2015, 7, 214–221. [Google Scholar] [CrossRef]
- Fan, Y.; Chenqin, W.; Yongquan, K.; Renquan, Z. Effect of Soil Available Nutrient Content on Yield of Chili. Tillage Cultiv. 2018, 3, 009. [Google Scholar]
- Reitz, N.F.; Shackel, K.A.; Mitcham, E.J. Differential effects of excess calcium applied to whole plants vs. excised fruit tissue on blossom-end rot in tomato. Sci. Hortic. 2021, 290, 110514. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Cano, A.; Johnson, J. Simultaneous determination of multiple soil enzyme activities for soil health-biogeochemical indices. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, Y.; Jiang, S.; Wu, P.; Zhang, X.; Liu, Q. Response of Soil Enzymes Activities and Microbial Biomass Carbonand Nitrogen Characteristics to Planting Years of Aralia elata. Shandong Agric. Sci. 2024, 56, 114–120. [Google Scholar]
- Li, Y.; Xing, X.; Qiu, X.; Xu, S.; Ni, F.; Zhao, L.; Yang, X. Effects of Phosphorus Application Rate on Phosphohydrolase Activityand Distribution of Phosphate Solubilizing Bacteria in Paddy Soil. Shandong Agric. Sci. 2024, 56, 111–117. [Google Scholar]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; McIntosh, M. Changes in soil biological activities under reduced soil pH during Thlaspi caerulescens phytoextraction. Soil Biol. Biochem. 2005, 38, 1451–1461. [Google Scholar] [CrossRef]
- Lacey, M.J.; Wilson, C.R. Relationship of Common Scab Incidence of Potatoes Grown in Tasmanian Ferrosol Soils with pH, Exchangeable Cations and other Chemical Properties of those Soils. J. Phytopathol. 2001, 149, 679–683. [Google Scholar] [CrossRef]
- Senechkin, I.V.; van Overbeek, L.S.; van Bruggen, A.H. Greater Fusarium wilt suppression after complex than after simple organic amendments as affected by soil pH, total carbon and ammonia-oxidizing bacteria. Appl. Soil Ecol. 2014, 73, 148–155. [Google Scholar] [CrossRef]
- Hiddink, G.A.; Bruggen, A.H.C.; Termorshuizen, A.J.; Raaijmakers, J.M. Effect of Organic Management of Soils on Suppressiveness to Gaeumannomyces graminis var. tritici and its Antagonist, Pseudomonas fluorescens. Eur. J. Plant Pathol. 2005, 113, 417–435. [Google Scholar] [CrossRef]
- Nam, M.H.; Lee, H.C.; Kim, T.I.; Lee, E.M.; Yoon, H.S. Effect of Nutrition Solution pH and Electrical Conductivity on Fusarium Wilt on Strawberry Plants in Hydroponic Culture. Res. Plant Dis. 2018, 24, 26–32. [Google Scholar] [CrossRef]
- Retamales, J.B.; Mena, C.; Lobos, G.; Morales, y. A regression analysis on factors affecting yield of highbush blueberries. Sci. Hortic. 2015, 186, 7–14. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.K.; Cai, L. Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat. Commun. 2022, 13, 7890. [Google Scholar] [CrossRef]
- Shujuan, C. Relationship Between Rhizosphere Soil Properties, Root Metabolome and Poor Plant Growth in North High-Bush Blueberry (Vaccinium corymbosum) and Its Mechanism; Tongfang Knowledge Network (Beijing) Technology Co., Ltd.: Beijing, China, 2019. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2006, 39, 1–23. [Google Scholar] [CrossRef]
- Quaiser, A.; Ochsenreiter, T.; Lanz, C.; Schuster, S.C.; Treusch, A.H.; Eck, J.; Schleper, C. Acidobacteria form a coherent but highly diverse group within the bacterial domain: Evidence from environmental genomics. Mol. Microbiol. 2003, 50, 563–575. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Jean, C.; Cheryl, K. Mobile genetic elements in the bacterial phylum Acidobacteria. Mob. Genet. Elem. 2012, 2, 179–183. [Google Scholar]
- de Castro, V.H.L.; Schroeder, L.F.; Quirino, B.F.; Kruger, R.H.; Barreto, C.C. Acidobacteria from oligotrophic soil from the Cerrado can grow in a wide range of carbon source concentrations. Can. J. Microbiol. 2013, 59, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Venturini, A.M.; Meyer, K.M.; Klein, A.M.; Tiedje, J.M.; Bohannan, B.J.; Nüsslein, K.; Tsai, S.M.; Rodrigues, J.L. Differential Response of Acidobacteria Subgroups to Forest-to-Pasture Conversion and Their Biogeographic Patterns in the Western Brazilian Amazon. Front. Microbiol. 2015, 6, 1443. [Google Scholar] [CrossRef]
- Sanguin, H.; Sarniguet, A.; Gazengel, K.; Moënne-Loccoz, Y.; Grundmann, G.L. Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture. New Phytol. 2009, 184, 694–707. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hamonts, K.; Anderson, C.; Singh, B.K. Keystone microbial taxa regulate the invasion of a fungal pathogen in agro-ecosystems. Soil Biol. Biochem. 2017, 111, 10–14. [Google Scholar] [CrossRef]
- Fajun, Y.; Weiqi, W.; Ziwei, W.; Hongda, X.; Maoquan, G.; Shaoying, L.; Xiande, L.; Yongxun, Z.; Chuanhui, W. Effects of Soil Nutrient and Measurement Ratioon Bacterial Community Structure in Fujian Tea Garden. J. Soil Water Conserv. 2023, 37, 209–218. [Google Scholar]
- Jing, W.; Huiping, Z.; Xiao, S.; Qiaojin, F.; Xuebang, L.; Fengqin, L.; Yizhen, S.; Yun, C.; Zhiliang, Y. Microbial diversity in rhizosphere soil of Anemone altaica. Guihaia 2023, 43, 1467–1477. [Google Scholar]
- Wang, H.; Zhu, W.; Cui, J.; Yang, P.; Du, J.; Sun, H.; Zhou, Y.; Jiang, S. Characteristics of Rhizosphere Soil Microbial Community Structure and Its Effects on Secondary Metabolites of Notopterygium incisum. J. Anhui Agric. Sci. 2023, 51, 173–178. [Google Scholar]
- Stone, B.W.; Li, J.; Koch, B.J.; Blazewicz, S.J.; Dijkstra, P.; Hayer, M.; Hofmockel, K.S.; Liu, X.J.A.; Mau, R.L.; Morrissey, E.M.; et al. Nutrients cause consolidation of soil carbon flux to small proportion of bacterial community. Nat. Commun. 2021, 12, 3381. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.L.; Zhao, Y.Y.; Wang, D.X.; Xie, T.Q.; Lv, J.G.; Jin, D.F.; Shi, H.Z. Effects of Organic Fertilizers on Nitrogen Mineralization, Soil Enzyme Activities and Microbial Communities in Tobacco-planting Soil. Soils 2023, 55, 1025–1034. [Google Scholar]
- Bollmann, A.; Sedlacek, C.J.; Norton, J.; Laanbroek, H.J.; Suwa, Y.; Stein, L.Y.; Klotz, M.G.; Arp, D.; Sayavedra-Soto, L.; Lu, M.; et al. Complete genome sequence of Nitrosomonas sp. Is79, an ammonia oxidizing bacterium adapted to low ammonium concentrations. Stand. Genom. Sci. 2013, 7, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, A.; Fu, W.; Peng, D.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Tobacco-associated with Methylophilus sp. FP-6 enhances phytoremediation of benzophenone-3 through regulating soil microbial community, increasing photosynthetic capacity and maintaining redox homeostasis of plant. J. Hazard. Mater. 2022, 431, 128588. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhou, S.; Zhou, C.; Zhang, Z.; Chen, W.; Dong, Z.; Li, X.; Tao, Y.; Zou, X.; Li, X. Differences and associations of endophytic microbial communities in different ecological niches of chilli pepper. Acta Microbiol. Sin. 2025, 65, 169–181. [Google Scholar]
- Wang, H.; Guo, S.; Huang, M.; Thorsten, L.H.; Wei, J. Ascomycota has a faster evolutionary rate and higher species diversity than Basidiomycota. Sci. China Life Sci. 2010, 53, 1163–1169. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Ma, C.X.; Yu, Q.; Liu, Y.J.; Li, J.G.; Yu, X.; Zhao, Y.; Qu, L.Y. The characteristics of rhizosphere soil fungal community of restored vegetation inInner Mongolia open pit mine dump. Acta Ecol. Sin. 2025, 4, 1–13. [Google Scholar]
- Guo, J.; Liu, W.; Zhu, C.; Luo, G.; Kong, Y.L.; Ling, N.; Wang, M.; Dai, J.; Shen, Q.; Guo, S. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Helgason, T.; Fitter, A.H. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J. Exp. Bot. 2009, 60, 2465–2480. [Google Scholar] [CrossRef]
- Wei, Y.; Dai, Y. Ecological function of wood-inhabiting fungi in forest ecosystem. Chin. J. Appl. Ecol. 2004, 10, 1935–1938. [Google Scholar]
- Chen, Y.Y.; Xia, W.Y.; Zhao, H.; Zeng, M. Effects of deep vertical rotary tillage on soil enzyme activity, microbial community structure and functional diversity of cultivated land. Acta Ecol. Sin. 2022, 42, 5009–5021. [Google Scholar]
- Gibbons, S.M. Microbial community ecology: Function over phylogeny. Nat. Ecol. Evol. 2017, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.N.; Wang, H.X.; Lu, C.H.; Liu, Z.; Shen, Y.M. Ansamycins Produced by Streptomyces sp. LZ35. Chin. Pharm. J. 2011, 46, 1317–1320. [Google Scholar]
- Ikeda, H.; Hideshima, T.; Fulciniti, M.; Lutz, R.J.; Yasui, H.; Okawa, Y.; Kiziltepe, T.; Vallet, S.; Pozzi, S.; Santo, L.; et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4028–4037. [Google Scholar] [CrossRef]
- Jana, F.; Simone, E.; Carsten, Z.; Andreas, K. Targeting heat-shock-protein 90 (Hsp90) by natural products: Geldanamycin, a show case in cancer therapy. Nat. Prod. Rep. 2013, 30, 1299–1323. [Google Scholar]
- Lounis, N.; Roscigno, G. In vitro and in vivo activities of new rifamycin derivatives against mycobacterial infections. Curr. Pharm. Des. 2004, 10, 3229–3238. [Google Scholar] [CrossRef]
- Bruce, D.; Elissa, R.M.; Jackson, S. Cost-benefit analysis comparing trough, two-level AUC, and Bayesian AUC dosing for vancomycin’ by V. Lee et al. Clin. Microbiol. Infect. 2021, 27, 927–928. [Google Scholar]
- Daniel, W.; Franziska, K.; Diederik, V.T.; Ghislaine, R.; Pierre-Emmanuel, C. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar]
- Zhang, M.; Che, R.; Cheng, Z.; Zhao, H.; Wu, C.; Hu, J.; Zhang, S.; Liu, D.; Cui, X.; Wu, Y. Decades of reforestation significantly change microbial necromass, glomalin, and their contributions to soil organic carbon. Agric. Ecosyst. Environ. 2023, 346, 108362. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Ai, Y.; Li, F.; Wu, Z. Research Progress on Effect of Arbuscular Mycorrhizal Fungi on Soil Carbon Balance. J. Agric. Sci. Technol. 2025, 1–14. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Sun, Q.; Niu, T.; Zhang, J. Arbuscular mycorrhizal fungus alleviates anthracnose disease in tea seedlings. Front. Plant Sci. 2023, 13, 1058092. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Sun, Q.; Niu, T.; Zhang, J. The relationship between arbuscular mycorrhizal fungi (AMF) and anthracnose occurrence in Camellia oleifera. J. Biol. 2023, 40, 35–40. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).